Abstract
Background
Pelvic lymph node dissection (PLND) is an important component of prostate cancer staging and treatment, especially for surgical patients with high-risk tumor features. It is not clear how the shift from open radical prostatectomy (ORP) to minimally invasive radical prostatectomy (MIRP) has affected use of PLND. Our objective was to identify predictors of PLND and assess the impact of surgical technique in a contemporary, population-based cohort.
Methods
In Surveillance, Epidemiology, and End Results (SEER) cancer registry data linked with Medicare claims, we identified men who had ORP or MIRP for prostate cancer in 2003–2007. We evaluated the impact of surgical approach on PLND and examined interactions between surgical procedure, PSA and Gleason score, controlling for patient and tumor characteristics.
Results
Of 6,608 men who had ORP or MIRP, 70% (n=4,600) had PLND. Use of PLND declined over time, overall and within subgroups defined by procedure type. PLND was 5 times more likely in men receiving ORP than MIRP, controlling for patient and tumor characteristics. Elevated PSA and biopsy Gleason score, but not clinical stage, were associated with greater odds of PLND in both ORP and MIRP groups. However, the magnitude of the association between these factors and PLND was significantly greater for ORP patients.
Conclusion
PLND was less common in men who received MIRP, independent of tumor risk factors. A decline in PLND rates was not fully explained by an increase in MIRP. These trends may signal a surgical approach-dependent disparity in prostate cancer staging and therapy.
Keywords: Prostate Cancer; Lymph Nodes; Lymphadenectomy; SEER-Medicare; Minimally Invasive Surgery; Radical Prostatectomy (Open Robotic, Laparoscopic)
INTRODUCTION
The incidence of prostate cancer in the general population is estimated at 192,280 new diagnoses and 27,360 mortalities in 20091. Prognosis improvements related to innovation in diagnosis and treatment are clearly identifiable 2. The surgical management of prostate cancer is a mainstay of therapy for men with localized disease, even for those with high-risk, clinically localized cancers 3. The standard of surgical care, open radical prostatectomy (ORP) has been overshadowed in the United States by robotic assisted laparoscopic techniques 4,5. This shift is related to the purported improved post operative recovery, diminished blood loss and improved quality-of-life outcomes with minimally invasive radical prostatectomy (MIRP) although the market appeal of robotic surgery has also played a central role. 6,7 Current literature emphasizes surgical experience 8 and case volume 9,10 as primary contributors to surgical outcomes rather then surgical modality. Additionally, although still debated, recent population-based analyses suggest that MIRP and ORP have similar oncologic and functional outcomes. 11, 12
PLND is a well-accepted staging modality in prostate cancer, although its clinical indication in lower-risk patients and therapeutic benefit are controversial 13. Despite this, similar patients undergoing either MIRP or ORP should theoretically be subjected to identical preoperative risk-based decision-making regarding PLND. Various prediction tools are used for this purpose 14, and serve as the basis for National Comprehensive Cancer Network [NCCN] 15 and American Urological Association [AUA] 16 guidelines. While investigations have evaluated how the uptake of MIRP is associated with surgical efficacy, potential disparities in PLND use according to the patient’s preoperative risk is currently unknown 17, 18. Our objective was to describe temporal trends and identify predictors of pelvic lymphadenectomy in a population-based cohort of older men with prostate cancer.
METHODS
Study Cohort
The study cohort was identified from Surveillance, Epidemiology, and End Results (SEER) cancer registry data linked with Medicare claims. SEER, sponsored by the National Cancer Institute (NCI), is a consortium of population-based cancer registries in selected geographic areas, covering approximately 25% of the US population 19. For all diagnosed cancer cases in their geographic areas, the SEER registries collect data regarding site and extent of disease, surgery and radiation therapy planned or administered in the first course of cancer-directed therapy, and socio-demographic characteristics, with active follow-up for date and cause of death 20. For cancer patients age 65 and older residing in SEER areas, Medicare claims have been linked to SEER files. Medicare is the primary health insurer for 97% of Americans 65 years and older, covering inpatient and outpatient hospital care (Part A, B respectively). Compared with the US elderly population, the SEER-Medicare population has similar age and sex distributions, but has a smaller proportion of nonwhites, and individuals in SEER-Medicare are more likely to live in urban areas and affluent areas 20,21.
Radical prostatectomy (RP) and PLND performed in 2003–2007 were identified by International Classification of Disease (ICD-9) (RP-60.5) and Current Procedural Terminology (CPT) codes (ORP-55866, MIRP-55840, 55842, 55845). Our analysis included all prostate cancers designated as stage 1–3 by the SEER modification of the American Joint Committee on Cancer’s staging system. Men who had T4 disease or presented with metastasis were excluded from analysis. Other exclusion criteria were prostate cancer diagnosis only at the time of death, history of prior malignancy, and or radiotherapy prior to prostate cancer surgery. We also excluded men who were not enrolled in both Part A and Part B of Medicare and those enrolled in managed care organization at the time of diagnosis or in the year prior to diagnosis, due to the absence of Medicare claims. The sample was limited to men age 66 and older so that a full year of claims prior to diagnosis was available for estimating comorbidity. We also excluded men missing information about preoperative PSA, Gleason score or clinical tumor stage.
