Abstract
Serum uric acid (UA) is emerging as a strong and independent marker for pulmonary arterial hypertension (PAH). PAH is well recognized as a life threatening complication of sickle cell disease (SCD). However, the association between UA and PAH in SCD is unknown. We reviewed electronic medical records (EMR) of 559 consecutive adult SCD patients from Kings County Hospital Center (KCHC) between January 2005 and February 2010. Patients (n = 96) with measurement of UA in close temporal proximity to the transthoracic echocardiography (TTE) were identified. PAH was defined as pulmonary artery systolic pressure (PASP) ≥30 mm Hg. Patients (n = 16) with other risk factors which may cause PAH and chronic renal insufficiency were excluded. In 18 patients, TTE could not measure PASP. Finally, 62 patients were selected. Statistical analysis was performed using Student t tests, Pearson correlation coefficient and multivariate regression analysis. Out of 62 patients, 30 had PAH. Patients with PAH had a higher UA level (8.67 ± 4.8 vs. 5.35 ± 2.1, P = 0.001). We found strong positive correlation between the UA level and PASP (r = 0.71; P < 0.0001). This correlation was independent of diuretic use. UA could be a potential marker for PAH in SCD. However, its’ prognostic and pathophysiologic role in SCD patients with PAH needs to be further investigated.
Keywords: Pulmonary arterial hypertension, Sickle cell disease, Uric acid, Pulmonary hypertension, Hemoglobinopathy
Introduction
SCD is one of the most common inherited diseases in the world [1]. Although more than 90% of children born with SCD living to adulthood [2], the life expectancy of adult SCD patients remains the same. The median age at death is 42 years for men and 48 years for women [2]. Pulmonary complications, acute chest syndrome (ACS) and PAH account for a large proportion of deaths in adults with SCD [3, 4]. One retrospective study reported a median survival of 25.6 months for SCD patients with PAH confirmed by right heart catheterization [5]. Autopsy studies revealed that up to 75% of SCD patients have histological evidence of PAH [6]. In recent years, it has become of growing importance to have precise early and convenient method to diagnose PAH in SCD [7]. Several biomarkers such as brain natriuretic peptide [8], endothelin-1 9 (ET1) and UA [10–12] were found to have good correlations in patients with PAH. However, the association between UA and PAH in SCD is unknown.
Methods
Patient Population
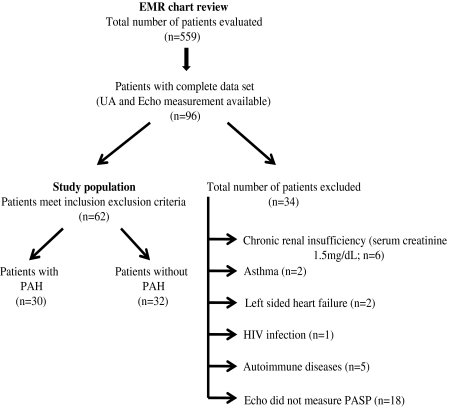
We retrospectively reviewed the EMR from adult sickle cell clinic at KCHC. We identified 559 adult SCD patients between January 2005 and February 2010. Out of which, 96 patients already had UA measurement and TTE under various clinical conditions. Patients (n = 16) were excluded with any known lung diseases, hepatic cirrhosis, autoimmune diseases, human immunodeficiency virus (HIV) infection, congenital heart disease, valvular heart disease, polycythemia, left heart failure, chronic renal insufficiency (serum creatinine ≥1.5) or with history of anorectic substance use. 18 patients in whom TTE failed to measure PASP were also excluded. The remaining 62 SCD patients were classified into two groups based on presence/absence of PAH (Fig. 1).
Fig. 1.
Study outline: Total 559 adult SCD patients were identified using EMR, 96 patients already had UA and echo measurement done under various clinical conditions. 34 patients were excluded. Study population (n = 62) was divided into two groups based on presence/absence of PAH. PAH was defined as PASP ≥30 mm Hg
Transthoracic Echocardiography
All TTE were performed by certified technician according to the KCHC echocardiography protocol. PASP was estimated using modified Bernoulli equation in the absence of right ventricular outflow obstruction. All studies were recorded and later reviewed by cardiologists. We defined PAH as PASP ≥30 mm Hg that was based on TRV ≥2.5 m/s [13] as marker of PAH in SCD. TTE performed while patients undergoing acute sickle crisis or ACS were excluded.
Blood Assays
EMR from the entire cohort revealed that UA and other blood markers (Table 1) had been measured in each patient in close temporal proximity to the TTE study. UA was measured using uricase-peroxidase method.
