Abstract
Patients with chronic HCV infection are prone to increased susceptibility bacterial infection due to neutropenia complicating the course of this disease. Neutropenia in those patients may stem from enhanced neutrophil apoptosis. However, the molecular mechanism of neutrophil apoptosis has not been clearly defined. Neutrophils harvested from 26 neutropenic patients with hepatitis C infection and nine age and sex-matched healthy control subjects were examined for the degree of apoptosis. Neutrophil apoptosis was quantified by flow cytometry through determination of annexin-V expression at 0 time (fresh neutrophil), and 24 h culture. Neutrophils from healthy subjects were also incubated with either 10% heterologous normal or neutropenic sera, with and without 10 µg GM-CSF. Caspases 3, 10 were assessed colormetrically in neutrophils at 0 times and after 24 h culture. At 0 time culture the neutrophil apoptosis of the HCV patients was in significantly higher as compared to that of normal control (P = 0.059). At 24 h culture patients neutrophils cultured with neutropenic patients own sera showed neutrophil apoptosis significantly increased as compared to that at 0 time culture and this effect was significantly attenuated in similar culture with addition of GM-CSF (P < 0.001). On the other hand patient’s neutrophil cultured with normal sera showed insignificantly increased neutrophil apoptosis at 24 h culture as compared to that at 0 time culture. Caspases 3 and 10 activities were significantly higher in patients neutrophil after 24 h cultured with patients own sera as compared to 0 time culture (P < 0.001 for both). Addition of GM-CSF to the neutrophil culture down regulates the caspases 3 and 10 activities. The correlation study between annexin-V expression and caspases activities revealed a borderline positive correlation between annexin-V and caspase 3 (r = 0.376, P = 0.058), and significant positive correlation with caspase 10 activity (r = 0.494, P = 0.01). In conclusion, these findings suggest that enhanced neutrophil apoptosis demonstrated in neutropenic patients with HCV infection might be induced through activation of caspase 10 and is attenuated by GM-CSF.
Keywords: Neutropenia, Apoptosis, HCV, Hepatitis
Introduction
Hepatitis C virus (HCV) infection is highly prevalent around the world and represents a major health problem in Egyptian patients (15–20%) [1]. It is a common cause of chronic liver disease, characterized by inflammation, cell damage, and fibrotic reactions in hepatocytes; it may lead to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma [2, 3], Eighty to 85% of people infected by HCV cannot resolve their infection and suffer from persistent chronic hepatitis [4]. This is characterized by histological evidence of liver inflammation and liver damage, although there is a very wide spectrum of severity and progression of the disease [5, 6].
Patients with decompensated cirrhosis are prone to bacterial infection due to neutropenia leading to high mortality rate. Although hypersplenism and increased clearance of polymorphnuclear leukocytes in the spleen are thought to contribute to neutropenia in those patients, other factors could not be excluded [7].
Human neutrophilic polymorphonuclear leucocytes (PMNs) have central role in innate immunity and host defense mechanism against bacterial infection and are responsible for clearance of pathogens. PMNs undergo a tightly regulated apoptosis program that allows for timely clearance of PMNs without extravasations of toxic intracellular contents [8, 9]. Neutrophil apoptosis was suggested as a cause of neutropenia in advanced liver disease, however, the trigger and the mechanism of accelerated apoptosis remains to be elucidated [10].
Caspases, the intracellular cystiene proteases that exist as proenzymes, become activated during the cascade of apoptosis. They cleave a variety of cellular molecules that contain the amino acid motif DEVD such as polyADP-ribose polymerase (PARP) [11]. Caspase-8, caspase-9 and caspase-3 are situated at pivotal junctions in apoptotic pathway. Caspase-8 initiates disassembly in response to extra-cellular apoptosis-inducing ligands and is activated in a complex associated with the receptors cytoplasmic death domains. Caspase-3 appears to amplify caspase-8 and caspase-9 to full activity. Caspase-3 may cleave via cellular proteins or activate additional caspases by proteolyic cleavage [12].
