Abstract
Background
To improve the prognosis of patients after resection of pancreatic cancer, the most appropriate and efficient treatment should be provided to specific subsets of patients. Our aim was to identify promising microRNAs as markers to predict responses to gemcitabine in patients with resected pancreatic cancer.
Methods
Two gemcitabine-resistant pancreatic cancer cell lines were established, and global microRNA expression analyses was performed by quantitative reverse transcription–polymerase chain reaction (qRT-PCR). Eleven miRNAs were selected as putative predictive markers and analyzed by means of macrodissected formalin-fixed, paraffin-embedded samples obtained from 90 patients with or without gemcitabine treatment after resection of pancreatic cancer.
Results
We identified 24 microRNAs whose expression was altered in gemcitabine-resistant cells. qRT-PCR analyses showed that patients with high miR-142-5p and miR-204 expression had significantly longer survival times than those with low miR-142-5p (P = 0.0077) and miR-204 (P = 0.0054) expression in the gemcitabine-treated group. This was not seen in the nontreated group. Multivariate analyses showed that miR-142-5p expression was an independent prognostic marker only in patients treated with gemcitabine (P = 0.034).
Conclusions
miR-142-5p is a promising predictive marker for gemcitabine response in patients with resected pancreatic cancer.
Pancreatic cancer is the fourth most common cause of tumor-related death in the industrialized world.1,2 Only 10–20% of pancreatic cancer patients are candidates for surgery at the time of presentation, and fewer than 20% of patients who undergo curative resection are alive after 5 years.3,4 A few recent reports have described the successful use of adjuvant chemotherapies such as 5-fluorouracil and gemcitabine.5,6 However, not all patients benefit from such adjuvant chemotherapy, and we cannot predict which patients will benefit most from this treatment. Therefore, to improve the prognosis of patients with resected pancreatic cancer, we need to identify specific markers that can predict responses to adjuvant therapy. Such a personalized therapy, based on the predictive markers, may provide the most appropriate and efficient treatment for a specific subset of patients.
Gemcitabine is a deoxycytidine analog with antitumor activity that bears a resemblance, both structurally and metabolically, to arabinosyl cytosine.7 Gemcitabine is widely accepted as the first-line treatment for patients with advanced or resected pancreatic cancer.6,8 However, recent reports showed that the complete plus partial response rate and the disease control rate in advanced pancreatic cancer are 8.0–13.5% and 49.2–62.1%, respectively, even with combination-treatment arms.9,10 The data suggest that approximately half of patients with resected pancreatic cancer do not benefit from gemcitabine-based combination therapies. Therefore, predictive markers are needed to select those patients who may benefit most from gemcitabine-based therapy. So far, research into the mechanism of resistance to gemcitabine has led to identification of several candidate predictive markers. These include genes related to gemcitabine metabolism and transport, such as deoxycytidine kinase, ribonucleotide reductase, and human equilibrative nucleoside transporter-1.11,12 However, the usefulness of such markers in a clinical setting remains unclear because of difficulties in evaluating their protein or mRNA levels. Even when well-established methods are used, immunohistochemical evaluation and its interpretation are different between laboratories.13 Also, accurate quantitative analyses of mRNA from clinical samples are often difficult as a result of degradation. Therefore, we need more reliable methods-based biomarkers to predict responses to gemcitabine.
MicroRNAs (miRNAs) are small noncoding RNA gene products of approximately 22 nt that are found in a variety of organisms. They play key roles in regulating the translation and degradation of mRNAs through base pairing to partially complementary sites, predominantly in the 3′-untranslated regions of mRNAs.14,15 Because microRNAs are well preserved, even in formalin-fixed, paraffin-embedded (FFPE) samples, the quantitative analysis of miRNA in many types of clinical sample is accurate and reliable.16,17 Therefore, miRNAs are promising molecular markers.
There is increasing evidence that miRNAs are mutated or differentially expressed in many types of cancers, and thus are also potential diagnostic markers.18–20 The expression levels of several miRNAs, such as miR-21, miR-196a-2, miR-155, and miR-210 in pancreatic cancer, are known to correlate with a poor prognosis.19,21,22 Thus, there is a possibility that specific miRNAs that can be used as predictive markers for a gemcitabine response exist.