Table 1.
Baseline characteristics of SCD patients from PAH and non PAH group
| Baseline characteristics | PAH (n = 30) | non PAH (n = 32) | P value |
|---|---|---|---|
| Age (years) | 39.47 ± 14.62 | 37.97 ± 13.27 | NS |
| Gender (m/f) | 11/19 | 12/20 | NS |
| Height (inch) | 170.7 ± 7.98 | 177.6 ± 51.92 | NS |
| Weight (Kg) | 68.19 ± 14.20 | 64.93 ± 13.26 | NS |
| Body surface area (m²) | 1.78 ± 0.18 | 1.78 ± 0.34 | NS |
| Ethnicity | African American | African American | – |
| Genotypes | |||
| SS | 27 | 23 | – |
| SC | 3 | 6 | – |
| Sickle cell trait | 0 | 0 | – |
| Sickle β+ thalassemia | 0 | 3 | – |
| Other types of sickle cell | 0 | 0 | – |
| UA (mg/dl) | 8.67 ± 4.8 | 5.35 ± 2.1 | 0.001 |
| Total hemoglobin (g/dl) | 7.99 ± 1.47 | 8.83 ± 1.62 | NS |
| Fetal hemoglobin (g/dl) | 6.49 ± 5.26 | 5.91 ± 4.03 | NS |
| Reticulocyte count (%) | 24.78 ± 69.45 | 11.6 ± 5.53 | NS |
| White blood cell count (K/μl) | 10.68 ± 3.4 | 9.8 ± 3.78 | NS |
| Platelet count (K/μl) | 425.7 ± 203.8 | 410.5 ± 189.4 | NS |
| Mean platelet volume (fl) | 9.01 ± 0.91 | 9.23 ± 0.95 | NS |
| Blood urea (mg/dl) | 12.73 ± 7.23 | 5.94 ± 1.05 | NS |
| Serum creatinine (mg/dl) | 0.77 ± 0.29 | 0.65 ± 0.21 | NS |
| Lactate dehydrogenase (U/l) | 548.0 ± 262.1 | 436.8 ± 179.6 | NS |
| Iron (μg/dl) | 90.53 ± 38.88 | 85.71 ± 43.22 | NS |
| Ferritin (μg/dl) | 778.6 ± 1398.7 | 1151.1 ± 2140.9 | NS |
| C-reactive protein (g/dl) | 35.12 ± 50.15 | 27.09 ± 36.22 | NS |
| Total bilirubin (mg/dl) | 2.96 ± 2.07 | 2.95 ± 1.86 | NS |
| Direct bilirubin (mg/dl) | 1.04 ± 1.43 | 0.61 ± 0.29 | NS |
| Ejection fraction (%) | 57.24 ± 4.54 | 57.90 ± 4.23 | NS |
| PASP (mm Hg) | 40.20 ± 9.92 | 22.06 ± 4.18 | <0.0001 |
| Medication | |||
| Folic acid (y/n) | 30/0 | 32/0 | NS |
| Pain medication (y/n) | 30/0 | 32/0 | NS |
| Hydroxyurea (y/n) | 9/21 | 8/24 | NS |
| Diuretics (y/n) | 3/27 | 1/31 | NS |
NS not significant, Y yes, N no, M male, F female, PAH pulmonary arterial hypertension, UA uric acid, PASP pulmonary artery systolic pressure, SS Hemoglobin SS, SC Hemoglobin SC
Statistical Analysis
Results were expressed as mean ± standard deviation (SD). Statistical analyses were performed by Student t tests and Pearson correlation coefficient. Multivariate regression analysis was applied to determine independent relations among clinical variables. A P value <0.05 was considered statistically significant.
Results
Patients’ Demographic
Total 559 SCD patients, 96 already had UA and TTE measurements done within 6 months interval under various clinical conditions. Based on inclusion and exclusion criteria, six patients were excluded because of chronic renal insufficiency, one patient was excluded because of HIV, two patient was excluded because of left sided heart failure, two patients were excluded because of asthma, five patients were excluded because of associated history of autoimmune disease and 18 patients were excluded as TTE failed to measure PASP. Finally, 62 patients with SCD were selected. Of these, 30 (48%) and 32 (52%) were classified into PAH and non PAH group, respectively. Our PAH population was comparable to non PAH population with respect to age, sex, BSA, genotypes, and ethnicity (Table 1).
Pulmonary Artery Systolic Pressure
PASP range from 13 to 29 mm Hg in non PAH. There were 23 (20 SS and 3 SC) patients with mild PAH (PASP ≤44 mm Hg) and seven (all type SS) with moderate PAH (45 < PASP ≤ 74 mm Hg). No patients with severe PAH (PASP >74 mm Hg).