Caspase 10 is one of the initiator caspases that are located upstream in death-signaling pathways and transducer or amplify apoptotic signaling via proteolytic cleavage and activation of effector and/or initiator caspases. Caspase-10 is known to initiate TNF-α mediated apoptosis in T-cells but there are no data to show the protein expression of caspase-10 in PMN or its involvement in PMN apoptosis [13, 14].
The aim of the current study was to investigate the role of caspases (3 and 10) and GM-CSF in neutrophil apoptosis ex vivo in patients with chronic HCV infection combined with neutropenia.
Subjects
The present study was carried out on 26 neutropenic HCV patients collected from at Mansoura University Hospitals after taking informed consent from every patient. All patients were neutopenic (absolute neutrophil count <2000/cmm). Moreover nine normal healthy control subjects served as a reference control group. The patients group was 10 females and 16 males. Their ages range from 43 to 72 years. The diagnosis was based on both clinical and laboratory findings including complete liver function tests [SGOT, SGPT (kinetic, BioMerieux, France), S albumin (BioMerieux, France), alkaline phosphatase (QCA. Quimica clinica aplicada), prothrombin time and INR (Diaplastin DiaMed, Switzerland) to assess synthetic function of the liver], HCV antibodies, HBsAg screening test, RT-PCR for positive HCV antibodies, in addition to abdominal ultrasonography to assess the degree of liver cirrhosis and splenomegely.
For comparative studies, nine apparently healthy individuals served as a control group. They were six males and three females. Their ages range from 38 to 52 years.
Methods
Study Design
To evaluate the degree of neutrophil apoptosis in hepatitis C, and healthy control groups, neutrophils isolated from the two groups were incubated in culture medium supplemented with either autologous sera (with and without addition of GM-CSF) or normal sera. After incubation period (24 h), neutrophil aliquots were processed for quantification of apoptosis.
To address the probability of the presence of serum factor in neutropenic hepatitis C patients, we evaluated apoptosis for neutrophils harvested from healthy normal subjects, which were incubated in culture medium supplemented by both normal and neutropenic patient sera. Also, we tested the neutrophil apoptosis harvested from neutropenic patients and incubated in culture medium supplemented with healthy control sera and patient own serum. After the incubation period, neutrophil aliquots were processed for quantification of apoptosis.
To address the suggestion, that GM-CSF could down regulate neutrophil apoptosis induced by patient own serum. We cultured patients neutrophil with patient own serum with and without addition of 10 µg GM-CSF.
Sample Collection
Blood was obtained by venipuncture of the forearm vein and it was drawn directly into heparinized plastic tubes for neutrophil isolation and culture and in an anti coagulant-free one for serum collection.
Neutrophil Viability
Neutrophil viability was assessed by trypan blue dye exclusion. One volume of 0.4% trypan blue was added to five volumes of cell suspension. After incubation at room temperature for 5 min, cells were counted using hemocytometer. All counts were performed in duplicate.
Light Microscopy
The apoptotic neutrophil was identified morphologically in Leishman-stained smears according to the following criteria: chromatin condensation and margination, formation of round nuclear profiles or nuclear fragmentation, cellular shrinking, cytoplasmic vacuolation, ruffling of the plasma membrane with eventually breaking up of the cell into apoptotic bodies, and cytoplasmic vacuolation.
Neutophil Isolation and Culture
We utilized an ex vivo assay for neutrophil apoptosis. Five millimeters of heparinized fresh blood samples were put directly and slowly on sides of falcon tubes containing equal amount of Ficoll (Biohrom AG 1077, Berlin). The tubes were centrifugated at 2500 rpm for 20 min. The mononuclear layer was discarded and neutrophil layer was withdrawn (thin whitish layer above erythrocytes directly). The cell pellet were washed twice in phosphate buffered saline (PBS) (Hyaclone, Logan, Utath, Kanada).
