Abstract
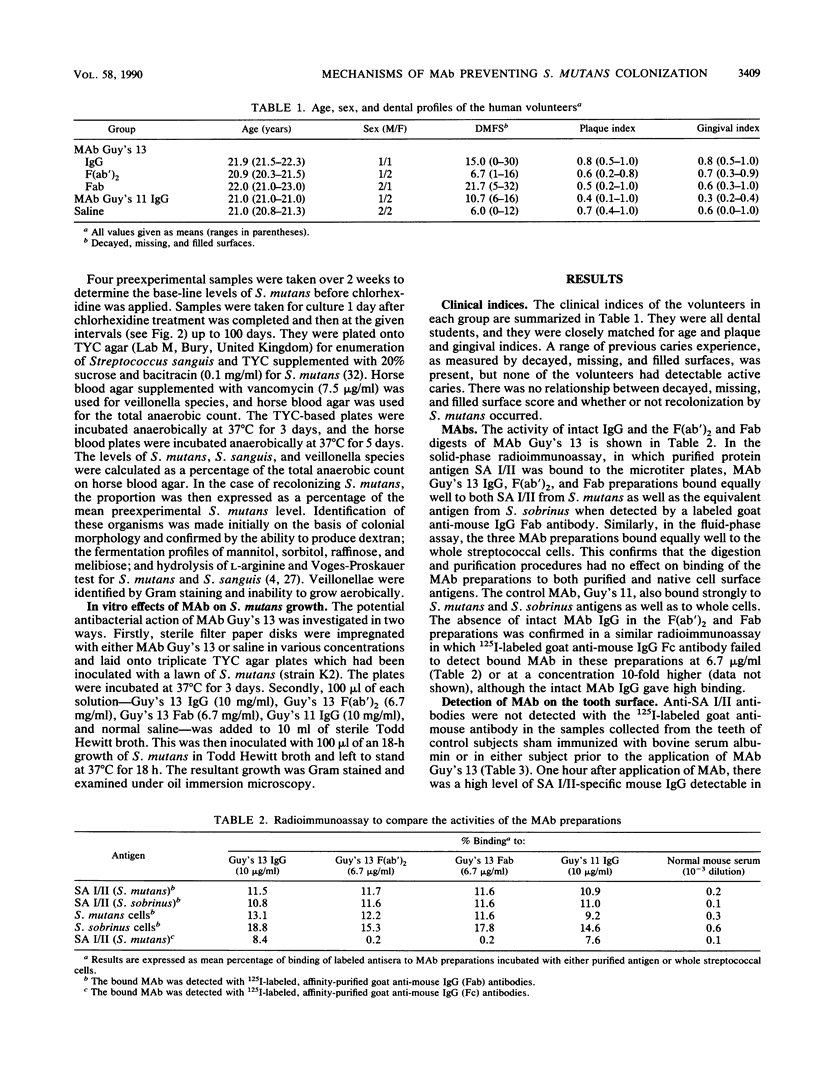
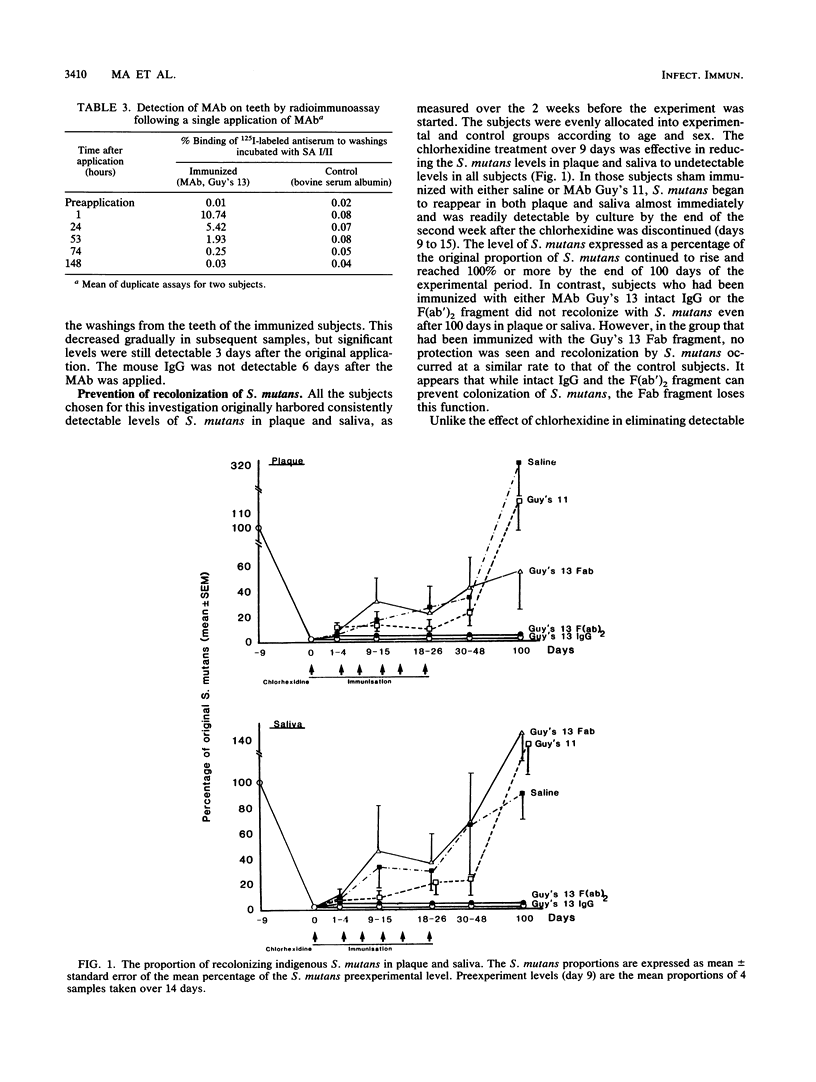
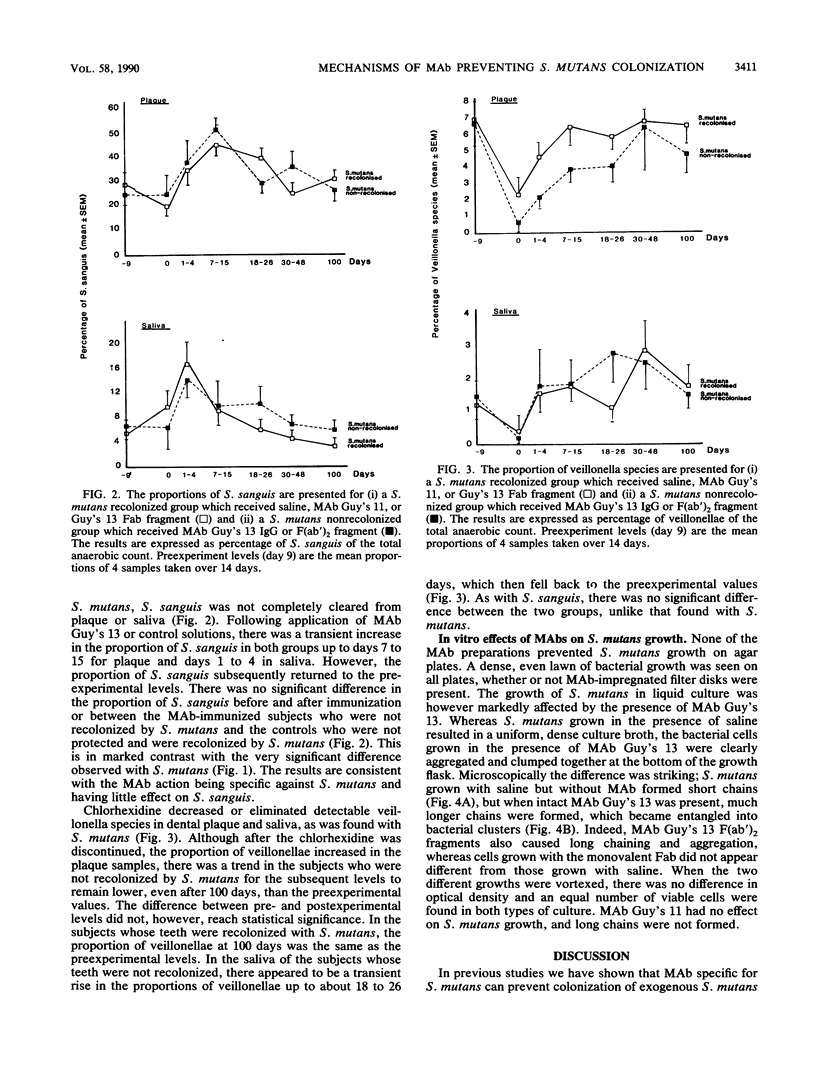
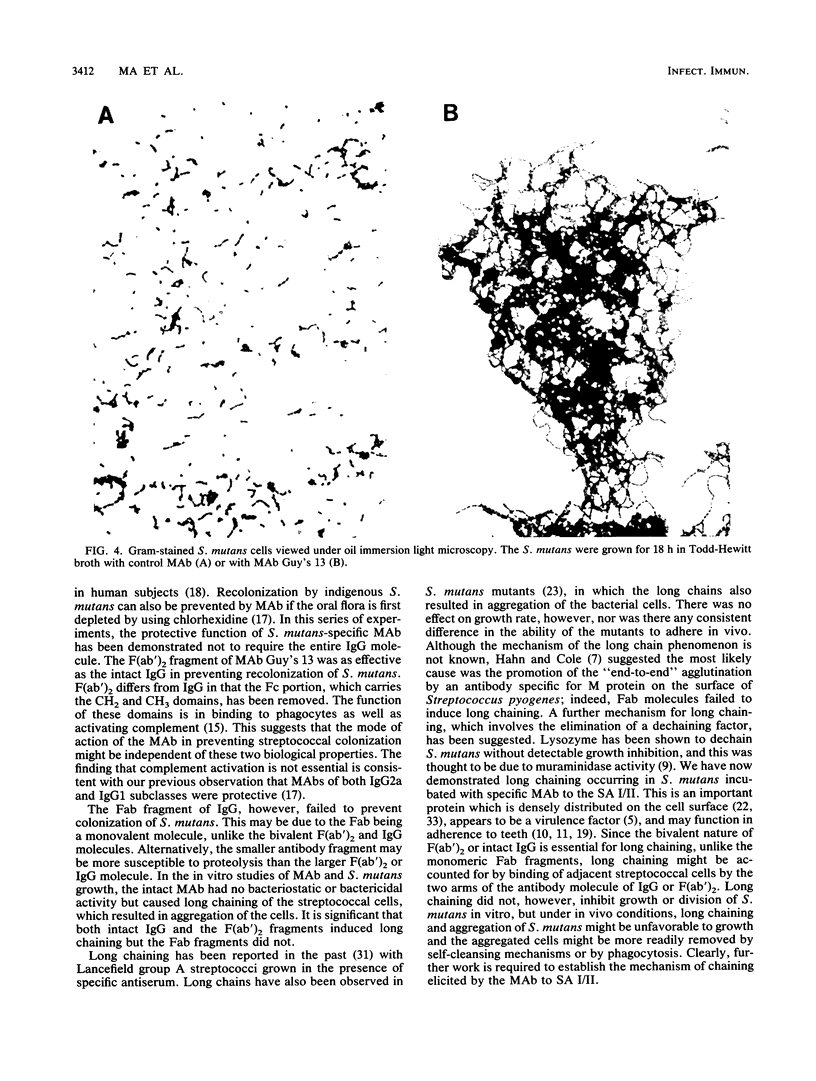
Local oral passive immunization with Streptococcus mutans-specific monoclonal antibody (MAb) (Guy's 13) prevented recolonization by indigenous S. mutans in human volunteers who had first been treated with a conventional antibacterial agent (chlorhexidine). The F(ab')2 fragment of the MAb was as protective as the intact immunoglobulin G, but the Fab fragment of the molecule failed to prevent recolonization of S. mutans. In subjects receiving the MAb Fab fragment, S. mutans levels in dental plaque and saliva reappeared at a similar rate to that found in sham-immunized subjects who received either saline or a nonprotective MAb. In vitro, MAb had no bacteriostatic or bacteriocidal effect on S. mutans. However, S. mutans grown in the presence of either intact immunoglobulin G MAb or the F(ab')2 fragment formed very long chains, which resulted in clumping of the cells. S. mutans grown with either saline or the MAb Fab fragment formed significantly shorter chains, more characteristic of streptococcal growth in liquid media. The results suggest that the two binding sites of the MAb molecule may be an essential feature for preventing streptococcal colonization but that the ability to bind to phagocytes and activate complement which resides in the Fc fragment is not essential. Protection against colonization by S. mutans lasting up to 2 years was observed in immunized subjects, although MAb was applied over a period of only 3 weeks. Furthermore, functional MAb was detected up to 3 days following application of MAb to the teeth. The long-term protection could not be accounted for by a persistence of MAb on the tooth surface, and we have suggested that it may be due to a shift in the balance of the oral flora which discouraged