Outcome
The outcome of interest was receipt of a bilateral PLND, identified in SEER. Using the SEER variable for the scope of regional lymph node surgery, we defined the outcome as removal of at least 4 regional lymph nodes. Patients who had only a nodal aspiration or biopsy and those for whom the scope of regional lymph node surgery was unknown were categorized as not having a lymph node dissection.
Predictors
We examined a number of characteristics hypothesized to predict receipt of PLND. Demographic characteristics included age, race, median income in the census tract of residence, urban-rural residence and geographic location. Marital status was included as a measure of social support. Metropolitan vs. Non-Metropolitan County was included to adjust for potential confounding by systematic practice variation among academic and community institutions. Tumor characteristics included clinical tumor stage, preoperative PSA, biopsy Gleason score, and surgical procedure (ORP vs. MIRP). Because SEER did not record exact PSA values until 2004, we used preoperative PSA categorized as elevated, borderline, normal, or unknown. In separate analyses we used numeric PSA values for cases diagnosed during or after 2004. Comorbidity was estimated using the Charlson comorbidity score, based on inpatient claims in the 365 days prior to prostate cancer diagnosis.
Statistical Analysis
We used multivariable logistic regression to estimate the adjusted effects of each variable on the likelihood of receiving a PLND. We also performed analyses stratified by surgical procedure (ORP vs. MIRP) to assess whether this modified the impact of important predictors, such as tumor stage, Gleason score and PSA, on receipt of PLND. In these analyses Gleason score was dichotomized at a threshold of 7 (Gleason score 2–6 vs. 7+) and PSA was dichotomized at 10 (PSA ≤10ng/ml vs. >10 ng/ml). These categories correspond with validated prognostic models, identifying Gleason score and PSA values associated with a 2% or greater probability of lymph node metastasis, a well-established criterion for PLND.14,22,23. These classifications also effectively distinguish between patients at low risk for biochemical recurrence after definitive local therapy from intermediate-risk and high-risk patients 24. Adjusted odds ratios, 95% confidence intervals, and two-sided p-values were estimated in all multivariable regressions. A Cochrane-Armitage test was used to assess trends in PLND over time, and an interaction between surgical procedure (ORP vs. MIRP) and year was included in a separate multivariable logistic regression model in order to test differences in trends by surgical procedure.
RESULTS
We identified 6,608 men diagnosed with clinical stage T1-T3 prostate cancer in 2003–2005 who had a radical prostatectomy in 2003–2007. Of these men, 4,534 (69%) had ORP, 1,190 (18%) had MIRP and 884 patients (13%) had a radical prostatectomy with surgical approach not specified. Overall, 4,600 men (70%) had a PLND. In unadjusted analysis, there were no significant associations between receipt of PLND and race, age or other demographic characteristics (Table 1). ORP patients represented 80% of men who had PLND but only 45% of those who did not have PLND (p<0.0001). The use of PLND increased with increasing preoperative PSA and Gleason score. However, a substantial fraction of men with high-risk tumor features did not receive PLND, and this relationship varied by surgical approach (Table 2). For example, among men diagnosed in 2004–2005 with a preoperative PSA of 10 ng/ml or greater, 80% received PLND, but PLND was omitted in 40% of those who had MIRP and only 15% of those who had ORP. We found similar patterns for other tumor features. Among men with stage T3 disease, 80% in the ORP group had a PLND, compared with 56% in the MIRP group. Among men with Gleason score >7, higher rates of PLND for were seen in the ORP group compared with the MIRP group.
Table 1.
