Abstract
Background
Prostatitis is a poorly understood disease and increasing evidence suggests inflammation is involved in other prostatic diseases, including prostate cancer.
Methods
The ability of pre-activated CD8 T cells to induce prostatitis was examined by adoptive transfer into POET-3 mice or POET-3/Luc/Pten−/+ mice. Characterization of the inflammatory response was determined by examining leukocyte infiltration by histological analysis, flow cytometry and by evaluating cytokine and chemokine levels in prostate tissue. The impact of inflammation on the prostate was evaluated by monitoring epithelial cell proliferation over time.
Results
Initiation of inflammation by ovalbumin specific CD8+ T cells (OT-I cells) resulted in development of acute prostatitis in the anterior, dorsolateral and anterior prostate of POET-3 and POET-3/Luc/Pten−/+ mice. Acute prostatitis was characterized by recruitment of adoptively transferred OT-I cells and importantly, autologous CD4+ and CD8+ T cells, myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg). In concert with leukocyte infiltration elevated levels of pro-inflammatory cytokines and chemokines were observed. Inflammation also resulted in marked epithelial cell proliferation that was sustained up to 80 days post adoptive-transfer of OT-I cells.
Conclusions
The POET-3 model represents a novel mouse model to study both acute and chronic prostate inflammation in an antigen-specific system. Further, the POET-3 mouse model can be crossed with other genetic models of disease such as the C57/Luc/Pten−/− model of prostate cancer, allowing the impact of prostatitis on other prostatic diseases to be evaluated.
Keywords: Prostate inflammation, prostatitis, prostate cancer, mouse model, POET-3, CD8 T cell, C57/Luc/Pten−/− mouse
Introduction
Prostatitis is the most frequently diagnosed outpatient condition in urologic practice, and epidemiological studies estimate that 2–16% of men worldwide suffer from this disease (1–8). Men who have been diagnosed with prostatitis experience symptoms resulting in reduced quality of life including urinary frequency, urgency, dysuria, pain with ejaculation, erectile dysfunction and reduced semen quality (6,9–11). The most common form of prostatitis, comprising 90–95% of cases, is chronic prostatitis/chronic pelvic pain syndrome (CP-CPPS), and is associated with other prostatic diseases such as benign prostatic hyperplasia (BPH), and prostate cancer (12–15). However, despite its prevalence prostatitis remains a poorly understood disease, with the majority of diagnosed cases in humans being of unknown etiology (3–4,9). Staggering medical costs and an increased risk of developing prostate cancer call for a more careful examination of both prostatitis and its impact on other prostatic diseases such as prostate cancer (16).
While the exact cause(s) of chronic prostatitis remain unknown, growing evidence suggests an immune origin for non-bacterial prostatitis. T cells isolated from men with chronic prostatitis proliferate and produce cytokines in response to seminal plasma, prostate specific antigen (PSA), or prostatic acid phosphatase (PAP) (1,17–18). These data suggest an auto-immune element is involved in some cases of chronic prostatitis; however, it remains unclear if chronic prostatitis is truly an auto-immune disease.
Several factors have contributed to the lack of knowledge about the cause(s) of chronic prostatitis, the largest being a lack of animal models to study inflammatory responses in the prostate (13,19). While several rodent models have been utilized to help better understand prostatitis, each has its own limitations (19). For example, Wistar, Lewis and Copenhagen rats, as well as NOD mice, appear to have some genetic susceptibility to prostatitis, as both models were found to spontaneously develop some level of prostate inflammation with age, but have the disadvantage of requiring aged animals (20–22). In the case of the NOD mice, spontaneous inflammation is in part directed against prostate steroid binding protein (PSBP) (23). However, while this model provides the ability to study antigen specific inflammation in the prostate, the data are complicated by altered tolerance mechanisms in the NOD background. Irritants, diet, hormones and mechanical obstruction have also been used to induce prostatitis, but these artificial methods also lack antigen specificity and may only represent a few of many potential inducers of prostatitis (14). Thymectomy 3 days after birth results in the development of prostate inflammation; however, inflammation was lobe specific and was strain dependent with approximately a 30% occurrence rate in C57BL/6 mice (24–26). Importantly, these models lack of antigen-specificity, and show large variations in age, strain susceptibility, anatomical site(s) affected, and are complicated by defective tolerance mechanisms, all of which makes data interpretation difficult. There is antigen specificity but altered tolerance mechanisms.
In order to study the initiation and regulation of prostatitis, we developed an antigen specific model of prostatitis, the POET (Prostate Ovalbumin Expressing Transgenic) mouse model. In the POET-1 mouse, tolerance mechanisms prevented severe prostate inflammation, even with mOVA specific CD8 and CD4 T cells (27–28). In contrast, using a different founder strain, the POET-3 mouse, we now present the POET-3 mouse as a suitable mouse model for studying the development of acute and chronic prostate. This model provides antigenic specificity, providing a basis for distinguishing the initiating process (OVA reactivity) from responses to other self proteins. Further, in prostatitis models where general tolerance mechanisms are altered inflammation is present in multiple organs. In contrast, the POET-3 model allows prostatitis to be monitored in a prostate specific manner. Looking simultaneously at inflammation in all three anatomical regions of the mouse prostate, we show inflammation is induced in dorsolaleral, anterior and ventral prostate lobes. Notably, prostatitis in the POET-3 model induces epithelial cell proliferation, increases collagen deposition, and enhances stromal cell responses. Finally, we demonstrate that crossing the POET-3 mouse with a mouse model of prostate cancer, C57/Luc/Pten−/− mice, results in an animal model wherein the effects of acute and chronic inflammation on the impact of prostate cancer can be studied.