recolonization by S. mutans. However, examination of the proportions of Streptococcus sanguis and veillonella species in the recolonization experiments failed to reveal a significant change in the proportions of either organism, which returned to approximately the preexperimental levels in both the immunized and control groups. These findings confirm the in vivo functional specificity of the MAb to S. mutans but are not consistent with the suggestion that S. sanguis or veillonella take over the niche vacated by S. mutans, unless the shift in the proportion of these organisms cannot be detected by the method used.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic analysis of Streptococcus mutans virulence. Curr Top Microbiol Immunol. 1985;118:253–277. doi: 10.1007/978-3-642-70586-1_14. [DOI] [PubMed] [Google Scholar]
- Emilson C. G., Krasse B. Support for and implications of the specific plaque hypothesis. Scand J Dent Res. 1985 Apr;93(2):96–104. doi: 10.1111/j.1600-0722.1985.tb01316.x. [DOI] [PubMed] [Google Scholar]
- HAHN J. J., COLE R. M. Studiey on the mechanism of the long chain phenomenon of group A streptococci. J Exp Med. 1963 Apr 1;117:583–594. doi: 10.1084/jem.117.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., Byrnes T. P., Crawford I. T., Grossbard B. L., Pollock J. J., MacKay B. J. Lysozyme-mediated de-chaining of Streptococcus mutans and its antibacterial significance in an acidic environment. J Dent Res. 1985 Jan;64(1):48–53. doi: 10.1177/00220345850640010901. [DOI] [PubMed] [Google Scholar]
- Koga T., Okahashi N., Takahashi I., Kanamoto T., Asakawa H., Iwaki M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990 Feb;58(2):289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOE H., SILNESS J. PERIODONTAL DISEASE IN PREGNANCY. I. PREVALENCE AND SEVERITY. Acta Odontol Scand. 1963 Dec;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Erdos G. W., Piacentini D. A., Ayakawa G. Y., Crowley P. J., Bleiweis A. S. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect Immun. 1989 Nov;57(11):3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Caldwell J., Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect Immun. 1985 Dec;50(3):796–799. doi: 10.1128/iai.50.3.796-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Russell M. W., Caldwell J. Immunisation with a purified protein from Streptococcus mutans against dental caries in rhesus monkeys. Lancet. 1980 May 10;1(8176):995–996. doi: 10.1016/s0140-6736(80)91437-3. [DOI] [PubMed] [Google Scholar]
- Ma J. K., Hunjan M., Smith R., Lehner T. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin Exp Immunol. 1989 Sep;77(3):331–337. [PMC free article] [PubMed] [Google Scholar]
- Ma J. K., Smith R., Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect Immun. 1987 May;55(5):1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Gregory R. L., Harmon C. C., Katz J., Richardson G. J., Hilton T., Filler S. J., McGhee J. R. Protection of gnotobiotic rats against dental caries by passive immunization with bovine milk antibodies to Streptococcus mutans. Infect Immun. 1987 Oct;55(10):2341–2347. doi: 10.1128/iai.55.10.2341-2347.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. G., Smith R., Lehner T. Recognition of carbohydrate and protein epitopes by monoclonal antibodies to a cell wall antigen from Streptococcus mutans. Infect Immun. 1987 Mar;55(3):810–815. doi: 10.1128/iai.55.3.810-815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro I., Russell M. W. Ultrastructural localization of protein antigens I/II and III in Streptococcus mutans. Infect Immun. 1983 Jul;41(1):410–413. doi: 10.1128/iai.41.1.410-413.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison H., Larrimore S., Hull S., Curtiss R., 3rd Isolation and characterization of Streptococcus mutans mutants with altered cellular morphology or chain length. Infect Immun. 