Characteristics of the sample by receipt of pelvic lymph node dissection (PLND)
| All Patients (n=6,608) | PLND (n=4,600) | No PLND (n=2,308) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | |
| Surgical procedure | <0.0001 | ||||||
| ORP | 4,534 | 69% | 3,638 | 79% | 896 | 45% | |
| MIRP | 1,190 | 18% | 515 | 11% | 675 | 34% | |
| RP, NOS | 884 | 13% | 447 | 10% | 437 | 21% | |
| Age at diagnosis | <0.001 | ||||||
| 66–69 | 3.896 | 59% | 2,691 | 58% | 1,205 | 60% | |
| 70–74 | 2,245 | 34% | 1,555 | 34% | 690 | 34% | |
| 75–79 | 425 | 6% | 323 | 7% | 102 | 5% | |
| 80+ | 42 | <1% | 31 | <1% | 11 | <1% | |
| Race | NS | ||||||
| White | 5,504 | 83% | 3, 858 | 84% | 1,646 | 82% | |
| Black | 492 | 8% | 330 | 7% | 162 | 8% | |
| Other | 612 | 9% | 412 | 9% | 200 | 10% | |
| Median income* | <0.01 | ||||||
| 1st quartile | 1,550 | 23% | 1,095 | 24% | 455 | 23% | |
| 2nd quartile | 1,679 | 25% | 1,165 | 25% | 514 | 26% | |
| 3rd quartile | 1,684 | 26% | 1,190 | 26% | 494 | 24% | |
| 4th quartile | 1,695 | 26% | 1,150 | 25% | 545 | 27% | |
| Urban residence | NS | ||||||
| Metro | 5.732 | 87% | 3.993 | 87% | 1,749 | 87% | |
| Non-metro | 866 | 13% | 607 | 13% | 259 | 13% | |
| Geographic area | <0.0001 | ||||||
| Northeast | 679 | 11% | 433 | 9% | 246 | 12% | |
| South | 858 | 13% | 563 | 12% | 295 | 15% | |
| Midwest | 814 | 12% | 534 | 12% | 280 | 14% | |
| West | 4,257 | 64% | 3,070 | 67% | 1,187 | 59% | |
| Married | 0.0001 | ||||||
| Yes | 5,360 | 81% | 3,812 | 83% | 1,548 | 77% | |
| No | 959 | 15% | 678 | 15% | 281 | 14% | |
| Unknown | 289 | 4% | 110 | 2% | 179 | 9% | |
| Clinical stage | <0.001 | ||||||
| T1 | 3,194 | 48% | 2,145 | 47% | 1,049 | 52% | |
| T2 | 3,303 | 50% | 2,371 | 52% | 932 | 47% | |
| T3 | 111 | 2% | 84 | <1% | 27 | <1% | |
| PSA | <0.0001 | ||||||
| Elevated | 5,627 | 85% | 3,952 | 86% | 1,675 | 83% | |
| Borderline | 441 | 7% | 281 | 6% | 160 | 8% | |
| Normal | 540 | 8% | 367 | 8% | 173 | 9% | |
| Numeric PSA** | <0.0001 | ||||||
| <4 ng/ml | 534 | 12% | 343 | 11% | 191 | 13% | |
| 4–10 ng/ml | 2,897 | 63% | 1,931 | 61% | 996 | 67% | |
| >10–20 ng/ml | 608 | 13% | 487 | 15% | 121 | 8% | |
| >20 ng/ml | 244 | 5% | 189 | 6% | 55 | 4% | |
| Gleason Score | <0.0001 | ||||||
| 2–6 | 3,144 | 48% | 2,022 | 44% | 1122 | 56% | |
| 7+ | 3,464 | 52% | 2,578 | 56% | 886 | 44% | |
| Comorbidity score | NS | ||||||
| 0 | 5,273 | 80% | 3,659 | 80% | 1,614 | 81% | |
| 1 | 1,040 | 16% | 735 | 16% | 305 | 15% | |
| 2+ | 295 | 4% | 206 | 4% | 89 | 4% | |
MIRP: minimally invasive radical prostatectomy, ORP: open radical prostatectomy, NS: Not statistically significant at p<0.05 P-value for chi-square test of association between each characteristic and receipt of pelvic lymph node dissection.
Unknown values not shown for census tract median income (n=19, <1% of entire cohort)
Analysis of numeric PSA limited to cases diagnosed 2004+
Table 2.
Use of pelvic lymph node dissection (PLND) by tumor risk factors and surgical approach, cases diagnosed 2004–2005
| All Patients (n=4,374) | MIRP (n=1,085) | ORP (n=3,289) | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | % PLND | N | % PLND | N | % PLND |
| Clinical stage | ||||||
| T1 | 1,561 | 68% | 273 | 42% | 1,288 | 78% |
| T2 | 1.464 | 73% | 175 | 53% | 1,289 | 82% |
| T3 | 65 | 74% | 10 | 56% | 55 | 80% |
| Numeric PSA* | ||||||
| ≤10 ng/ml | 2072 | 69% | 317 | 40% | 1,775 | 80% |
| >10 ng/ml | 593 | 81% | 91 | 60% | 502 | 86% |
| Gleason Score | ||||||
| 2–6 | 1,200 | 63% | 150 | 30% | 1,050 | 75% |
| 7+ | 1,890 | 76% | 308 | 52% | 1,582 | 84% |
MIRP: minimally invasive radical prostatectomy, ORP: open radical prostatectomy
Men missing numeric PSA information (n=647 of those diagnosed 2004–2005) excluded from analysis.
Predictors of PLND
Controlling for patient and tumor characteristics, the odds of PLND was more than five times greater with ORP than with MIRP (Table 3). Using the SEER categorical PSA variable, men with an “elevated” preoperative PSA were not significantly more likely to receive PLND. However, in analysis limited to men diagnosed in 2004–2005, a numeric PSA value of >10 ng/ml was associated with increased odds of PLND (adjusted OR 1.77, 95% CI 1.46–2.15). Among all men, a biopsy Gleason score of 7 or greater was also associated with increased odds of PLND (adjusted OR 2.41, 95% CI 1.88–3.10). Advanced tumor stage was not associated with PLND, controlling for other patient and tumor characteristics. Use of PLND was not influenced by socio-demographic characteristics or comorbidity.
Table 3.