Here, to identify predictive markers, we established two gemcitabine-resistant pancreatic cancer cell lines and performed global miRNA expression analyses. We then further investigated 11 selected miRNAs as potential predictive markers using macrodissected FFPE samples derived from 90 patients with resected pancreatic cancer.
Materials and Methods
Cell Lines and Establishment of Gemcitabine-Resistant Cells
Two human pancreatic cancer cell lines, SUIT-2 and CAPAN-1 (the gift of Dr. H. Iguchi, National Shikoku Cancer Center, Matsuyama, Japan), were used in this study. Gemcitabine-resistant cells were generated by exposing these cell lines to gradually increasing concentrations of gemcitabine. The initial concentration of gemcitabine was 1 nM, which did not seem to affect the proliferation of either the SUIT-2 or CAPAN-1 cells. When the cells had adapted to the drug, the concentration of gemcitabine was gradually increased by 10–100 nM per week to a final concentration of 200 nM (SUIT-2) and 1 μM (CAPAN-1). Gemcitabine was dissolved in phosphate-buffered saline and added to the cell culture media.
Propidium Iodide Assay
Cell proliferation was evaluated by measuring the fluorescence intensity of propidium iodide as described previously.23 The fluorescence intensity corresponding to the total cells was measured with a CytoFluor multiwell plate reader (PerSeptive Biosystems, Framingham, MA, USA). The results were converted to percentage survival rates by comparing treated cells with untreated cells.
miRNA Expression Profiling
Expression profiles for the 365 mature miRNAs were obtained by quantitative polymerase chain reaction (qPCR) using the TaqMan Array Human MiRNA (TLDA) or TaqMan MiRNA Assays (Applied Biosystems; ABI) on an ABI Prism 7900HT according to the manufacturer’s instructions. Mature miRNAs were normalized to RNU44 and are expressed as fold changes relative to controls.
Patients and Pancreatic Tissues
From 1992 through 2008, a total of 104 patients underwent pancreatic resection for pancreatic cancer at the Department of Surgery and Oncology, Kyushu University Hospital (Fukuoka, Japan). Survival was measured from the time of pancreatic resection, and death was the end point. The follow-up data for 103 cases were available. Thirteen of the 103 patients were excluded from the present study because they had received combination therapy with gemcitabine and other chemotherapeutic agents such as S1(tegafur/gimestat/potassium oxonate).
Our final study series consisted of 90 patients with resected pancreatic cancer with available follow-up data. The patients (57 men and 32 women) had a median age of 65 years (range, 36–86 years). The median observation time for overall survival was 14.7 months, ranging 0.5–108 months. Sixty-three patients died during follow-up; the other patients were alive and censored.
All resected specimens were fixed in formalin and embedded in paraffin for pathological diagnosis. All tissues adjacent to the specimens were evaluated histologically according to the criteria of the World Health Organization.24 Diagnoses were confirmed independently by two pathologists with regard to the pathological features of all cases. Tumor stage was assessed according to the International Union Against Cancer classification.25 Clinicopathological characteristics of the tumors are shown in Table 1. The study was approved by the Ethics Committee of Kyushu University and was conducted according to the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese government and the Helsinki Declaration.
Table 1.