Serum Uric Acid Levels and its Correlation with Pulmonary Arterial Hypertension
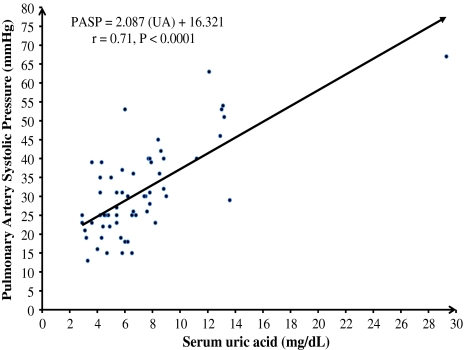
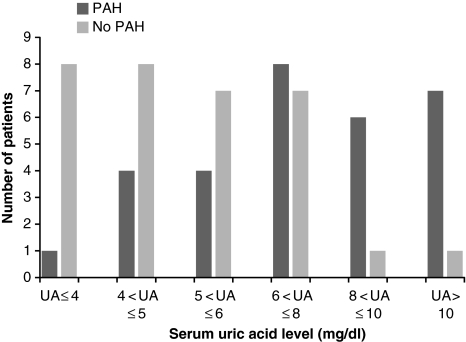
Compared with non PAH population, UA levels in PAH population were significantly elevated (8.67 ± 4.8 vs. 5.35 ± 2.1, P = 0.001). For the entire cohort UA levels also showed a trend of increase in proportion to the increase in PAH (r = 0.71; P < 0.0001; Fig. 2). This correlation was statistically much stronger in PAH group. The upper normal values for UA in the general population are 6 mg/dl in females and 7 mg/dl in males [14]. Based on this definition, hyperuricemia was found in 22 of 30 (73%) patients (10 of 11 (91%) males and 12 of 19 (63%) females] in PAH group and seven of 32 (19%) patients (1 of 12 (8%) males and 6 of 20 (30%) females) in non PAH group (Fig. 3). The correlation between UA levels and PAH remained statistically significant when males (r = 0.79; P < 0.0001) and females (r = 0.70; P < 0.0001) were analyzed separately.
Fig. 2.
Correlation between UA and PASP: UA levels and their corresponding PASP from both groups, PAH and non PAH
Fig. 3.
UA levels among adult SCD patients in PAH and non PAH group: In PAH group, most patients are on the right side of the graph indicating higher UA levels. Majority patients from non PAH group is on the left side indicating low UA levels among them
Since loop diuretics and thiazides are known to impair renal UA excretion and may cause hyperuricemia. We identified four SCD patients (three with PAH and one without PAH) who were treated with diuretics. However, their exclusion gave results similar to the whole study population suggests that, at least in SCD with PAH, hyperuricemia cannot simply be explained by diuretic therapy. There were two outliers in this study, one in PAH and one in non PAH group. Exclusion of outliers did not affect the study results.
Multivariate Regression Analysis
We performed regression analysis to model the relationship among PAH, UA and other clinical variables (Table 1). We found the relationship between UA and PAH were independent from all clinical variables. When tested individually, PAH did not correlate with any of these clinical variables.
Discussion
We showed a significant positive correlation between the UA level and PAH in patients with SCD. This is the first study that demonstrated this correlation in adult SCD patient. Furthermore, we found that PAH did not correlate with age, gender, body surface area, total hemoglobin, fetal hemoglobin, white blood cell count, reticulocyte count, platelet count, mean platelet volume, iron, ferritin, C-reactive protein, total and direct bilirubin, serum creatinine, lactate dehydrogenase, and use of hydroxyurea.
In SCD, the early symptoms of PAH are difficult to interpret in presence of chronic anemia. Early detection of PAH has become more important recently because of the availability of therapies that might treat and alter its course. Biological marker could also be of great clinical importance for initial screening. Elevated UA can be used as a potential biomarker of PAH in SCD. This also raises a question whether an elevated UA promote or protect against the development of PAH in SCD, or simply a consequence of right heart failure [15]. Earlier study has shown that an otherwise clinically stable adult SCD patient maintains normouricemia by increasing renal UA clearance [16]. Additionally, SCD patients with tubular proteinuria did not have significantly higher UA concentrations than others [17]. Therefore, an elevated UA in SCD patients could point toward its pathophysiological importance.