Lysis of erythrocytes was done using nine volumes of an ice-cold isotonic chloride solution (NH4Cl 155 mmol/l, KHCO3 10 mmol/l and EDTA 0.1 mmol/l) to one volume of cell pellet at room temperature for 7 min, then the neutrophil were washed twice by PBS to reach purity. The neutrophils were resuspended at concentration of 3 × 106/ml in RPMI 1640 (Biowest, South America). The medium of culture was supplemented with 10% fetal bovine serum (Biowest, South America), 300 μg/ml glutamine (Biowhttaker, Belgium), 100 Iu/ml penicillin (10,000 units/ml) and 10,000 μg/ml streptomycin (Hyclone, Utath). The cell suspensions were seeded into tissue culture flasks (Tipp, Europe) then cultured at 37°C in 5% CO2 incubator for 24 h.
We put the cell suspension in different conditions. Firstly, we applied patient neutrophils (100 μl containing 3 × 104 neutrophil) with 10% healthy normal control serum, then patient neutrophils, own serum, with and without 10 μg GM-CSF. To assess the role of patient’s sera in enhancing apoptosis, we added patient sera to normal healthy control neutrophils.
Assessment of Caspases 3 and 10 by Colorimetric Assay (R&D System USA)
The cells were lysed and the protease activity was assessed by addition of a caspase—specific peptide that is conjugated to the color reporter molecule p-nitroanaline (pNA). The cleavage of the peptide by the caspase releases the chromophore pNA, which can be quantified spectrophotometrically at a wave length of 405 nm. The level of caspase enzymatic activity in the cell lysate is directly proportional to the color reaction.
Assessment of Apoptosis by Flowcytometry
Neutrophil apoptosis was assessed by flowcytometry through annexin-V (TACS Annexin V-FITC). We followed the protocol manufactured by (R&D system, USA). Briefly, cells were harvested and washed. Then, 100 μl of neutrophil suspension (adjusted to 1 × 106 cells/100 μl) were incubated with of annexin-V incubation reagent (10 μl 10 × binding buffer, 10 μl propidium iodide (PI), 1 μl annexin-V FITC, 79 μl d H2O). The suspended neutrophil was then incubated in the dark for 15 min at room temperature. Analysis was done by flowcytometry (EPICS XL, Coulter, USA) within 1 h. Based on analysis of the physical parameters forward scatter (FSC) is indicator of the cell size while cellular complexity and granularity on the side scatter. Granular polymorphs were identified. Electronic emission was detected in the FL-1 channel (x-axis) and PI was detected in the FL-2 (y-axis). Electronic compensation required for annexin was done in three stages: (1) by analyzing a sample without staining to determine the level of auto fluorescence, (2) by analyzing an annexin-V labeled cell population, (3) PI-labeled population was analyzed on these settings. PI staining is a dye—exclusion assay that discriminates between cells with intact membranes (PI−) and permeabilized membranes (PI+). Cell population annexin V−/PI− was regarded as alive cell. Annexin-V+/PI− was considered as an early apoptotic while late stage apoptotic or necrotic was represented by annexin-V+/PI+ population.
Data Analysis
SPSS version 15 (statistical package for social science) was used for data analysis. Quantitative data were presented as median and range. Comparison between two groups was done by Mann-Whitney U test. Spearman’s rank correlation coefficient was used to measure the correlation between two variables. Statistical significance was defined as P < 0.05.
Results
Assessment of Neutrophil Apoptosis Before Culture
Neutrophil apoptosis which was presented by percentage of annexin-V expression was slightly increased in patient group as compared to normal healthy controls, but the difference was not statistically significant. Moreover, both caspases 3, and 10 activity were not significantly different in neutrophils separated from healthy controls or patients group at time 0 culture (P > 0.05 for all) (Figs. 1, 2, 3; Table 1).