Correlation between median OS and clinical and pathological factors in patients with resected pancreatic cancer
| Factor | n | OS (95% CI) | P value |
|---|---|---|---|
| Age | 0.675 | ||
| ≥65 years | 47 | 19 (13.07–27) | |
| <64 years | 42 | 19 (12–26) | |
| Sex | 0.7544 | ||
| Male | 57 | 23 (13.07–30.17) | |
| Female | 32 | 14 (10–24.2) | |
| pT category | 0.0019 | ||
| pTl/pT2/pT3 | 55 | 23 (16.30–43) | |
| p4 | 33 | 12 (9.43–19) | |
| pN category | 0.0070 | ||
| pN0 | 26 | 43 (16.93–) | |
| pN1 | 62 | 13.27 (11.6–22) | |
| Histological grade | 0.0804 | ||
| G1 | 19 | 31 (9–) | |
| G2 | 33 | 23 (14–30.16) | |
| G3 | 35 | 12 (10–23) | |
| Residual tumor | <0.0001 | ||
| R0 | 53 | 26 (19–45) | |
| R1 | 34 | 12 (9–13.73) | |
| Vessel invasion | 0.0204 | ||
| Positive | 57 | 13.73 (12–23) | |
| Negative | 31 | 27 (15–) | |
| Lymphatic invasion | 0.3004 | ||
| Positive | 76 | 15 (12–24.2) | |
| Negative | 18 | 27 (14.53–) | |
| Neural invasion | 0.6849 | ||
| Positive | 73 | 16.93 (13.07–26) | |
| Negative | 15 | 23 (4.7–) | |
| Adjuvant therapy | 0.0382 | ||
| Yes | 59 | 23 (14.53–30.17) | |
| No | 30 | 12 (7.7–24.2) |
OS overall survival, CI confidence interval
Macrodissection
After a review of representative hematoxylin and eosin-stained slides, 4–7 sections (5 μm thick) were cut from FFPE blocks for macrodissection. Adjacent normal tissues, including normal acinar tissues and adipose tissues, in the sections were removed macroscopically with a scalpel. Only the cancerous parts were used for the isolation of miRNA.
Isolation of miRNA
miRNA was extracted from the macrodissected FFPE samples with the RNeasy FFPE kit (Qiagen, Tokyo, Japan) using a method modified from the manufacturer’s instructions. Briefly, macrodissected FFPE sections were deparaffinized with xylene, washed with ethanol, and dried. Lysis buffer and proteinase K were added to the dried sections. The sections were incubated and Binding buffer was then added to the lysate and transferred to a gDNA Eliminator spin column (Qiagen) to remove genomic DNA. After eliminating DNA, 100% ethanol was added to the flow-through. After mixing, the samples were transferred to an RNeasy MinElute column (Qiagen), which binds total RNA. After washing, the purified RNA was eluted with 50 μl of RNase-free water.
Quantitative Reverse Transcription–Polymerase Chain Reaction
Quantitative reverse transcription–polymerase chain reaction (qRT-PCR) was performed in a Chromo4 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) using TaqMan MicroRNA Reverse Transcription Kit and TaqMan Universal PCR Master Mix (Applied Biosystems, Tokyo, Japan). For the measurement of miRNA expression, we performed two-step qRT-PCR with specific primers for the indicated miRNAs (designed by Applied Biosystems) following the manufacturer’s protocol. Each sample was run in triplicate. The level of miRNA expression was calculated from a standard curve constructed by using small RNAs from CAPAN-1 cells. The expression levels of the indicated miRNAs were normalized to those of RNU6B. The accuracy and integrity of the PCR products were confirmed with an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Palo Alto, CA, USA).
Statistical Analysis
A data mining technique provided by the SAS Institute was used to split gene expression in high- and low-level groups on the basis of a platform that recursively partitions data according to a relationship between the X and Y values, creating a tree of partitions (recursive descent partition analysis).26 By searching all possible cuts, it finds a set of cut points of X values (gene expression) that best predict the Y value (survival time). These data splits are done, recursively forming a tree of decision rules until the desired fit is reached; the most significant split is determined by the largest likelihood ratio chi-square statistic. In either case, the split is chosen to maximize the difference in the responses between the two branches of the split. Categorical variables were compared by a chi-square test (Fisher’s exact probability test). Survival curves were constructed using the Kaplan-Meier product-limit method and compared by the log rank test. To evaluate the independent prognostic factors associated with patient survival (with or without gemcitabine treatment), multivariate Cox proportional hazard regression analysis was used, with miR-142-5p expression, miR-34a expression, pN status, and/or residual tumor status (R factor) as covariates. Statistical significance was defined as a P value of <0.05. All statistical analyses were performed by JMP 7.01 software (SAS Institute, Cary, NC, USA).