Adjusted impact of patient and tumor characteristics on receipt of pelvic lymph node dissection (PLND), all patients
| Characteristic | Adjusted odds ratio (95% CI) | p |
|---|---|---|
| Surgical procedure | <0.0001 | |
| MIRP | Reference | |
| ORP | 5.28 (4.56–6.81) | |
| RP, NOS | 1.26 (1.05–1.52) | |
| Age at diagnosis | NS | |
| 66–69 | Reference | |
| 70–74 | 0.95 (0.84–1.07) | |
| 75–79 | 1.33 (1.03–1.72) | |
| 80+ | 1.00 (0.48–2.12) | |
| Race | NS | |
| White | Reference | |
| Black | 0.96 (0.77–1.22) | |
| Other | 0.91 (0.75–1.11) | |
| Median income | NS | |
| 1st quartile | Reference | |
| 2nd quartile | 0.96 (0.81–1.43) | |
| 3rd quartile | 1.07 (0.89–1.28) | |
| 4th quartile | 1.08 (0.90–1.30) | |
| Urban residence | NS | |
| Metro | Reference | |
| Non-Metro | 0.94 (0.78–1.14) | |
| Geographic region | NS | |
| Northeast | Reference | |
| South | 0.88 (0.69–1.13) | |
| Midwest | 1.16 (0.90–1.46) | |
| West | 1.39 (1.15–1.68) | |
| Married | <0.0001 | |
| Yes | Reference | |
| No | 0.84 (0.70–1.10) | |
| Unknown | 0.26 (0.20–0.34) | |
| Clinical stage | NS | |
| T1 | Reference | |
| T2 | 1.08 (0.96–1.22) | |
| T3 | 1.35 (0.83–2.19) | |
| PSA | <0.001 | |
| Normal | Reference | |
| Borderline | 0.74 (0.55–1.22) | |
| Elevated | 1.35 (0.83–2.19) | |
| Numeric PSA* | <0.0001 | |
| ≤10 ng/ml | Reference | |
| >10 ng/ml | 1.77 (1.46–2.15) | |
| Gleason Score | <0.0001 | |
| 2–6 | Reference | |
| 7+ | 2.41 (1.88–3.10) | |
| Comorbidity score | NS | |
| 0 | Reference | |
| 1 | 0.89 (0.63–1.25) | |
| 2+ | 0.54 (0.27–1.09) | |
| Year of surgery | 0.67 (0.58–0.73) | <0.0001 |
MIRP: minimally invasive radical prostatectomy, ORP: open radical prostatectomy, RP, NOS: radical prostatectomy, not otherwise specified, NS: Not statistically significant at p<0.05
Odds ratio for numeric PSA from separate multivariable analysis limited to men diagnosed with prostate cancer in 2004–2006, adjusted for all other characteristics in table except for qualitative PSA.
Analyses stratified by surgical procedure (ORP vs. MIRP) suggested that surgical approach modified the effects of Gleason score, but not PSA, on the likelihood of PLND (Table 4). The impact of PSA on PLND was similar in both surgical groups. In analyses limited to men diagnosed in 2004–2005, the impact of preoperative PSA >10 ng/ml doubled the odds of PLND among men who had MIRP (95% CI 1.42–3.08) and increased the odds by about 1.7 (95% CI 1.33–2.08) in men who had ORP. In a separate multivariable regression model (not shown), the coefficient on an interaction term for type of surgery (MIRP vs. ORP) by numeric PSA score (≤10 vs. >10 ng/ml) was not statistically significant. The impact of Gleason score did vary by surgical approach. In men who had MIRP, a Gleason score ≥7 more than doubled the odds of PLND (95% CI 1.87–3.10), while in men who had ORP the odds ratio for Gleason score ≥7 was about 1.8 (95% CI 1.54–2.10). In a separate model (not shown), the coefficient on an interaction term for type of surgery by Gleason score was positive and statistically significant (p<0.001), suggesting that while Gleason score greater than 7 increased the odds of PLND in both groups, the magnitude of this effect was greater in men who had MIRP.
Table 4.