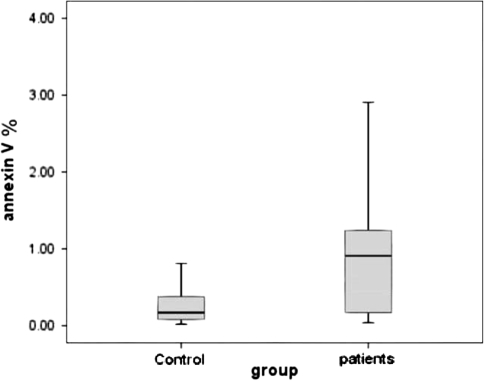
Fig. 1.
Annexin-V expression in freshly isolated neutrophil from control and patients groups. The percentage of annexin-V expression was insignificantly higher in patients neutrophils 0.96 (0.08–2.89) as compared to that from normal controls [0.17(0.02–0.81) (P = 0.059). The data were presented as median and range. The annexin-V expression was determined by flowcytometry and expressed as percentage
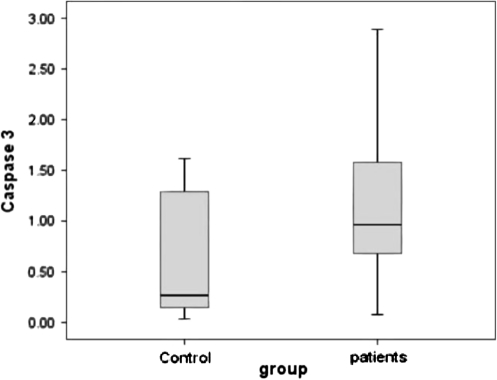
Fig. 2.
Caspase 3 activities in freshly isolated neutrophils from patients and controls groups were determined calorimetrically. The median and range of caspase 3 in HCV patients was 0.96 (0.08–2.89) and in healthy control was 0.23 (0.18–1.52). This difference was insignificant (P = 0.521)
Fig. 3.
Caspase 10 activity in freshly isolated neutrophils from patients and controls groups was determined calorimetrically. The median and range of caspase 10 in HCV patients was 1.26 (0.33–2.28) and in healthy control was 1.02 (0.27–1.72). This difference did not reach the level of significance (P = 0.521). The data were presented as median and range
Table 1.
Laboratory and hematological parameters in cirrhotic patients and controls
| Cirrhotic patients n = 26 | Healthy control n = 9 | P | |
|---|---|---|---|
| Hb (g/dl) | 9.95 (4.3–13.5) | 13.2 (12.7–14.0) | 0.002 |
| WBCs (×109/l) | 2.8 (1.5–3.7) | 5.27 (5.4–9.4) | 0.000 |
| Platelet (×109/l) | 71.79 (11–241) | 275 (190–320) | 0.000 |
| Albumin (g/dl) | 3.1 (2.1–4.2) | 4.9 (4.3–5.6) | 0.001 |
| SGPT (U/L) | 43 (15–266) | 32 (19–42) | 0.030 |
| SGOT (U/L) | 44.5 (22–173) | 31 (18–40) | 0.023 |
| INR | 1.58 (1.2–1.9) | 1.0 (1.0–1.1) | 0.000 |
| Annexin-V | 0.47 (0.04–3.27) | 0.17 (0.02-.81) | 0.059 |
| Caspase-3 | 96 (0.08–2.89) | 0.23 (0.18–1.52) | 0.450 |
| Caspase-10 | 1.26 (0.33–2.28) | 1.02 (0.27–1.72) | 0.521 |
The data were presented as median and range
Neutrophil Apoptosis After 24 h Culture
Patients sera induced significantly higher apoptosis in patient and control neutrophils culture ex vivo as compared with normal sera (P = 0.000). The addition of GM-CSF to patient’s sera cultured with patients or control neutrophil significantly attenuate neutrophil apoptosis (Fig. 4).
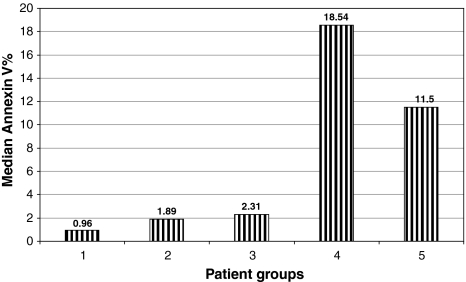
Fig. 4.