Materials and Methods
Mice
Prostate ovalbumin expressing transgenic-3 mice (POET-3) were generated as previously described (27–28). C57BL/6 animals were obtained from National Cancer Institute (NCI; Bethesda, MD, USA). Thy1.1+ OT-I (OT-I) mice were generated by breeding C57BL/6 Thy1.1+ mice (Jackson Laboratories, Bar Harbor, ME) to OT-I mice (a gift of Dr. W.E. Heath, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia). B6.Cg-Tg(Pbsn-cre)4Prb (Pb-Cre4+) mice were obtained from the NIH Mouse Models of Human Cancer Consortium. C;129S4-Ptentm1Hwu/J (Ptenfl/fl) mice were obtained from Jackson laboratories. FVB.129S6 (B6)-Gt (ROSA)26Sortm1(Luc)Kael/J (ROSA26-LSL-Luc) mice were a kind gift from Dr. William Kaelin (Dana-Farber Cancer Institute). Mice were intercrossed to combine alleles and backcrossed to C57BL/6J-Tyrc-2J/J (Jackson Labs) for 7 generations to generate C57/Luc/Pten −/− mice. To obtain POET 3/Luc/Pten +/− mice, C57/Luc/Pten −/− mice were crossed to POET 3 mice. All animals were bred and maintained under specific pathogen free conditions at the University of Iowa and Purdue University animal care facilities. Unless stated otherwise in text animals were used between 7 and 14 weeks of age. All protocols for the reported animal studies were approved by The Lab Animal Programs and the University of Iowa and Purdue University.
Induction of Inflammation
Splenocytes were isolated from Thy1.1+OT-I mice and cultured at 1×106/mL with 1µg/mL SIINFEKL (Ova peptide 257–264, American Peptide, Sunnyvale, CA) for 48hrs. Live cells were purified by Fico/Lite (Atlanta Biologicals, Lawrenceville, GA) and 1×107 cells were injected iv into POET-3 or C57BL/6 mice.
Flow Cytometry
Prostate tissue was placed in a solution of 1 µg/mL Collagenase D (Roche Diagnostics, Indianapolis, IN) in RPMI containing 10% FBS with DNASE (Sigma-Aldrich, Saint Louis, MO). Tissue was minced and placed at 37°C for 1 hr for digestion followed by passing through a 70 µm filter. Spleens and lymph nodes were removed and ground between frosted microscope slides in supplemented RMPI. Red blood cells were lysed with ACK buffer and passed through a 70 µm filter. Single cell suspensions were then stained with directly conjugated antibodies (eBioscience and BioLegend, San Diego, CA) according to manufacturer instructions. PE conjugated OVA Tetramer was purchased from BD (Franklin Lakes, NJ). Flow-cytometric analysis was performed at the University of Iowa and Purdue University Flow Cytometry Shared Resources. Data was analyzed using FlowJo software (Tree Star, Ashland, OR).
Absolute numbers of leukocytes in tissue digests
To assess the absolute numbers of infiltrating cells in each prostate lobe, tissues were weighed, processed and stained for flow cytometry as described above. Fluoresbrite Fluorescent Monodisphere Carboxylated Microspheres (Polysciences INC, Warrington, PA) were added to equal volumes of filtered whole prostate digest. Prior to addition number of beads are determined using a hemocytometer and light microscope. The following equation is used to determine absolute number of cells in a sample:
(# cells counted/# beads counted) ×((# beads/ml)×#mls of added beads). This number was then divided by the number of mg of prostate tissue in order to account for differences in prostate lobe size.
Histology and Immunohistochemistry
Prostate lobes were dissected and placed in 10% neutral buffered formalin or zinc-formalin (BD, Franklin Lakes, NJ), embedded in paraffin, sectioned, and processed for hematoxylin and eosin staining. Hematoxylin and eosin stained sections were scored in a blinded manner by a board-certified pathologist using the following scoring system. 0: no detectable inflammation; 1: mild inflammation (scattered leukocyte infiltration, lack of tissue alteration/damage); 2: moderate inflammation (few focal leukocyte aggregates ± mild interstitial edema and tissue alteration/damage); 3: severe inflammation (multifocal to coalescing aggregates of infiltrating leukocytes, ± interstitial edema, tissue alteration/damage). For Ki-67 staining, antigen retrieval was done using citrate buffer (pH6). Blocking was performed using DAKO serum free solution (DAKO, Carpinteria, CA) followed by incubation with a rabbit poly-clonal antibody to Ki-67 (1:100, Abcam, Cambridge, CA). Rabbit on mouse HRP polymer (Biocare, Concord CA) was applied to sections, after which slides were developed using the DAB kit (Vector Laboratories, Burlingame, CA). The slides were counterstained with hematoxylin (Vector Laboratories), dehydrated, cleared and mounted using Vectamount (Vector Laboratories). The number of Ki-67 positive epithelial cells was counted by a board-certified pathologist blinded to the study.
Bioplex
Prostate lobes were harvested, placed in lysis buffer and frozen at −80°C. Prostate lysates were homogenized and the protein concentration was determined using a BCA kit (Pierce, Rockford, IL). Samples were normalized to 500ug/ml and prepared for cytokine and chemokine protein analysis using a bioplex kit and conjugated beads (Bio-Rad, Berkeley, CA) and read by a Luminex machine (Bio-Rad).
Results
Induction of prostatitis in POET-3 mice
Prostatitis remains a highly prevalent yet poorly understood disease in part due to the lack of appropriate animal models (13,19). We therefore initiated studies with the goal of developing an antigen specific model system in order to dissect initiation and regulation of inflammatory processes in the prostate. To develop a mouse prostatitis model where consistency and timing of inflammation could be more carefully controlled, OT-I cells were pre-activated and transferred iv into sexually mature male POET-3 mice or C67BL/6 control mice. Seven days after adoptive transfer of OT-I cells, the prostates of POET-3 but not C57BL/6 controls had evidence of perivascular and periglandular infiltration of lymphocytes, macrophage/monocytes and neutrophils, and to a lesser extent mast cells and plasma cells (Figure 1A, B). Further, infiltration was accompanied by histopathological changes in the morphology of the prostate epithelium and stroma 7 days after adoptive transfer (Figure 1A, B). Both the epithelial and stromal layers of POET-3 prostates had signs of thickening, with marked increases in epithelial cell proliferation and apoptotic events, including anisocytosis, anisokaryosis, and luminal cellular and nuclear debris, as well as vacuolation of the myoepithelial layer (Figure 1A, B).