Adjusted impact of patient and tumor characteristics on receipt of pelvic lymph node dissection (PLND), stratified by surgical procedure
| Characteristic | Surgical Procedure |
|||
|---|---|---|---|---|
| MIRP (n=1190) | ORP (n=4951) | |||
| AOR (95% CI) | p | AOR (95% CI) | p | |
| Age at diagnosis | NS | |||
| 66–69 | Reference | Reference | ||
| 70–74 | 0.90 (0.69–1.17) | NS | 1.04 (0.88–1.22) | |
| 75–79 | 1.32 (0.81–2.14) | 1.48 (1.03–2.12) | ||
| 80+ | 1.38 (0.28–6.77) | 0.79 (0.33–1.89) | ||
| Race | NS | |||
| White | Reference | NS | Reference | |
| Black | 0.76 (0.41–1.38) | 0.88 (0.70–1.17) | ||
| Other | 0.86 (0.58–1.27) | 1.10 (0.83–1.45) | ||
| Median income | NS | |||
| 1st quartile | Reference | Reference | ||
| 2nd quartile | 1.29 (0.83–2.00) | NS | 0.95 (0.77–1.17) | |
| 3rd quartile | 1.48 (0.92–2.24) | 1.04 (0.83–1.30) | ||
| 4th quartile | 1.38 (0.90–2.13) | 1.06 (0.83–1.35) | ||
| Urban residence | <0.01 | |||
| Metro | Reference | NS | Reference | |
| Non-Metro | 1.15 (0.67–1.85) | 0.80 (0.63–1.00) | ||
| Geographic region | NS | |||
| Northeast | Reference | <0.05 | Reference | |
| South | 0.84 (0.46–1.54) | 1.07 (0.79–1.45) | ||
| Midwest | 1.28 (0.75–2.16) | 1.27 (0.93–1.93) | ||
| West | 1.58 (1.07–2.32) | 1.43 (1.13–1.84) | ||
| Married | <0.0001 | |||
| Yes | Reference | <0.0001 | Reference | |
| No | 1.03 (0.71–1.47) | 0.92 (0.75–1.14) | ||
| Unknown | 0.27 (0.14–0.52) | 0.26 (0.19–0.36) | ||
| Clinical stage | NS | |||
| T1 | Reference | NS | Reference | |
| T2 | 0.97 (0.75–1.23) | 1.12 (0.96–1.31) | ||
| T3 | 1.62 (0.59–4.42) | 1.23 (0.67–1.50) | ||
| PSA | NS | |||
| Normal | Reference | <0.01 | Reference | |
| Borderline | 0.32 (0.16–0.64) | 1.00 (0.82–1.22) | ||
| Elevated | 0.90 (0.59–1.37) | 0.98 (0.73–1.30) | ||
| Numeric PSA* | <0.001 | <0.0001 | ||
| ≤10 ng/ml | Reference | Reference | ||
| >10 ng/ml | 2.09 (1.42–3.08) | 1.67 (1.33–2.08) | ||
| Gleason score | <.0001 | |||
| 2–6 | Reference | <.0001 | Reference | |
| 7+ | 2.41 (1.87–3.10) | 1.80 (1.54–2.10) | ||
| Comorbidity score | NS | |||
| 0 | Reference | NS | Reference | |
| 1 | 0.87 (0.63–1.25) | 1.00 (0.82–1.22) | ||
| 2+ | 0.54 (0.27–1.10) | 0.97 (0.69–1.34) | ||
| Year of treatment | 0.67 (0.58–0.78) | <0.0001 | 0.88 (0.82–0.95) | <0.01 |
MIRP: minimally invasive radical prostatectomy, ORP: open radical prostatectomy, NS: Not statistically significant at p<0.05
Odds ratios for numeric PSA from separate multivariable analysis limited to men diagnosed with prostate cancer in 2004–2006, adjusted for all other characteristics in table except for qualitative PSA.
Temporal trends
There was a statistically significant decline in the use of PLND from 2003–2007 (Figure 1, p<0.001 for trend test). This trend was not solely attributable to the increased use of MIRP over the time period of analysis. Adjusting for surgical procedure (MIRP vs. ORP), year of surgery remained a significant predictor of PLND (Table 2), and the decline was observed in both surgery subgroups (Table 3). In a separate analysis of all patients, we found a statistically significant (p<0.05) and negative coefficient on an interaction between MIRP and year of surgery, suggesting that the decline in PLND over time was steeper in the MIRP group compared with the ORP group, controlling for other patient and tumor characteristics.
Figure 1.
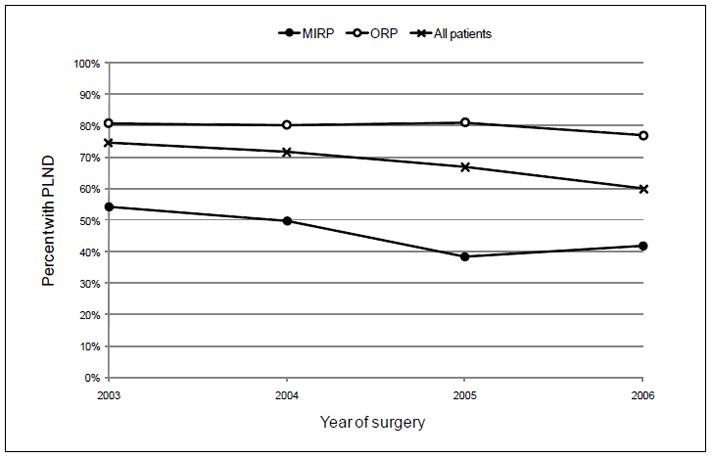
Trends in pelvic lymph node dissection (PLND) with minimally invasive (MIRP) or open radical prostatectomy (ORP), 2003–2006
DISCUSSION
In this population-based analysis of radical prostatectomy patients, the use of PLND was more common in men with higher-risk tumor features, consistent with current practice guidelines.15 However, we also observed an independent effect of surgical procedure type. The odds of receiving a PLND were more than five times greater in men who had ORP compared with their peers who had MIRP, controlling for PSA, Gleason score, age, comorbidity and other demographic and clinical factors. Moreover, surgical approach modified the effect of Gleason score on the likelihood of PLND. While a higher Gleason score increased the odds of PLND in all patients, the magnitude of this effect was greater in the MIRP group than in the ORP group.