Several recent studies have shown that the UA might contribute in the pathophysiology of PAH. For example, UA increases cyclo-oxygenase-2 and ET-1 expression in vascular smooth muscle cells (VSMC) [18, 19]. UA also inhibits vasodilatation in the lung by reducing nitric oxide production by endothelial cells [20]. In rats, acute elevation of UA with uricase inhibitor allantoxanamide leads to increase in medial thickness of pulmonary arterioles [18].
This study has several limitations. Firstly, it is an observational study with retrospective data collection. Secondly, PASP was measured by TTE, not from the right heart catheterization. Furthermore, TTE data were generated by multiple operators and all TTE were not reviewed by the same cardiologist. Lastly, the interval between the UA and echo measurement was highly variable.
In conclusion, in this retrospective study, we demonstrated that in SCD, an increase in UA level was associated with increase in PAH. UA may provide a reproducible biological marker to assess the severity of PAH in SCD. However, role of UA in early diagnosis, predicting long term survival and genesis of PAH needs further studies.
Acknowledgments
The authors thank Mr. Peter L Flom, Ph.D. for his assistance with the statistical analysis. This study was supported by Department of Veterans Affairs Merit Review Funds and Empire Clinical Research Investigator Program (ECRIP) of New York.
Conflict of Interests None.
Contributor Information
Keval Joshi, Email: keval.joshi@downstate.edu.
Raj Wadgaonkar, Email: raj.wadgaonkar@downstate.edu.
References
- 1.Mentzer WC, Kan YW. Prospect for research in hematologic disorders: sickle cell disease and thalassemia. J Am Med Assoc. 2001;285(5):640–642. doi: 10.1001/jama.285.5.640. [DOI] [PubMed] [Google Scholar]
- 2.Castro LM, Jonassaint JC, Graham FL, et al. Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcome. Am J Hematol. 2008;83(1):19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 3.Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85(1):36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darbari DS, Kple-Faget P, Kwagyan J, et al. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81(11):858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 5.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101(4):1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 6.Haque AK, Gokhale S, Rampy BA, et al. Pulmonary hypertension in sickle cell hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol. 2002;33(10):1037–1043. doi: 10.1053/hupa.2002.128059. [DOI] [PubMed] [Google Scholar]
- 7.Barst RJ, Mubarak KK, Machado RF, et al. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan. Br J Haematol. 2010;149(3):426–435. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol. 2004;43:764–770. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 9.Rubens C, Ewert R, Halank M, et al. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120:1562–1569. doi: 10.1378/chest.120.5.1562. [DOI] [PubMed] [Google Scholar]
- 10.Nagaya N, Uematsu M, Satoh T, et al. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:487–492. doi: 10.1164/ajrccm.160.2.9812078. [DOI] [PubMed] [Google Scholar]
- 11.Bendayan D, Shitrit D, Ygla M, et al. Hyperuricemia as a prognostic factor in pulmonary arterial hypertension. Respir Med. 2003;97:130–133. doi: 10.1053/rmed.2003.1440. [DOI] [PubMed] [Google Scholar]
- 12.Zharikov SI, Swenson ER, Lanaspa M, et al. Could uric acid be a modifiable risk factor in subjects with pulmonary hypertension. Med Hypotheses. 2010;74(6):1069–1074. doi: 10.1016/j.mehy.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anthi A, Machado RF, Jison ML, et al. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175(12):1272–1279. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley WN, Palella TD, et al. Gout and other disorders of purine metabolism. In: Wilson JD, Braunwald E, Isselbacher KJ, et al., editors. Harrisons principles of internal medicine. 12. New York: MacGraw-Hill; 1991. pp. 1834–1843. [Google Scholar]
- 15.Hoeper MM, Hohlfeld JM, Fabel H, et al. Hyperuricaemia in patients with right or left heart failure. Eur Respir J. 1999;13:682–685. doi: 10.1183/09031936.99.13368299. [DOI] [PubMed] [Google Scholar]
- 16.Olukoga AO, Adewoye HO, Erasmus RT, et al. Maintenance of normouricaemia during adolescence in sickle-cell anaemia. J Trop Pediatr. 1992;38(4):199–201. doi: 10.1093/tropej/38.4.199. [DOI] [PubMed] [Google Scholar]
- 17.Morgan AG, Ceulaer K, Serjeant GR. Glomerular function and hyperuricaemia in sickle cell disease. J Clin Pathol. 1984;37:1046–1049. doi: 10.1136/jcp.37.9.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.ASN.0000034910.58454.FD. [DOI] [PubMed] [Google Scholar]
- 19.Chao HH, Liu JC, Lin JW, et al. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol Sin. 2008;29:1301–1312. doi: 10.1111/j.1745-7254.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 20.Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295(5):C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]