Figure 1. Adoptive transfer of OT-I cells results in acute prostatitis is in POET-3 mice.

OT-I cells were adoptively transferred into POET-3 or C57BL/6 mice and after 7 days, tissues were harvested for histology. (A–B) Representative H&E sections of POET-3 (top panels) or C57BL/6 (bottom panels) prostates. Images were taken with a 10x (A) or 20x objective (B). (C) Inflammatory scores assigned according to criteria outlined in materials and methods. Open symbols: POET-3 OT-I cells, closed symbols C57BL/6 OT-I cells.
Histological scoring was performed using the following guidelines: inflammatory cell infiltrates and aggregates, edema and tissue alteration/injury, and extensiveness and severity of these inflammatory phenomena. All prostate lobes from POET-3 mice harvested 7 days post transfer of pre-activated OT-I cells had characteristics of severe inflammation (Figure 1C). No inflammation was observed in other organs, including the urinary bladder and seminal vesicles (data not shown). Likewise, C57BL/6 controls, which do not express OVA, had no detectable inflammation in the anterior prostate and mild inflammation in the dorsolateral and ventral prostate without tissue alterations (Figure 1C). Thus, we conclude that inflammation is specific to the expression of OVA in POET-3 mice.
Characterization of inflammatory infiltrate
To more specifically assess the number and type of leukocytes infiltrating the inflamed prostate, inflammation was induced as described above and after 7 days infiltrating populations of leukocytes were examined by flow cytometry. Seven days post adoptive transfer of OT-I cells, significantly greater numbers of CD45+ cells were present all three prostate lobes of POET-3 compared to C57BL/6 controls (Figure 2A). In multiple experiments using naïve C57BL/6 mice, numbers of inflammatory cells as measured by infiltration of CD45+ leukocytes, were observed to be very low in the anterior, dorsolateral and ventral lobes (654± 194; 2571 ± 1330.6; 5165 ± 3168 CD45+ cells per 500,000 events, mean ± SD, n=10). These data demonstrate adoptive transfer of OT-I cells into POET-3 mice results in a strong acute inflammatory response, confirming the histological findings of severe, tissue-wide inflammation in POET-3 mice as described above.
Figure 2. T cell subsets accumulate in inflamed prostates during acute and chronic prostatitis.
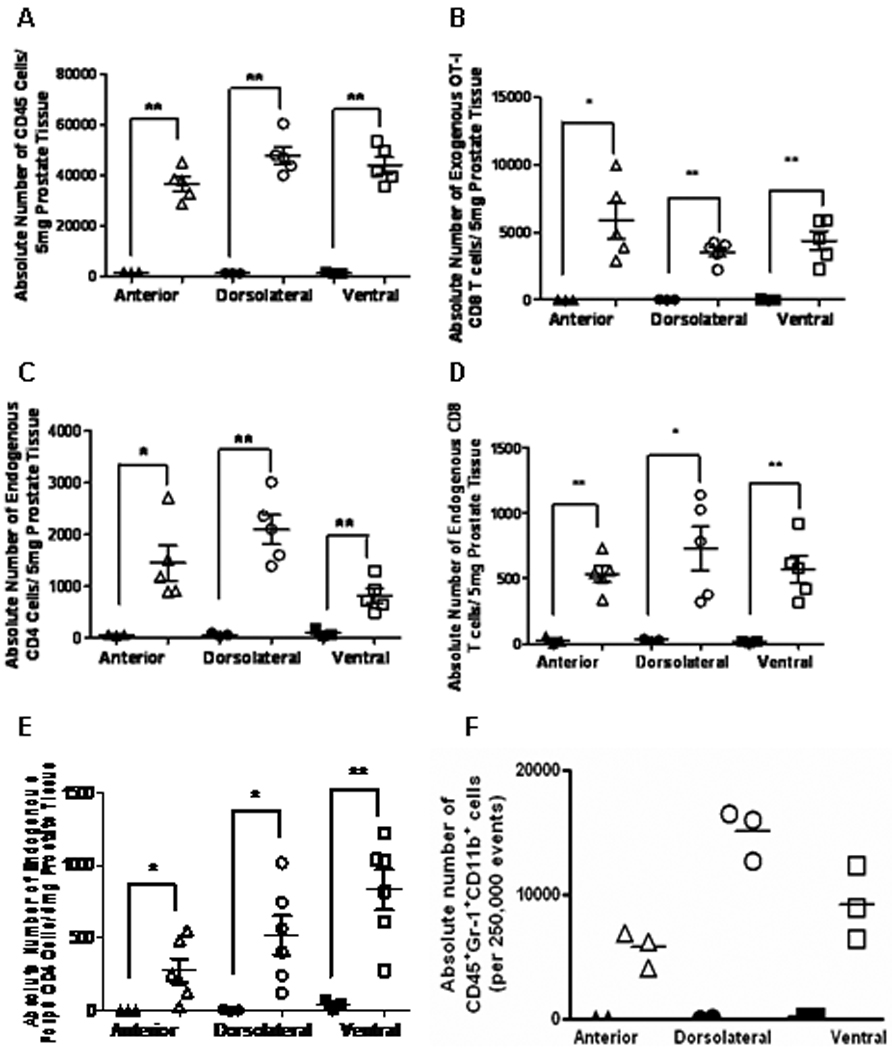
OT-I cells were adoptively transferred into POET-3 or C57BL/6 mice and after 7 days, tissues were harvested processed for analysis by flow cytometry. The absolute number of (A) CD45+ cells, (B) Thy1.1+CD8+, (C) Thy1.2+CD4+, (D) Thy1.2+CD8+ T cells, (E) CD45+Thy1.2+Foxp3+CD4+, (F) Gr-1+CD11b+ cells. Significance between groups was determined as * p<0.05 and **p<0.01. Statistics using two-tailed unpaired t test with Welch’s correction for unequal variances are reported (A, B, E). Statistics are reported using two-tailed nonparametric t test assuming unequal variances (C, D).