There are several possible explanations for the differential use of PLND by surgical approach. Surgeons who are recent adopters of MIRP, and therefore practicing on the steep, early segment of the surgical learning curve may omit PLND in order to reduce operative times 25,26. Alternatively, surgeons performing MIRP may be more concerned about technical aspects of the procedure associated with functional outcomes, such as continence and erectile function, than any possible oncologic benefit associated with PLND. Mastery of the prostatectomy segment has dominated the learning curve for MIRP and PLND has not figured centrally in the early dissemination of this procedure 27,28.
Despite the comparable safety profile and overall feasibility of MIRP compared with ORP 29, disparities in the nodal counts have been reported 17. However, when properly performed, minimally invasive PLND can achieve a nodal yield similar to open PLND, without increasing the risk of complications 27. Recent prospective series have further supported the technical feasibility of robotic PLND 30, especially with extended dissection templates 31,32. Thus MIRP should not be a prohibitive factor in accomplishing a complete oncologic resection including PLND 33,34.
Based on early experience in which a concomitant PLND with radical prostatectomy was routinely performed for staging purposes35, current treatment guidelines are now stage-specific, recommending PLND routinely for high-risk cancers, and selectively for patients with lower risk disease 15,16. This prompted the development of statistical models to predict the likelihood of lymph node metastasis based on preoperative PSA, Gleason score and clinical stage 14,22. Validation of these models suggests that their predictive accuracy does not vary by surgical approach 36–38. Thus, in a man whose tumor features suggest a high risk of nodal metastasis, it would be inappropriate to arbitrarily omit PLND because a minimally invasive technique is being utilized.
A properly performed PLND improves post-operative risk assessment, and may provide a therapeutic benefit in selected patients with microscopic nodal disease 39,40. While most men with nodal metastasis will experience biochemical recurrence, some men achieve a sustained non-detectable PSA 41–43. However, the prognosis for men with node-positive prostate cancer is still good, with cancer-specific survival exceeding 80% at 10 years 44. While the benefit of PLND in low-risk patients is probably modest 45, patients with elevated Gleason score, PSA or clinical stage have considerable predicted rates of nodal metastasis 13. Although comorbidity has been associated with a decrease in the odds of receiving a PLND 18, we did not observe this relationship, controlling for other patient and tumor characteristics. Moreover, we found that 26% of men with clinical stage T3 disease, 19% with a serum PSA greater than 10 ng/ml, and 24% with a Gleason score greater than 7 did not receive PLND. Contemporary criteria for identifying men at high risk of nodal metastasis suggest that most, if not all of these men with high-risk features should have received PLND 23.
The time period of our study was characterized by rapid uptake of MIRP. However, the decline in PLND over time cannot be explained by the increased use of MIRP, as we observed a decline in PLND in both the MIRP and ORP subgroups. In fact, the decline was steeper among those who received MIRP. If early MIRP cases were selected for more favorable characteristics, representing men with lower risk disease on average, then we would have expected an increase in PLND among MIRP patients over time, contrary to the trend we observed. Similarities in baseline tumor risk factors in both cohorts did not support preferential MIRP for lower risk-patients, similar to other series.18 Thus, the accelerated decline in PLND in the MIRP cohort was likely fueled by other factors, such as more swift avoidance of PLND in cases of marginal or unproven benefit in the minimally invasive setting. Although not the focus of this investigation, this may be more common in low-volume community practices than in high-volume academic medical centers10,18, both of which are included in the population-based SEER-Medicare dataset 17.
It is also unlikely that our results are explained by financial incentives for providers. In fact, Medicare reimbursement favors greater use of PLND with MIRP, where it can be billed separately, than with ORP, where it is included in a bundled payment. In 2010, Medicare reimbursement for MIRP with PLND is about $1,000, or 50%, greater than ORP with PLND17. The higher rate of PLND with ORP that we observed suggests that this difference in reimbursement is not promoting overuse of PLND in MIRP cases.
Features of the SEER-Medicare population and dataset could explain some of our findings, and may limit the generalizability of our conclusions. While the robotic platform is now estimated to be the most common approach for radical prostatectomy today in the United States 46, MIRP represented only 18% of the procedures in our dataset. Although the states and metropolitan areas in the SEER program were not likely MIRP-underserved areas during the time period of the study, the uptake of MIRP may vary considerably across surgeons, even among those who have access to minimally invasive surgical equipment. The relatively low rate of MIRP we observed could also be explained by under use of MIRP or prostatectomy in general in men over 66 years of age 47. In addition, while we were able to assess the impact of several important patient, tumor and treatment characteristics on the likelihood of receiving PLND, there may be other, unobserved factors that explain differential use of this procedure, such as physician experience, hospital characteristics such as community vs. academic designation, or patient comorbidity not captured in the Charlson index. Also, given the limited information regarding tumor characteristics in SEER, our estimates of preoperative risk of nodal disease were based on preoperative PSA and Gleason score only. Finally, while we were able to determine whether a patient received bilateral PLND, definitions of standard, limited and extended templates are not standardized across providers and not identifiable in either SEER or Medicare data. And while SEER includes information regarding the number of nodes removed, the anatomic location of these nodes is not specified.