Results
Establishment of Gemcitabine-Resistant Pancreatic Cancer Cells
Gemcitabine-resistant SUIT-2 and CAPAN-1 cells were generated by exposure to gradually increasing concentrations of gemcitabine. The final concentration of gemcitabine was 200 nM for SUIT-2 cells and 1 μM for CAPAN-1 cells. The viability of the parental SUIT-2 cells significantly decreased to less than 20% after treatment with 10 nM gemcitabine, while the viability of gemcitabine-resistant SUIT-2 cells remained unchanged after treatment with 10 nM gemcitabine. The viability of the parental CAPAN-1 cells significantly decreased to less than 10% after treatment with 1 μM gemcitabine, while the viability of gemcitabine-resistant CAPAN-1 cells remained unchanged after treatment with 1 μM gemcitabine.
miRNA Differentially Expressed between Parent and Gemcitabine-Resistant Cells
We used the TaqMan miRNA array to obtain the 365 miRNA expression profiles from the parent and gemcitabine-resistant pancreatic cancer cell lines. The results show that the expression of 10 miRNAs was more than 2-fold higher in both SUIT-2 and CAPAN-1 gemcitabine-resistant cells compared with the parental cells. We also identified 14 miRNAs in both SUIT-2 and CAPAN-1 gemcitabine-resistant cells that were downregulated to less than 30% of that in the parental cells.
To confirm our TaqMan miRNA array data, we subjected 11 of the differentially expressed miRNAs (5 upregulated miRNAs: miR-9, miR-27a, miR-424, miR-449b, and miR-34a; 6 downregulated miRNAs: miR-152, miR-181c, miR-518b, miR-125a, miR-142-5p, and miR-204), which were selected on the basis of expression levels and fold changes as promising candidate of clinical markers, to triplicate qRT-PCR analysis, and obtained consistent results (data not shown).
Analysis of miRNA Expression in Relation to Survival Time of Patients with Pancreatic Cancer after Curative Resection
Conventional prognostic factors, such as pT category, pN category, R factor, positive vessel invasion, and adjuvant therapies, including 5-fluorouracil or gemcitabine, reached significance for overall survival (Table 1). We also found that the patients treated with gemcitabine had a better prognosis than those without, although the difference was not significant (P = 0.058).
Next, to investigate the correlation between gemcitabine response and miRNA expression identified by miRNA expression profiling, patients with resected pancreatic cancer were divided into two groups: patients who were treated with gemcitabine and patients who were not. Within each group, we investigated the correlation between the expression levels of the 11 miRNAs identified by miRNA expression profiling and the prognosis of patients with resected pancreatic cancer. miRNA expression was also divided into high- and low-level groups by recursive descent partition analysis, as described by Hoffmann et al.26
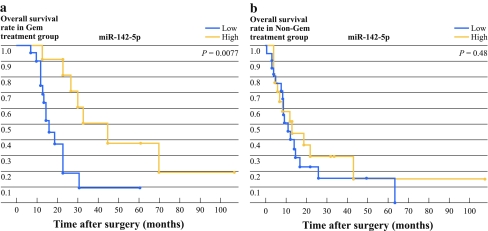
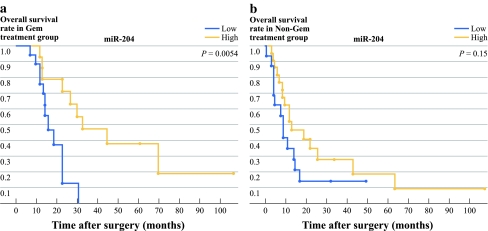
First, we investigated the correlation between prognosis and the six downregulated miRNAs: miR-152, miR-181c, miR-518b, miR-125a, miR-142-5p, and miR-204. For the gemcitabine group, the high miR-142-5p and miR-204 patients had a significantly longer survival time than the low miR-142-5p and miR-204 patients (Figs. 1 and 2, P = 0.0077 for miR-142-5p; P = 0.0054 for miR-204). The median survival time was 45 months and 33 months, respectively, for the high miR-142-5p and high miR-204 patients, and 16.3 months in both the low miR-142-5p and low miR-204 patients (Table 2). We also found that the high miR-125a patients had significantly longer survival times than the low miR-125a patients when the Wilcoxon test was used (P = 0.035), although the difference was not statistically significant when the log rank test was used (Table 2, P = 0.085). However, for the nongemcitabine group, there were no differences in the survival times between the high and low miR-142-5p, miR-204, or miR-125a patients (Table 2). Analysis of both the gemcitabine and nongemcitabine groups did not revealed any significant differences in survival time between the high and low miR-152, miR-181c, and miR-518b patients (data not shown).