1982 Oct;38(1):282–291. doi: 10.1128/iai.38.1.282-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- SILNESS J., LOE H. PERIODONTAL DISEASE IN PREGNANCY. II. CORRELATION BETWEEN ORAL HYGIENE AND PERIODONTAL CONDTION. Acta Odontol Scand. 1964 Feb;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- STOLLERMAN G. H., EKSTEDT R. Long chain formation by strains of group A streptococci in the presence of homologous antiserum: a type-specific reaction. J Exp Med. 1957 Sep 1;106(3):345–356. doi: 10.1084/jem.106.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J. A biochemical scheme for the separation of the five varieties of Streptococcus mutans. Arch Oral Biol. 1974 Nov;19(11):1079–1081. doi: 10.1016/0003-9969(74)90099-5. [DOI] [PubMed] [Google Scholar]
- Smith R., Lehner T. A radioimmunoassay for serum and gingival crevicular fluid antibodies to a purified protein of Streptococcus mutans. Clin Exp Immunol. 1981 Feb;43(2):417–424. [PMC free article] [PubMed] [Google Scholar]
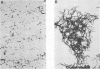
- Smith R., Lehner T. Characterisation of monoclonal antibodies to common protein epitopes on the cell surface of Streptococcus mutans and Streptococcus sobrinus. Oral Microbiol Immunol. 1989 Sep;4(3):153–158. doi: 10.1111/j.1399-302x.1989.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Van Palenstein Helderman W. H., Ijsseldijk M., Huis in 't Veld J. H. A selective medium for the two major subgroups of the bacterium Streptococcus mutans isolated from human dental plaque and saliva. Arch Oral Biol. 1983;28(7):599–603. doi: 10.1016/0003-9969(83)90007-9. [DOI] [PubMed] [Google Scholar]
- Zanders E. D., Lehner T. Separation and characterization of a protein antigen from cells of Streptococcus mutans. J Gen Microbiol. 1981 Feb;122(2):217–225. doi: 10.1099/00221287-122-2-217. [DOI] [PubMed] [Google Scholar]