Histological analysis revealed recruitment of lymphocytes into inflamed prostates (Figure 1A). Therefore, we next determined the numbers CD4+ or CD8+ lymphocytes present in the inflamed prostate regions. Additionally, we determined whether the T cells belonged to the transferred OT-I cell population or were autologous (endogenous) T cells. To do this, we utilized the Thy-1.1/1.2 T cell congenic markers. In this system transferred OT-I cells are Thy1.1+, whereas endogenous T cells are Thy1.2+. In all three lobes of POET-3 prostates, numbers of infiltrating Thy1.1+ OT-I CD8+ T cells were significantly increased than in C57BL/6 prostates (Figure 2B). Numbers of both infiltrating endogenous CD4+ and CD8+ cells were present at significantly higher numbers in all three lobes of POET-3 prostates compared with C57BL/6 controls (Figure 2C, D). Accumulation of endogenous CD4+ T cells into inflamed prostates led us to next investigate if any of these cells were CD4+Foxp3+ regulatory T cells (Treg). Numbers of Treg were significantly increased in all three lobes of inflamed prostates compared to C57BL/6 controls (Figure 2E). Likewise, we tested for the infiltration of myeloid derived suppressor cell phenotype. Gr-1+CD11b+ cells were present in increased numbers in all lobes (Figure 2F).
Cytokine and chemokine levels in inflamed prostate tissue
To further characterize the type of inflammation established in this model system, we evaluated the cytokine profiles for each lobe of the prostate isolated from POET-3 mice or C57BL/6 controls. Seven days post transfer of OT-I cells, levels of several pro-inflammatory cytokines were elevated in all three regions of the POET-3 prostate relative to C57BL/6 controls (Figure 3). Seven days after adoptive transfer of OT-I cells, levels of IL-1β were found to be significantly increased in both the dorsolateral and ventral prostates of POET-3 mice given OT-I cells compared to naïve controls. IL-2 was significantly increased in the anterior and ventral prostate lobes relative to naïve control prostates. IL-6 levels were significantly increased relative to the naive controls only in anterior prostates. Levels of IL-12(p70) were significantly higher in the dorsolateral and ventral prostate lobes in inflamed POET-3 prostates. Surprisingly, at the time point tested levels of IFN-γ were slightly but not statistically significantly increased in inflamed prostates relative to naïve controls. TNF-α expression was significantly increased in the anterior and dorsolateral prostates relative to non-inflamed controls. MIP-1α (CCL2), MCP-1(CCL3) and RANTES (CCL5) were expressed at statistically significantly higher levels in all three lobes of POET-3 prostates relative to naïve controls. IL-4, IL-10 and IL-17 levels were below detectable limits (data not shown). Together, these findings provide evidence that induction of inflammation by adoptive transfer of OT-I cells initiates a strong pro-inflammatory environment characterized by both inflammatory cytokines and chemokines in the prostate.
Figure 3. Pro-inflammatory cytokines and chemokine expression is elevated in inflamed prostates.

OT-I cells were adoptively transferred into POET-3 and after 7 days prostate tissues from inflamed POET-3 (filled shapes) or naïve controls (open shapes) were placed in lysis buffer, homogenized and total protein quantified. The protein expression level of the indicated cytokine or chemokine in POET-3 prostates lysates was examined by bioplex assay. Significance between groups was determined as* p<0.05 ** p<0.01 using an unpaired t test with Welch’s correction.
Development of chronic prostatitis in POET-3 mice
After characterization of the acute inflammatory response in POET-3 mice, we next asked if adoptive transfer of OT-I cells was able to induce a chronic inflammatory response. To test this, pre-activated OT-I cells were adoptively transferred into POET-3 or C57BL/6 controls and prostate lobes were harvested 80 days following initial transfer. Approximately 12 weeks after initial transfer, most prostate lobes in the majority of POET-3 mice had some evidence of inflammation. Most often POET-3 prostate lobes had focal points of peri-vascular and peri-glandular inflammatory infiltrates, which included mast cells, macrophages, lymphocytes and less frequently, neutrophils and plasma cells. Occasionally macrophages were found in the interstitium and some areas of the ventral prostate had evidence of necrosis, apoptosis, and anisokaryosis (Figure 4A). Remarkably, even after 80 days, mild inflammation was still present in the anterior and dorsolateral prostates of POET-3 mice, whereas inflammation in the ventral prostate ranged from mild to severe (Figure 4B). No inflammation was noted in prostates of C57BL/6 mice 80 days after OT-I cell transfer (Figure 4B). Together, these data indicate sustained infiltration of leukocytes and histopathological changes characteristic of a smoldering chronic inflammatory response.
Figure 4. POET-3 mice develop chronic prostatitis.

OT-I cells were adoptively transferred into POET-3 or C57BL/6 mice and after 80 days, tissues were harvested for histology. (A) Representative H&E sections of POET-3 (top panels) or C57BL/6 (bottom panels) prostates. Images were taken with 10x objective. (B) Inflammatory scores assigned according to criteria outlined in materials and methods. Open symbols: POET-3 OT-I cells, closed symbols C57BL/6 OT-I cells.