Fig. 1.
Correlation between the expression levels of miR-142-5p identified by miRNA expression profiling and the prognosis of patients with resected pancreatic cancer in the gemcitabine and nongemcitabine groups. The levels of miRNA expression were normalized against RNU6B. High miR-142-5p expression was significantly associated with longer survival times in the gemcitabine group (P = 0.0077), but not in the nongemcitabine group (P = 0.48)
Fig. 2.
The correlation between the expression levels of miR-204 identified by miRNA expression profiling and the prognosis of patients with resected pancreatic cancer in the gemcitabine and nongemcitabine groups. The levels of miRNA expression were normalized against RNU6B. High miR-204 expression was significantly associated with longer survival times in the gemcitabine group (P = 0.0054), but not in the nongemcitabine group (P = 0.15)
Table 2.
Correlation between median overall survival and microRNA expression in patients with resected pancreatic cancer
| Factor | Overall survival (95% CI) | P value | |
|---|---|---|---|
| High | Low | ||
| miR-142-5p | |||
| Gem group | 45 (23–) | 16.3 (12–23) | 0.0077 |
| Non-Gem group | 13.07 (4.7–43) | 11 (7.7–14.7) | 0.4880 |
| miR-204 | |||
| Gem group | 33 (13.27–) | 16.3 (12–23) | 0.0054 |
| Non-Gem group | 13.07 (8.47–26) | 9 (4–14.7) | 0.1527 |
| miR-125a | |||
| Gem group | 30.17 (19–) | 14.57(12–31) | 0.0854 |
| Non-Gem group | 12 (8.47–16.93) | 14(3–) | 0.8990 |
| miR-34a | |||
| Gem group | 27 (20.87–47.9) | 16.3 (12–) | 0.1754 |
| Non-Gem group | 16.9 (9–24.4) | 8.5 (3–12) | 0.0127 |
CI confidence interval, Gem gemcitabine
Next, we investigated the correlation between prognosis and the six upregulated miRNAs: miR-9, miR-27a, miR-424, miR-449b, and miR-34a. For the nongemcitabine group, the high miR-34a patients had a significantly longer survival times than the low miR-34a patients (Table 2, P = 0.012), while there were no significant differences in survival time between the high and low miR-34a patients in the gemcitabine group (Table 2, P = 0.175). The median survival time was 16.9 months in the high miR-34a patients and 8.5 months in the low miR-34a patients in the nongemcitabine group (Table 2). We also found no significant differences in survival between the high and low miR-9, miR-27a, miR-424, and miR-449b patients in the gemcitabine and nongemcitabine groups (data not shown).
Multivariate Analysis of miR-142-5p and miR-34a Expression in Relation to Survival Time of Patients with or without Gemcitabine Treatment after Curative Resection
In three miRNAs identified as potential prognostic markers in the gemcitabine group, miR-142-5p was the best candidate and was the most specific for the gemcitabine group compared with the nongemcitabine group. Therefore, we focused on miR-142-5p as the most promising predictive marker for gemcitabine. Also, univariate analysis of the gemcitabine group showed that the only significant clinical factors were pN status (pN1 vs. pN0), and R factor (R1 vs. R0). Multivariate survival analysis, based on the Cox proportional hazard model, was performed by using miR-142-5p expression (high versus low), pN status, and R factor. Overall survival time was significantly dependent on miR-142-5p expression (Table 3, P = 0.034), but not on pN status (P = 0.24) or R factor (P = 0.11). In the gemcitabine group, miR-142-5p expression was an independent prognostic marker for pancreatic cancer patients, with a relative risk of 3.109 (Table 3).