Represents the median percentage of annexin-V expression in patient groups. At the basal level group 1 (before culture) 0.96 (0.08–2.89) and after culture under different condition, (group 2) patients neutrophil cultured with sera of healthy control 1.89 (0.18–3.58), (group 3) patients neutrophil cultured with their own sera after addition of GM-CSF 2.31(0.88–9.81), (group 4) patients neutrophil cultured with their own sera without addition of GM-CSF 18.54 (1.53–69.1). (group 5) healthy neutrophil cultured with patients sera [11.5 (1.07–54.9)]. The difference is statistically significant (P < 0.001). The neutropenic patient’s sera induce the highest apoptotic effect on both patient neutrophil and healthy control neutrophil and addition of GM-CSF downregulate the apoptotic effect of patient’s sera
Activities of Caspses 3 and 10
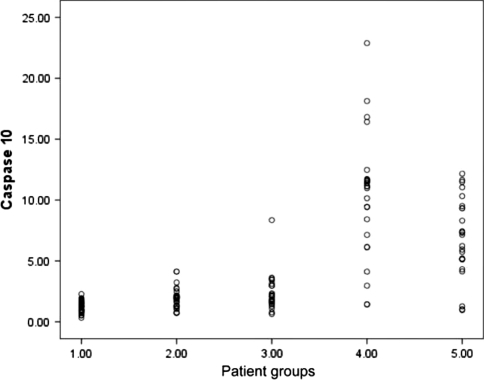
Caspases 3 and 10 activities were determined in cultured neutrophils under different conditions. The highest activities in caspases 3 and 10 were in neutrophil cultured with patient own sera, followed by control neutrophils cultured with patients sera, and this activities were down regulated by addition of GM-CSF (Figs. 5, 6).
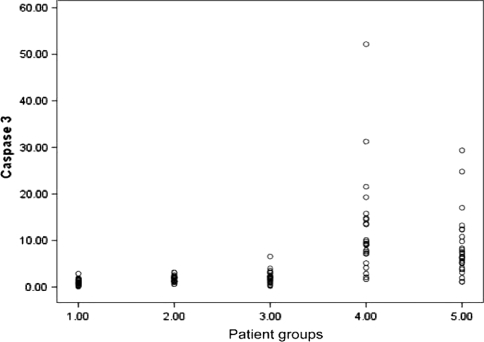
Fig. 5.
Represents scatter plot diagram of Caspase-3 activity at the basal level (1.00) before culture [1.09(0.08–2.89)] and after 24 h culture under different condition, (2.00) neutrophils patients with sera of healthy control [1.69(0.62–3.14)], (3.00) cultured patients neutrophils with their own sera after addition of GM-CSF [2.09(0.24–6.57)], (4.00) Patients neutrophils with their own sera [12.21(1.67–52.17)], (5.00) cultured healthy neutrophils with patients sera [6.57(1.12–29.36)]. The difference was statistically significant (P < 0.001). The neutropenic patient’s sera induce the highest apoptotic effect on both patient neutrophil and healthy control neutrophil and addition of GM-CSF down regulate the apoptotic effect of patient’s sera
Fig. 6.