Prostate inflammation induces sustained epithelial cell proliferation
Histological analysis revealed increased proliferation of the prostate epithelium in POET-3 mice after adoptive transfer of OT-I cells. To confirm these findings, tissue sections from POET-3 or C57BL/6 were evaluated for Ki-67 staining, a known marker expressed in the nuclei of proliferating cells. During acute prostatitis (day 7) significantly increased epithelial cell proliferation was observed in all lobes of POET-3 prostates relative to C56BL/6 controls (Figure 5A, C). The dorsolateral and anterior prostate lobes demonstrated the highest percentage of proliferating epithelial cells, with both basal and columnar epithelial cells predominating (Figure 5A. C). In contrast, increased proliferation of epithelial cells in the ventral prostate appeared to primarily be basal epithelial cells (Figure 5A, C). Remarkably, even after 80 days post adoptive transfer of OT-I cells into POET-3 mice proliferation of prostate epithelial cells in the anterior and dorsolateral prostate lobes remained significantly elevated compared to control mice (Figure 5B, C). Together these data demonstrate that inflammation has a significant and long lasting impact on the prostate epithelium.
Figure 5. Increased proliferation of prostate epithelium during acute and chronic prostate inflammation.
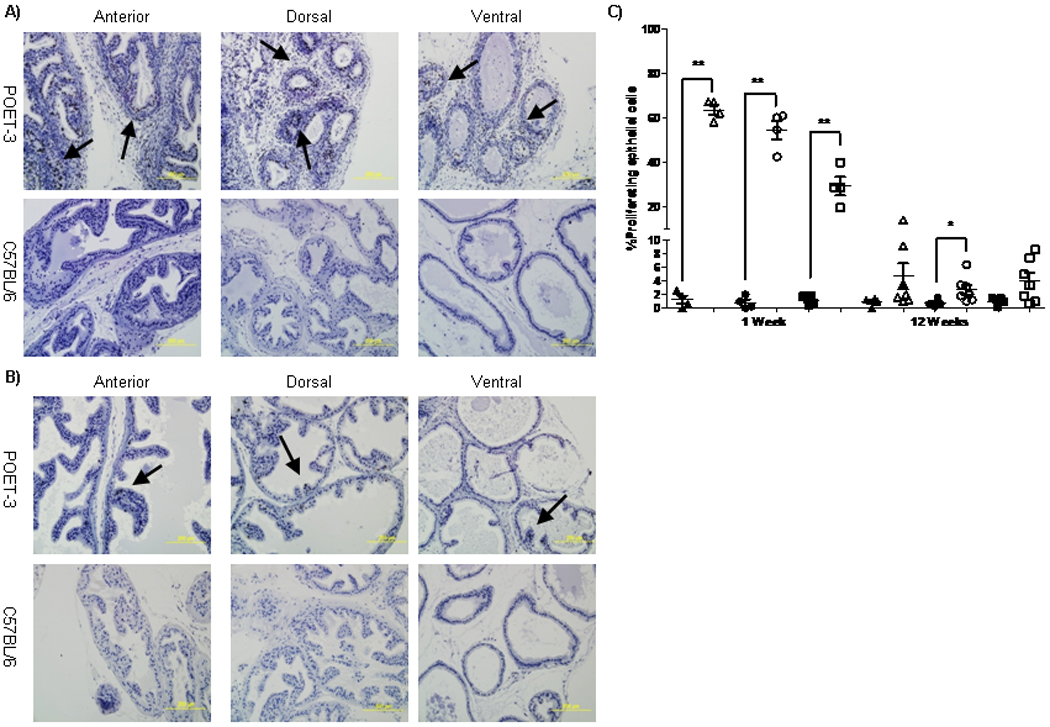
OT-I cells were adoptively transferred into POET-3 or C57BL/6 mice and after 7 (A) or 80 days (B) tissues were harvested for immunohistochemistry and the number of proliferating epithelial cells was evaluated by Ki-67 staining. (C) The percent proliferating epithelial cells in C57BL/6 (filled shapes) and POET-3 (open shapes) anterior (triangle), dorsolateral (circle) and ventral (square) prostates as determined by pathologist based on Ki67 staining. Significance between groups was defined as* p<0.05 ** p<0.01 using an unpaired t test with Welch’s correction.
Induction of inflammation in a genetically susceptible model of prostate cancer
The development of chronic prostatitis in POET-3 mice makes this model uniquely suitable for probing the impact of inflammation on the development and progression of prostate cancer. To develop a model in which to test this, we crossed the POET-3 mouse with a genetically susceptibility prostate cancer model, the C57/Luc/Pten−/− mouse model. Aged POET-3/Luc/Pten−/+ mice had low levels of spontaneous inflammation, especially in the dorsolateral prostate, as measured by increased infiltrates comprised of leukocyte populations including CD45+Gr-1+CD11b+ cells (Figure 6C, D, top panels). Importantly, adoptive transfer of pre-activated OT-I cells into POET-3/Luc/Pten−/+ mice induced prostatitis in all three lobes of the prostate, resulting in increased leukocyte infiltration including CD45+Gr-1+CD11b+ cells 6 days after adoptive transfer of OT-I cells (Figure 6A,C, D, bottom panels).
Figure 6. Aged POET-3/Luc/Pten−/+ mice exhibit spontaneous inflammation which worsens after adoptive transfer of OT-I cells.

OT-I cells were adoptively transferred into POET-3/Luc/Pten−/+ mice and after 7 days (A, C, D) or 30 days (B) lobes were harvested for histology (A, B) or for analysis by flow cytometry (C, D). (A) Representative H&E images of naïve or inflamed POET-3/Luc/Pten−/+ prostate tissues. (B) Representative histological changes as demonstrated by proliferative lesions characterized by giant cells (arrows) and cellular atypia characteristic of neoplasia in 7 month old POET-3/Luc/Pten−/+ mice (left panel), and by hyperplastic acini with proliferating epithelium characterized by increased apoptotic cells (arrows) and decreased lumen in inflamed 7 month old POET-3/Luc/Pten−/+ mice (right panel). (C, D) Representative data showing the percentage of CD45+ leukocytes (C) or CD45+Gr-1+CD11b+ cells (D) in prostates of POET-3/Luc/Pten−/+ mice.
Induction of chronic inflammation, 30 days after adoptive transfer of OT-I cells into aged POET-3/Luc/Pten−/+ mice resulted in hyperplastic acini characterized by a proliferative epithelium with apoptotic cells (arrows) and a reduced lumen (Figure 6 B). These data demonstrate chronic inflammation in POET-3/Luc/Pten−/+ mice resulted in alterations in the prostate epithelium.