Table 3.
Multivariate survival analysis (Cox regression model) of clinical prognostic factors and miR-142-5p expression in patients with gemcitabine treatment
| Factor | Relative risk | 95% CI | P value |
|---|---|---|---|
| miR-142-5p | 3.109 | 1.087–10.01 | 0.0340 |
| pN status | 2.055 | 0.646–9.219 | 0.2395 |
| R factor | 2.197 | 0.823–5.904 | 0.1146 |
CI confidence interval
Univariate analysis of the nongemcitabine group showed that the only significant clinical factor was pN status. Therefore, multivariate survival analysis was performed by using miR-34a expression and pN status. The results show that the overall survival time was significantly dependent on both miR-34a expression and pN status (Table 4, P = 0.0103 for miR-34a; P = 0.0035 for pN status). In the nongemcitabine group, miR-34a expression was an independent prognostic marker for pancreatic cancer patients with a relative risk of 2.920 (Table 4).
Table 4.
Multivariate survival analysis (Cox regression model) of clinical prognostic factors and miR-34a expression in patients without gemcitabine treatment
| Factor | Relative risk | 95% CI | P value |
|---|---|---|---|
| miR-34a | 2.920 | 1.303–6.295 | 0.0103 |
| pN status | 2.957 | 1.410–6.812 | 0.0035 |
CI confidence interval
Discussion
The present miRNA profiling study using two gemcitabine-resistant pancreatic cancer cell lines and their parent cell lines identified 24 miRNAs candidates, which were up- or downregulated in gemcitabine resistant cells. The present results also showed that high miR-142-5p and miR-204 patients had statistically significantly longer survival times than the low miR-142-5p and miR-204 patients in the gemcitabine group, but not in nongemcitabine group, although further examination is needed because the number of patients in the nongemcitabine group is too small to conclude that there were no differences in survival between high and low expression patients in the nongemcitabine group. Liu et al. reported that miR-142-5p was repressed in human lung cancer, and the transfection of miR-142-5p significantly repressed lung cancer cell growth.27 miR-204 has been also reported to be downregulated in intrahepatic cholangiocarcinoma, and the level of miR-204 expression was inversely correlated with that of Bcl-2 expression, possibly leading to chemotherapeutic drug-triggered apoptosis.28 Taken together, these data suggest that miR-142-5p and miR-204 are promising predictive markers for chemotherapeutic responses in patients with resected pancreatic cancer.
In neuroblastomas, miR-34a was generally expressed at lower levels in unfavorable primary tumors, and the reintroduction of miR-34a results in a dramatic reduction in cell proliferation.29 miR-34a expression was decreased in 9 of 25 (36%) colon cancers, and transient introduction of miR-34a suppressed the in vitro and in vivo growth of colon cancer.30 In non-small-cell lung cancer, the miR-34 family was downregulated in tumors compared with normal tissues, and low levels of miR-34a expression correlated with a high probability of relapse.31 These data suggest that miR-34a functions as a potential tumor suppressor. Our results show that miR-34a is a favorable prognostic marker in patients without gemcitabine treatment after resection. We also found no correlation between miR-34a expression and survival time in patients treated with gemcitabine, possibly suggesting that pancreatic cancers with low levels of miR-34a are more sensitive to gemcitabine treatment than those with high levels of miR-34a, although larger studies are needed to confirm this.
In conclusion, miR-142-5p expression is correlated with survival time in patients treated with gemcitabine after surgical resection of pancreatic cancer, but not in patients without gemcitabine treatment. miR-142-5p would be a promising predictive marker for gemcitabine treatment in patients with resected pancreatic cancer, although further examination are needed to analyze the functional role of these microRNAs. In the present study, we used FFPE samples to measure the indicated miRNAs. Measurement of such miRNAs may be possible using plasma, serum, and pancreatic fluids, suggesting that these miRNAs may be useful in predicting the effects of chemotherapy for unresectable pancreatic cancer and of neoadjuvant chemotherapy for resectable pancreatic cancer, where it is difficult to obtain tissues samples without the use of invasive procedures.