Represents scatter plot diagram of Caspase-10 activity at the basal level, (1.00) before culture [1.26(0.33–2.28)] and after culture under different condition,(2.00) neutrophils patients with sera of healthy control [1.97(0.72–4.13)], (3.00) cultured patients neutrophils with their own sera after addition of GM-CSF [2.29(0.03–8.35)], (4.00) Patients neutrophils with their own sera [10.31(1.42–22.89),)], (5.00) cultured healthy neutrophils with patients sera [6.57(0.96–12.17)]. The difference was statistically significant (P < 0.001). The neutropenic patient’s sera induce the highest apoptotic effect on both patient neutrophil and healthy control neutrophil and addition of GM-CSF downregulate the apoptotic effect of patient’s sera
Interactions Between Annexin-V and Caspases 3 and 10
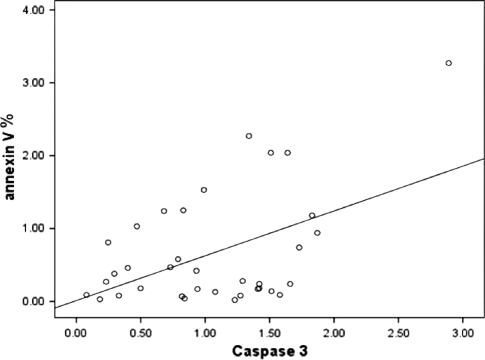
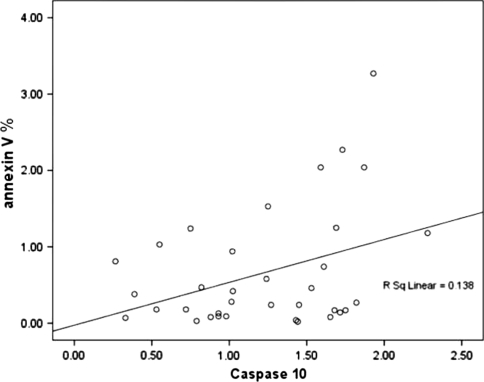
The correlation study between annexin-V expression and caspases activities revealed a borderline positive correlation between annexin–V and caspase 3 (r = 0.376, P = 0.058), a nearly significant positive correlation with caspase 10 activity (r = 0.494, P = 0.010) as shown in Figs. 7 and 8.
Fig. 7.
Correlation between annexin-V expression and caspase 3 activity. A borderline insignificant positive correlation (r = 0.376, P = 0.058)
Fig. 8.
Correlation between annexin-V and caspase 10 activity; significant positive correlation was presented (r = 0.494, P = 0.001)
Discussion
Neutropenia is a common complication during the course of chronic HCV infection, predisposing those patients to bacterial infection, with a high mortality. One of the postulated mechanisms for neutropenia is enhanced neutrophil apoptosis which is induced by Fas ligand secreted from spleen. However, the mechanistic approach underlying this process is still unclear.
In the present study, no significant difference of neutrophil apoptosis % was observed between patients group and normal healthy controls. This was explained by Savill et al. [15] who reported that apoptotic neutrophils are swiftly recognized and phagocytesed by macrophages. Shi et al. [16] studied neutrophil kinetics in rat and observed that apoptotic neutrophils were phagocytosed by kupffer cells or macrophage in hepatic sinusoids showing that the liver serves as major role in the clearance of apoptotic neutrophils, hence, we performed ex vivo culture of neutrophil.
After culture of isolated neutrophils, we observed increased loss of neutrophil viability when compared with controls. The percentage of annexin-V expression was highly significantly increased in patients group as compared to normal controls. This was in agreement with Aref et al. [17], who found enhanced neutrophil apoptosis in schisotosomiasis splenomegaly by annexin–V.
Cultured neutrophils with neutropenic sera harvested either from neutropenic HCV patients or from healthy normal control showed highly significant increase in annexin-V expression as compared with 0 time culture. This finding point out for the presence of serum factor in the neutropenic patients sera that induce neutrophils apoptosis. This finding confirm our previous finding but in a cases of schistosomiasis [10].
The increased neutrophils apoptosis in neutrophils cultured with neutropenic patient’s sera is down regulated by addition of GM-CSF. The mechanistic pathway of neutrophil apoptosis is still unclear. The increased caspases 10 and 3 activity as well positive correlation with annexin-V expression points out for their role in this process. Similar findings were stated regarding caspase 3. Molloy et al. [12] reported an increase in caspase 3 activity in hepatitis C patients, and Ramirez et al. [7] found significantly higher caspase 3 activities with marked accelerated apoptosis and decreased PMN viability in cirrhotic patients with ascites than control.