The POET-3/Luc/Pten−/+ model is clinically relevant to human prostate cancer, as alterations in PTEN expression are implicated in the development of human prostate cancer (29–30). Secondly, C57/Luc/Pten −/+ mice slowly develop focal murine prostate intraepithelial neoplasia (mPIN) and do not progress to fulminate prostate cancer (manuscript submitted). This slow progression of disease makes for an ideal background on which to determine whether inflammation can accelerate progression of prostate cancer.
Discussion
The multiplicity of associations between prostate diseases and inflammation provide a strong rationale for better understanding the origin and regulation of prostatitis. Our results demonstrate that CD8 T cells specific for a transgenic prostate antigen, OVA, are capable of inducing both acute and chronic prostatitis in POET-3 mice. Importantly, inflammation is specifically induced in all lobes of the prostate in the POET-3 model, demonstrating the inflammatory response is not restricted to one area of the prostate as has been seen in other rodent models of prostate inflammation (19). Further, breeding these mice to other genetic models such as the POET-3/Luc/Pten−/+ mice allows for the induction of prostatitis in the context of a genetically predisposed animal model of prostate cancer. Thus, the POET-3/Luc/Pten−/+ mice for the first time allow the impact of chronic abacterial inflammation on the initiation and progression of prostate cancer to be evaluated.
There are several bacterial models of prostatitis (31–32); however, these models are not representative of the majority of human prostatitis cases where no bacterial agents can be identified (33). Numerous groups have utilized hormone treatments with 17β-estradiol to model prostate inflammation in rats (34–35). Hormonal treatment can modulate recruitment and function of lymphocytes, and therefore these models are not ideal for determining the role of specific immune populations in prostatitis (36–37). Another popular method to induce prostatitis used in both mice and rats involves injecting mice with male accessory gland or prostate homogenate or purified prostate specific proteins (23,38–39). Further, autoimmune disease models, such as the non-obese diabetic (NOD) and Aire−/− mice develop spontaneous prostatitis (23,40) among other inflammatory processes. Though the Aire−/− model develops prostatitis that is likely to mimic human prostatitis, the multi-organ autoimmunity in these mice complicates long-term studies, which are ultimately impacted by the survival rate of these mice. Further, in these models development of prostatitis is a result of circumvented peripheral tolerance mechanisms (23,40). In contrast to the existing animal models, the POET-3 model allows prostatitis to be studied in an animal in which immune tolerance mechanisms are intact and provides an antigen-specific model for studying regulatory mechanisms of inflammation.
The initiation of prostate inflammation by CD8+ T cells and the resulting accumulation of CD4+ T cell populations demonstrate that the POET-3 model is a clinically relevant model system in which to study the function of T cell subpopulations in prostatitis. Studies using human prostate tissue suggest the majority of BPH cases are characterized by CD8+ T cell infiltration (41). Further, another study using prostate samples from autopsies found that normal prostate tissue is characterized by leukocytes, comprised largely of T cells, with higher numbers located in the stroma then in the epithelium (42). While these data support the clinical relevance of the POET-3 model, they do not address the function of infiltrating cells.
One important issue concerning the presence of leukocytes in the prostate is determining what factors influence their migration into the tissue. The migration of leukocytes into the prostate may be influenced by the secretion of pro-inflammatory stimuli produced either by resident lymphocytes or by prostate epithelial cells. This hypothesis is supported by data showing prostate epithelial cells are directly involved in the recruitment of inflammatory cells (43). We found increased levels of RANTES, MCP-1 and MIP-1α during acute prostate inflammation. RANTES recruits T cells into inflamed tissues, and may play a role in recruitment of endogenous T cells into inflamed prostates. MCP-1 recruits monocytes, memory T cells and dendritic cells. MIP-1α can induce the production of IL-1 and TNFα from macrophages, both of which were found to be elevated in inflamed prostate tissue. Therefore, it is possible that MCP-1 recruited monocytes become activated in the prostate and their production of IL1, and TNFα is supported by MIP-1α.
Current evidence suggests inflammation promotes the risk of developing neoplastic changes by increasing the proliferation rate of epithelial cells in the presence of an inflammatory environment rich in pro-inflammatory cytokines, chemokines, growth factors and DNA damaging agents (44). Induction of prostatitis resulted in proliferation of approximately 80% of the epithelial cells during acute inflammation. Importantly, this proliferative response was prolonged and sustained up to 80 days post adoptive transfer of OT-I cells. These data are supported by recent in vitro experiments showing human prostate epithelial cell lines proliferate in response to CD4+ and CD8+ T cells (45). These data, together with earlier data showing that pro-inflammatory cytokines and chemokines are increased in the prostate demonstrate the inflammatory environment may support an environment promoting neoplastic or pre-neoplastic changes in the epithelium. Further, these data strongly support the use of the POET-3 model in the study of inflammation on prostate cancer by providing evidence that events linked with transformation are present during acute inflammation in the POET-3 system.
Induction of inflammation in POET-3/Luc/Pten−/+ mice resulted in histopatholgical changes and in the recruitment of CD45+ leukocytes and CD45+Gr-1+CD11b+ cells into inflamed prostate tissue (Figure 6). Myeloid derived suppressor cells are identified in mice as CD45+Gr-1+CD11b+ cells with suppressive function, and have been shown to play a role in regulation of immune responses to tumor antigens (46–47). The presence of CD45+Gr-1+CD11b+ cells in prostates of POET-3/Luc/Pten−/+ mice suggests these cells may play a role in development or progression of prostate cancer in part by dampening anti-tumor immunity. Together, these data provide a strong foundation for use of the POET-3/Luc/Pten−/+ model for studying the role of prostate inflammation and prostate cancer.