Acknowledgment
Supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from the Kato Memorial Bioscience Foundation. We are grateful to Emiko Manabe, and Miyuki Omori (Department of Surgery and Oncology, Kyushu University) for skillful technical assistance.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Kenoki Ohuchida, Phone: +81-92-6425440, FAX: +81-92-6425458, Email: kenoki@surg1.med.kyushu-u.ac.jp, Email: kenoki@med.kyushu-u.ac.jp.
Kazuhiro Mizumoto, Email: mizumoto@med.kyushu-u.ac.jp.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Hirata K, Egawa S, Kimura Y, et al. Current status of surgery for pancreatic cancer. Dig Surg. 2007;24:137–147. doi: 10.1159/000102067. [DOI] [PubMed] [Google Scholar]
- 3.Hawes RH, Xiong Q, Waxman I, Chang KJ, Evans DB, Abbruzzese JL. A multispecialty approach to the diagnosis and management of pancreatic cancer. Am J Gastroenterol. 2000;95:17–31. doi: 10.1111/j.1572-0241.2000.01699.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsuno S, Egawa S, Fukuyama S, et al. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28:219–230. doi: 10.1097/00006676-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 6.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 7.Heinemann V, Hertel LW, Grindey GB, Plunkett W. Comparison of the cellular pharmacokinetics and toxicity of 2′, 2′-difluorodeoxycytidine and 1-beta-d-arabinofuranosylcytosine. Cancer Res. 1988;48:4024–4031. [PubMed] [Google Scholar]
- 8.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 9.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 11.Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–195. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 12.Nakano Y, Tanno S, Koizumi K, et al. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457–463. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 14.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 15.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Smyth P, Flavin R, et al. Comparison of miRNA expression patterns using total RNA extracted from matched samples of formalin-fixed paraffin-embedded (FFPE) cells and snap frozen cells. BMC Biotechnol. 2007;7:36. doi: 10.1186/1472-6750-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xi Y, Nakajima G, Gavin E, et al. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA. 2007;13:1668–1674. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebanony D, Benjamin H, Gilad S, et al. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J Clin Oncol. 2009;27:2030–2037. doi: 10.1200/JCO.2008.19.4134. [DOI] [PubMed] [Google Scholar]
- 19.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y, Todd NW, Liu Z, et al. Altered miRNA expression in sputum for diagnosis of non–small cell lung cancer. Lung Cancer. 2010;67:170–176. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greither T, Grochola L, Udelnow A, Lautenschlager C, Wurl P, Taubert H. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumours associates with poorer survival. Int J Cancer. 2010;126(1):73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- 22.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–2176. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohuchida K, Mizumoto K, Murakami M, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64:3215–3222. doi: 10.1158/0008-5472.CAN-03-2464. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton R, Gray C, Belanger SA, et al. Cardiac risk assessment before the use of stimulant medications in children and youth: A joint position statement by the Canadian Paediatric Society, the Canadian Cardiovascular Society and the Canadian Academy of Child and Adolescent Psychiatry. J Can Acad Child Adolesc Psychiatry. 2009;18:349–355. [PMC free article] [PubMed] [Google Scholar]
- 25.Sobin C, Gutierrez M, Alterio H. Polymorphisms of delta-aminolevulinic acid dehydratase (ALAD) and peptide transporter 2 (PEPT2) genes in children with low-level lead exposure. Neurotoxicology. 2009;30:881–887. doi: 10.1016/j.neuro.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann AC, Mori R, Vallbohmer D, et al. High expression of HIF1a is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF, and bFGF. Neoplasia. 2008;10:674–679. doi: 10.1593/neo.08292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Sempere LF, Galimberti F, et al. Uncovering growth-suppressive microRNAs in lung cancer. Clin Cancer Res. 2009;15:1177–1183. doi: 10.1158/1078-0432.CCR-08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Yan HX, Yang W, et al. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol. 2009;50:358–369. doi: 10.1016/j.jhep.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 30.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallardo E, Navarro A, Vinolas N, et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis. 2009;30:1903–1909. doi: 10.1093/carcin/bgp219. [DOI] [PubMed] [Google Scholar]