Recent evidence demonstrates that the in vitro life span of mature human neutrophils can be extended significantly by incubation with pro inflammatory mediators and cytokines including granulocyte colony–stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF) [12].
GM-CSF is the major haematopoitic growth factor regulating the production of neutrophils. Treatment by G-CSF leads to an increase in neutrophil count to >1000 cells per cubic millimeter in 90% of patients resulting in significant improvements in survival and quality of life [18, 19].
Many reports [20–22] stated that GM-CSF plays an important role in the maintenance of hematopoietic homeostasis by regulating hematopoietic cell growth, survival. GM-CSF counteracts programmed cell death through induction of BCL-2 and BCL-Xl expression in a dose- and time-dependent manner and through the activation of a number of intracellular signaling pathways that ultimately results in the activation of specific transcription factors and subsequent modulation of target gene expression [23]. The mitogen-activated protein kinase (MAPK) cascade is one of the key signaling pathways that couples the signals from cell-surface cytokine receptors to trigger downstream pathways [24, 25]. Three major groups of MAPKs have been characterized in mammals, including extracellular signal-regulated protein kinases (ERKs), c-Jun NH2-terminal kinases, and p38MAPKs [26, 27]. Activation of MAPK is regulated by an evolutionary conserved kinase cascade and MAPKKKs are serine/threonine kinases capable of phosphorylating and activating MAPKKs, which in turn stimulate MAPK activity through dual phosphorylation on threonine and tyrosine residues within a tripeptide motif (Thr-X-Tyr). On activation, MAPK itself can phosphorylate specific target substrates on serine or threonine residues [28, 29].
In the present study the percentage of neutrophil apoptosis induced by neutropenic patient sera was down regulated by addition of GM-CSF. The data of Nagarsekar et al. [8] supported our findings. They reported that the PMN survival factor G-CSF, GM-CSF and interleukin-10 each prolonged PMN survival at 37 and 39.5°C. This finding will give the hope to those patients for improvement from neutopenia in advanced liver cirrhosis and minimize the risk of bacterial infection.
Conclusion
These findings suggest that enhanced neutrophil apoptosis demonstrated in neutropenic patients with HCV infection might be due to activation of caspase 10 and is attenuated by GM-CSF.
References
- 1.Frank C, Mohamed MK, Strickland GT, et al. The role of parentral antishistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/S0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 2.Marusawa H, Hijikata M, Chiba T, Shimotohno K. Hepatitis C Virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF- B activation. J Virol. 1999;73:4713–4720. doi: 10.1128/jvi.73.6.4713-4720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo JC, Hwang SJ, Chang FY, et al. Simple blood tests can predict compensated liver cirrhosis in patients with chronic hepatitis C. Hepatogastroenterology. 2002;49:478–481. [PubMed] [Google Scholar]
- 4.Srinivas D, Mani H, Crumpler C, Thiel DH. Daily interferon therapy for chronic hepatitis C: a report of a community practice experience. Hepatogastroenterology. 2002;49:1053–1057. [PubMed] [Google Scholar]
- 5.Calabrese F, Pontiso P, Perrenazzo E, et al. Liver cell apoptosis in chronic hepatitis C correlates with histological but not biochemical activity or serum HCV-RNA levels. Hepatology. 2000;31:1153–1159. doi: 10.1053/he.2000.7123. [DOI] [PubMed] [Google Scholar]