Conclusions
The POET-3 model is a suitable model for dissecting out the role of individual inflammatory stimuli and specific leukocyte subsets in prostatitis. Existing models of prostatitis are complicated by variability related to age, susceptibility, anatomical sites affected, genetic background and severity of inflammation. Importantly, the POET-3 model decreases all these variables, providing a model in which prostatitis can be induced in mice of the same age at relatively similar levels, making it a good model for testing clinical therapies. Finally, as preliminary data from the POET-3/Luc/Pten−/+ mice demonstrate, this model will prove to be a tool for examining how inflammation impacts prostate cancer initiation and progression.
Acknowledgments
Funding:
This work was supported by NIH grant DL084454.
References
- 1.Alexander RB, Brady F, Ponniah S. Autoimmune prostatitis: evidence of T cell reactivity with normal prostatic proteins. Urology. 1997;50(6):893–899. doi: 10.1016/S0090-4295(97)00456-1. [DOI] [PubMed] [Google Scholar]
- 2.Collins MM, Stafford RS, O'Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998;159(4):1224–1228. [PubMed] [Google Scholar]
- 3.Clemens JQ, Meenan RT, O'Keeffe-Rosetti MC, Gao SY, Brown SO, Calhoun EA. Prevalence of prostatitis-like symptoms in a managed care population. J Urol. 2006;176(2):593–596. doi: 10.1016/j.juro.2006.03.089. discussion 596. [DOI] [PubMed] [Google Scholar]
- 4.Nickel JC, Downey J, Hunter D, Clark J. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. J Urol. 2001;165(3):842–845. [PubMed] [Google Scholar]
- 5.Moon TD, Hagen L, Heisey DM. Urinary symptomatology in younger men. Urology. 1997;50(5):700–703. doi: 10.1016/S0090-4295(97)00336-1. [DOI] [PubMed] [Google Scholar]
- 6.Tan JK, Png DJ, Liew LC, Li MK, Wong ML. Prevalence of prostatitis-like symptoms in Singapore: a population-based study. Singapore Med J. 2002;43(4):189–193. [PubMed] [Google Scholar]
- 7.Kunishima Y, Matsukawa M, Takahashi S, Itoh N, Hirose T, Furuya S, Takatsuka K, Mori M, Tsukamoto T. National institutes of Health Chronic Prostatitis Symptom Index for Japanese men. Urology. 2002;60(1):74–77. doi: 10.1016/s0090-4295(02)01636-9. [DOI] [PubMed] [Google Scholar]
- 8.Cheah PY, Liong ML, Yuen KH, Teh CL, Khor T, Yang JR, Yap HW, Krieger JN. Chronic prostatitis: symptom survey with follow-up clinical evaluation. Urology. 2003;61(1):60–64. doi: 10.1016/s0090-4295(02)02081-2. [DOI] [PubMed] [Google Scholar]
- 9.Yang CC, Lee JC, Kromm BG, Ciol MA, Berger RE. Pain sensitization in male chronic pelvic pain syndrome: why are symptoms so difficult to treat? J Urol. 2003;170(3):823–826. doi: 10.1097/01.ju.0000082710.47402.03. discussion 826–827. [DOI] [PubMed] [Google Scholar]
- 10.McNaughton Collins M, Pontari MA, O'Leary MP, Calhoun EA, Santanna J, Landis JR, Kusek JW, Litwin MS. Quality of life is impaired in men with chronic prostatitis: the Chronic Prostatitis Collaborative Research Network. J Gen Intern Med. 2001;16(10):656–662. doi: 10.1111/j.1525-1497.2001.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RU, Wise D, Sawyer T, Chan CA. Sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome: improvement after trigger point release and paradoxical relaxation training. J Urol. 2006;176(4 Pt 1):1534–1538. doi: 10.1016/j.juro.2006.06.010. discussion 1538–1539. [DOI] [PubMed] [Google Scholar]
- 12.Krieger JN, Nyberg L, Jr., Nickel JC. NIH consensus definition and classification of prostatitis. Jama. 1999;282(3):236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 13.Haverkamp J, Charbonneau B, Ratliff TL. Prostate inflammation and its potential impact on prostate cancer: a current review. J Cell Biochem. 2008;103(5):1344–1353. doi: 10.1002/jcb.21536. [DOI] [PubMed] [Google Scholar]
- 14.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsasser-Beile U, Przytulski B, Gierschner D, Grussenmeyer T, Katzenwadel A, Leiber C, Deckart A, Wetterauer U. Comparison of the activation status of tumor infiltrating and peripheral lymphocytes of patients with adenocarcinomas and benign hyperplasia of the prostate. Prostate. 2000;45(1):1–7. doi: 10.1002/1097-0045(20000915)45:1<1::aid-pros1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Pontari MA, Joyce GF, Wise M, McNaughton-Collins M. Prostatitis. J Urol. 2007;177(6):2050–2057. doi: 10.1016/j.juro.2007.01.128. [DOI] [PubMed] [Google Scholar]
- 17.Motrich RD, Maccioni M, Molina R, Tissera A, Olmedo J, Riera CM, Rivero VE. Presence of INFgamma-secreting lymphocytes specific to prostate antigens in a group of chronic prostatitis patients. Clin Immunol. 2005;116(2):149–157. doi: 10.1016/j.clim.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Klyushnenkova EN, Ponniah S, Rodriguez A, Kodak J, Mann DL, Langerman A, Nishimura MI, Alexander RB. CD4 and CD8 T-lymphocyte recognition of prostate specific antigen in granulomatous prostatitis. J Immunother. 2004;27(2):136–146. doi: 10.1097/00002371-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Vykhovanets EV, Resnick MI, MacLennan GT, Gupta S. Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Dis. 2007;10(1):15–29. doi: 10.1038/sj.pcan.4500930. [DOI] [PubMed] [Google Scholar]
- 20.Rivero VE, Cailleau C, Depiante-Depaoli M, Riera CM, Carnaud C. Non-obese diabetic (NOD) mice are genetically susceptible to experimental autoimmune prostatitis (EAP) J Autoimmun. 1998;11(6):603–610. doi: 10.1006/jaut.1998.0248. [DOI] [PubMed] [Google Scholar]