Conclusion
Controlling for patient and tumor characteristics, the odds of PLND in men receiving ORP was more than 5 times that of men receiving MIRP. This association was evident even in higher-risk patients, where the odds of PLND varied significantly with surgical approach. Oncologic principles in prostatectomy should transcend surgical technique, thereby requiring urologists to provide the same operation regardless of surgical modality. A renewed emphasis of PLND in MIRP in an effort to standardize practice patterns across techniques is warranted.
Acknowledgments
This work was supported in part by funds from the National Institutes of Health [T32-CA82088 to P.S. and W.L.]; the National Cancer Institute [P50-CA92629 SPORE to P.S., CA118189-01A2 to E.E]; Sidney Kimmel Center for Prostate and Urologic Cancers; and David H. Koch provided through the Prostate Cancer Foundation; and a Challenge Grant funded by the NIH (Grant Number: 1RC1CA146516-01 to J.E.)
The authors gratefully acknowledge Joshua Mirkin for assistance with Medicare billing codes and reimbursement calculations and Michael Newman for editorial assistance. The authors also acknowledge the Applied Research Program (National Cancer Institute), the SEER program registries, the Centers for Medicare and Medicaid Services and Information Management Services, Inc, for the SEER-Medicare linkage and guidance in the use and interpretation of these data.
References
- 1.Health NCI-USNIo. http://www.cancer.gov/cancertopics/types/prostate.
- 2.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Natl Cancer Inst. 2009;101:1325–9. doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yossepowitch O, Eggener SE, Serio AM, et al. Secondary therapy, metastatic progression, and cancer-specific mortality in men with clinically high-risk prostate cancer treated with radical prostatectomy. Eur Urol. 2008;53:950–9. doi: 10.1016/j.eururo.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh PC. Radical prostatectomy for localized prostate cancer provides durable cancer control with excellent quality of life: a structured debate. J Urol. 2000;163:1802–7. [PubMed] [Google Scholar]
- 5.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557–64. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 6.Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: the Montsouris experience. J Urol. 2000;163:418–22. doi: 10.1016/s0022-5347(05)67890-1. [DOI] [PubMed] [Google Scholar]
- 7.Mulhall JP, Rojaz-Cruz C, Muller A. An analysis of sexual health information on radical prostatectomy websites. BJU Int. 2009 doi: 10.1111/j.1464-410X.2009.08762.x. [DOI] [PubMed] [Google Scholar]
- 8.Klein EA, Bianco FJ, Serio AM, et al. Surgeon experience is strongly associated with biochemical recurrence after radical prostatectomy for all preoperative risk categories. J Urol. 2008;179:2212–6. doi: 10.1016/j.juro.2008.01.107. discussion 2216–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickers AJ, Bianco FJ, Gonen M, et al. Effects of pathologic stage on the learning curve for radical prostatectomy: evidence that recurrence in organ-confined cancer is largely related to inadequate surgical technique. Eur Urol. 2008;53:960–6. doi: 10.1016/j.eururo.2008.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briganti A, Capitanio U, Chun FK, et al. Impact of surgical volume on the rate of lymph node metastases in patients undergoing radical prostatectomy and extended pelvic lymph node dissection for clinically localized prostate cancer. Eur Urol. 2008;54:794–802. doi: 10.1016/j.eururo.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Lowrance WT, Elkin EB, Jacks LM, et al. Comparative effectiveness of prostate cancer surgical treatments: a population based analysis of postoperative outcomes. J Urol. 2010;183:1366–72. doi: 10.1016/j.juro.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowrance WT, Elkin EB, Eastham JA. Minimally invasive vs open radical prostatectomy. JAMA. 303:619–20. doi: 10.1001/jama.2010.126. author reply 620. [DOI] [PubMed] [Google Scholar]
- 13.Briganti A, Blute ML, Eastham JH, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55:1251–65. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Cagiannos I, Karakiewicz P, Eastham JA, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–803. doi: 10.1097/01.ju.0000091805.98960.13. [DOI] [PubMed] [Google Scholar]
- 15.Scardino P. Update: NCCN prostate cancer Clinical Practice Guidelines. J Natl Compr Canc Netw. 2005;3 (Suppl 1):S29–33. [PubMed] [Google Scholar]
- 16.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Cooperberg MR, Kane CJ, Cowan JE, et al. Adequacy of lymphadenectomy among men undergoing robot-assisted laparoscopic radical prostatectomy. BJU Int. 2009 doi: 10.1111/j.1464-410X.2009.08699.x. [DOI] [PubMed] [Google Scholar]
- 18.Prasad SM, Keating NL, Wang Q, et al. Variations in surgeon volume and use of pelvic lymph node dissection with open and minimally invasive radical prostatectomy. Urology. 2008;72:647–52. doi: 10.1016/j.urology.2008.03.067. discussion 652–3. [DOI] [PubMed] [Google Scholar]
- 19.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–48. [PubMed] [Google Scholar]