- 6.Bantel H, Lugering A, Poremba C, et al. Caspase activation correlates with the degree of inflammatory liver injury in chronic hepatitis C virus infection. Hepatology. 2001;34:758–767. doi: 10.1053/jhep.2001.28229. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez MJ, Titos E, Claria J, et al. Increased apoptosis dependent on caspase-3 activities in polymorphonuclear leukocyte from patients with cirrhosis and ascites. J Hepatol. 2004;41:44. doi: 10.1016/j.jhep.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Nagarsekar A, Greenberg RS, Shah NG, et al. Febrile-range hyperthermia accelerates caspase-dependent apoptosis in human neutrophils. J Immunol. 2008;15:2636–2643. doi: 10.4049/jimmunol.181.4.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El bim C, Katsikis D, Estaquier P. Neutrophil apoptosis during viral infections. Open Virol J. 2009;3:52–59. doi: 10.2174/1874357900903010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aref S, El-Refaaei M, Goda T, et al. Accelerated neutrophil apoptosis in neutropenic patients with hepatosplenic schistosomiasis is induced by serum Fas ligand. Hematol J. 2004;5:434–443. doi: 10.1038/sj.thj.6200542. [DOI] [PubMed] [Google Scholar]
- 11.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-flip in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 12.Molloy EJ, O Neill AJ, Grantham JJ et al (2005) Increase caspase-3 activity in hepatitis C. Pediatr Res 57:806
- 13.Majewska E, sulowska Z, Baj Z. Spontaneous apoptosis of neutrophils in whole blood and its relation to apoptosis gene proteins. Scan J Immunol. 2000;52:496–501. doi: 10.1046/j.1365-3083.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- 14.Geopel F, Weinmann P, Schymeinsky J, Walzog B. Identification of caspase-10 in human neutrophils and its role in spontenous apoptosis. J Leuk Biol. 2004;75:836. doi: 10.1189/jlb.0703317. [DOI] [PubMed] [Google Scholar]
- 15.Savil JS, Wyllie AH, Henson JE, et al. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J, Fujjeda H, Kokubo Y, Wake K. Apoptosis of neutrophil and their elimination by kupffer cells in rat liver. Hepatology. 1996;24:1256–1263. doi: 10.1002/hep.510240545. [DOI] [PubMed] [Google Scholar]
- 17.Aref S, Mahmoud L, El-Refie M, Abdel-Wahaab M, Abou Samara N. Assessment of neutrophil apoptosis in hepatosplenic patients with neutropenia before and after splenectomy. Hematology. 2003;8:221–228. doi: 10.1080/1024533031000153630. [DOI] [PubMed] [Google Scholar]
- 18.Dale DCMA, Bonillia MW, Davis AM, et al. A randomized controlled phase III trial of recombinant human granulocyte colony–stimulating factor (filgrastism) for treatment of severe chronic neutropenia. Blood. 1993;81:2496–2502. [PMC free article] [PubMed] [Google Scholar]
- 19.Bonilla MAD, Date C, Zeidler L, et al. Long-term safty of treatment with recombinant human granulocyte colony stimulating factor (r- met HUG-CSF) in patients with severe congenital neutropenia. Br J Haematol. 1994;88:723–730. doi: 10.1111/j.1365-2141.1994.tb05110.x. [DOI] [PubMed] [Google Scholar]
- 20.Schäbitz W, Krüger C, Pitzer C, et al. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF) J Cereb Blood Flow Metab. 2008;28:29–43. doi: 10.1038/sj.jcbfm.9600496. [DOI] [PubMed] [Google Scholar]
- 21.Geest RC, Buitenhuis M, Koerkamp M, et al. Tight control of MEK-ERK activation is essential in regulating proliferation, survival, and cytokine production of CD34+-derived neutrophil progenitors. Blood. 2009;114:3402–3412. doi: 10.1182/blood-2008-08-175141. [DOI] [PubMed] [Google Scholar]
- 22.Cowburn AS, Summers C, Dunmore BJ et al (2010) GM-CSF causes a Paradoxical Increase in the BH3-only Pro-apoptotic protien BIM in human neutrophils. Am J Respir Cell Mol Biol [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 23.Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724–6737. doi: 10.1038/sj.onc.1210757. [DOI] [PubMed] [Google Scholar]
- 24.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/S0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 25.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 26.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang CI, Xu B, Akella R, et al. Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol Cell. 2002;9:1241–1249. doi: 10.1016/S1097-2765(02)00525-7. [DOI] [PubMed] [Google Scholar]
- 29.Tanoue T, Adachi M, Moriguchi T, et al. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]