- 21.Muntzing J, Sufrin G, Murphy GP. Prostatitis in the rat. Scand J Urol Nephrol. 1979;13(1):17–22. doi: 10.3109/00365597909179995. [DOI] [PubMed] [Google Scholar]
- 22.Lundgren R, Holmquist B, Hesselvik M, Muntzing J. Treatment of prostatitis in the rat. Prostate. 1984;5(3):277–284. doi: 10.1002/pros.2990050305. [DOI] [PubMed] [Google Scholar]
- 23.Penna G, Amuchastegui S, Cossetti C, Aquilano F, Mariani R, Giarratana N, De Carli E, Fibbi B, Adorini L. Spontaneous and prostatic steroid binding protein peptide-induced autoimmune prostatitis in the nonobese diabetic mouse. J Immunol. 2007;179(3):1559–1567. doi: 10.4049/jimmunol.179.3.1559. [DOI] [PubMed] [Google Scholar]
- 24.Taguchi O, Nishizuka Y. Self tolerance and localized autoimmunity. Mouse models of autoimmune disease that suggest tissue-specific suppressor T cells are involved in self tolerance. J Exp Med. 1987;165(1):146–156. doi: 10.1084/jem.165.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setiady YY, Ohno K, Samy ET, Bagavant H, Qiao H, Sharp C, She JX, Tung KS. Physiologic self antigens rapidly capacitate autoimmune disease-specific polyclonal CD4+ CD25+ regulatory T cells. Blood. 2006;107(3):1056–1062. doi: 10.1182/blood-2005-08-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14(1–2):15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 27.Lees JR, Charbonneau B, Hayball JD, Diener K, Brown M, Matusik R, Cohen MB, Ratliff TL. T-cell recognition of a prostate specific antigen is not sufficient to induce prostate tissue destruction. Prostate. 2006;66(6):578–590. doi: 10.1002/pros.20307. [DOI] [PubMed] [Google Scholar]
- 28.Lees JR, Charbonneau B, Swanson AK, Jensen R, Zhang J, Matusik R, Ratliff TL. Deletion is neither sufficient nor necessary for the induction of peripheral tolerance in mature CD8+ T cells. Immunology. 2006;117(2):248–261. doi: 10.1111/j.1365-2567.2005.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60(6):1541–1545. [PubMed] [Google Scholar]
- 30.Whang YE, Wu X, Suzuki H, Reiter RE, Tran C, Vessella RL, Said JW, Isaacs WB, Sawyers CL. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci U S A. 1998;95(9):5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elkahwaji JE, Hauke RJ, Brawner CM. Chronic bacterial inflammation induces prostatic intraepithelial neoplasia in mouse prostate. Br J Cancer. 2009;101(10):1740–1748. doi: 10.1038/sj.bjc.6605370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elkahwaji JE, Ott CJ, Janda LM, Hopkins WJ. Mouse model for acute bacterial prostatitis in genetically distinct inbred strains. Urology. 2005;66(4):883–887. doi: 10.1016/j.urology.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Krieger JN, Riley DE, Cheah PY, Liong ML, Yuen KH. Epidemiology of prostatitis: new evidence for a world-wide problem. World J Urol. 2003;21(2):70–74. doi: 10.1007/s00345-003-0329-0. [DOI] [PubMed] [Google Scholar]
- 34.Vykhovanets EV, Resnick MI, Marengo SR. Intraprostatic lymphocyte profiles in aged wistar rats with estradiol induced prostate inflammation. J Urol. 2006;175(4):1534–1540. doi: 10.1016/S0022-5347(05)00652-X. [DOI] [PubMed] [Google Scholar]
- 35.Stoker TE, Robinette CL, Cooper RL. Perinatal exposure to estrogenic compounds and the subsequent effects on the prostate of the adult rat: evaluation of inflammation in the ventral and lateral lobes. Reprod Toxicol. 1999;13(6):463–472. doi: 10.1016/s0890-6238(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 36.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, Waters WB, Kast WM, Kwon ED. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, Allison JP, Kwon ED. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173(10):6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 38.Liu KJ, Chatta GS, Twardzik DR, Vedvick TS, True LD, Spies AG, Cheever MA. Identification of rat prostatic steroid-binding protein as a target antigen of experimental autoimmune prostatitis: implications for prostate cancer therapy. J Immunol. 1997;159(1):472–480. [PubMed] [Google Scholar]
- 39.Fong L, Ruegg CL, Brockstedt D, Engleman EG, Laus R. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J Immunol. 1997;159(7):3113–3117. [PubMed] [Google Scholar]
- 40.Hou Y, DeVoss J, Dao V, Kwek S, Simko JP, McNeel DG, Anderson MS, Fong L. An aberrant prostate antigen-specific immune response causes prostatitis in mice and is associated with chronic prostatitis in humans. J Clin Invest. 2009;119(7):2031–2041. doi: 10.1172/JCI38332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robert G, Descazeaud A, Nicolaiew N, Terry S, Sirab N, Vacherot F, Maille P, Allory Y, de la Taille A. Inflammation in benign prostatic hyperplasia: a 282 patients' immunohistochemical analysis. Prostate. 2009;69(16):1774–1780. doi: 10.1002/pros.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bostwick DG, de la Roza G, Dundore P, Corica FA, Iczkowski KA. Intraepithelial and stromal lymphocytes in the normal human prostate. Prostate. 2003;55(3):187–193. doi: 10.1002/pros.10224. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004;61(1):60–72. doi: 10.1002/pros.20061. [DOI] [PubMed] [Google Scholar]
- 44.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDowell KL, Begley LA, Mor-Vaknin N, Markovitz DM, Macoska JA. Leukocytic promotion of prostate cellular proliferation. Prostate. 2010;70(4):377–389. doi: 10.1002/pros.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13(7):828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]