- 20.Bach PB, Guadagnoli E, Schrag D, et al. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40:IV-19-25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 21.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 22.Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–8. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 23.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8:145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 24.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 25.Zorn KC, Orvieto MA, Gong EM, et al. Robotic radical prostatectomy learning curve of a fellowship-trained laparoscopic surgeon. J Endourol. 2007;21:441–7. doi: 10.1089/end.2006.0239. [DOI] [PubMed] [Google Scholar]
- 26.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–7. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 27.Zorn KC, Katz MH, Bernstein A, et al. Pelvic lymphadenectomy during robot-assisted radical prostatectomy: Assessing nodal yield, perioperative outcomes, and complications. Urology. 2009;74:296–302. doi: 10.1016/j.urology.2009.01.077. [DOI] [PubMed] [Google Scholar]
- 28.Yates J, Haleblian G, Stein B, et al. The impact of robotic surgery on pelvic lymph node dissection during radical prostatectomy for localized prostate cancer: the Brown University early robotic experience. Can J Urol. 2009;16:4842–6. [PubMed] [Google Scholar]
- 29.Guillonneau B, Cappele O, Martinez JB, et al. Robotic assisted, laparoscopic pelvic lymph node dissection in humans. J Urol. 2001;165:1078–81. [PubMed] [Google Scholar]
- 30.Atug F, Castle EP, Srivastav SK, et al. Prospective evaluation of concomitant lymphadenectomyin robot-assisted radical prostatectomy: preliminary analysis of outcomes. J Endourol. 2006;20:514–8. doi: 10.1089/end.2006.20.514. [DOI] [PubMed] [Google Scholar]
- 31.Feicke A, Baumgartner M, Talimi S, et al. Robotic-assisted laparoscopic extended pelvic lymph node dissection for prostate cancer: surgical techniqueand experience with the first 99 cases. Eur Urol. 2009;55:876–83. doi: 10.1016/j.eururo.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Touijer K. Editorial comment on: Robotic-assisted laparoscopic extended pelvic lymph node dissection for prostate cancer: surgical technique and experience with the first 99 cases. Eur Urol. 2009;55:884. doi: 10.1016/j.eururo.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Vanni AJ, Moinzadeh A. Lymphadenectomy in minimally invasive urologic oncology. Curr Opin Urol. 2008;18:163–6. doi: 10.1097/MOU.0b013e3282f4f00b. [DOI] [PubMed] [Google Scholar]
- 34.Silberstein JL, Derweesh IH, Kane CJ. Lymph node dissection during robot-assisted radical prostatectomy: where do we stand? Prostate Cancer Prostatic Dis. 2009;12:227–32. doi: 10.1038/pcan.2009.17. [DOI] [PubMed] [Google Scholar]
- 35.Catalona WJ. Pelvic lymphadenectomy is essential to staging accuracy in most patients with stages A-2 and B prostate cancer before radical prostatectomy. Semin Urol. 1983;1:212–6. [PubMed] [Google Scholar]
- 36.Yu JB, Makarov DV, Sharma R, et al. Validation of the partin nomogram for prostate cancer in a national sample. J Urol. 2010;183:105–11. doi: 10.1016/j.juro.2009.08.143. [DOI] [PubMed] [Google Scholar]
- 37.Graefen M, Karakiewicz PI, Cagiannos I, et al. International validation of a preoperative nomogram for prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2002;20:3206–12. doi: 10.1200/JCO.2002.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Graefen M, Karakiewicz PI, Cagiannos I, et al. A validation of two preoperative nomograms predicting recurrence following radical prostatectomy in a cohort of European men. Urol Oncol. 2002;7:141–6. doi: 10.1016/s1078-1439(02)00177-1. [DOI] [PubMed] [Google Scholar]
- 39.Daneshmand S, Quek ML, Stein JP, et al. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J Urol. 2004;172:2252–5. doi: 10.1097/01.ju.0000143448.04161.cc. [DOI] [PubMed] [Google Scholar]
- 40.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–65. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 41.Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–23. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 42.Masterson TA, Bianco FJ, Jr, Vickers AJ, et al. The association between total and positive lymph node counts, and disease progression in clinically localized prostate cancer. J Urol. 2006;175:1320–4. doi: 10.1016/S0022-5347(05)00685-3. discussion 1324–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carver BS, Bianco FJ, Jr, Scardino PT, et al. Long-term outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. JUrol. 2006;176:564–8. doi: 10.1016/j.juro.2006.03.093. [DOI] [PubMed] [Google Scholar]
- 44.Cheng L, Zincke H, Blute ML, et al. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer. 2001;91:66–73. doi: 10.1002/1097-0142(20010101)91:1<66::aid-cncr9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 45.Klein EA, Kattan M, Stephenson A, et al. How many lymphadenectomies does it take to cure one patient? Eur Urol. 2008;53:13–5. doi: 10.1016/j.eururo.2007.09.017. discussion 18–20. [DOI] [PubMed] [Google Scholar]
- 46.Available from: http://www.intuitivesurgical.com
- 47.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]


