Abstract
Hypoxia is thought to be critical in regulating physiological processes within the female reproductive system, including ovulation, composition of the fluid in the oviductal/uterine lumens and ovarian follicle development. This study examined the localisation of exogenous (pimonidazole) and endogenous [hypoxia inducible factor 1α and 2α (HIF1α, -2α), glucose transporter type 1 (GLUT1) and carbonic anhydrase 9 (CAIX)] hypoxia-related antigens within the oviduct and uterus of the rat reproductive tract. The extent to which each endogenous antigen co-compartmentalised with pimonidazole was also assessed. Female Wistar Furth rats (n = 10) were injected intraperitoneally with pimonidazole (60 mg/kg) 1 h prior to death. Reproductive tissues were removed immediately following death and fixed in 4% paraformaldehyde before being embedded in paraffin. Serial sections were cut (6–7 μm thick) and antigens of interest identified using standard immunohistochemical procedures. The mucosal epithelia of the ampulla, isthmus and uterus were immunopositive for pimonidazole in most sections. Co-compartmentalisation of pimonidazole with HIF1α was only expressed in the mucosa of the uterus whilst co-compartmentalisation with HIF2α was observed in the mucosa of the ampulla, isthmus and uterus. Both GLUT1 and CAIX were co-compartmentalised with pimonidazole in mucosa of the isthmus and uterus. This study confirms that mucosal regions of the rat oviduct and uterus frequently experience severe hypoxia and there are compartment specific variations in expression of endogenous hypoxia-related antigens, including the HIF isoforms. The latter observation may relate to target gene specificity of HIF isoforms or perhaps HIF2α’s responsiveness to non-hypoxic stimuli such as hypoglycaemia independently of HIF1α.
Keywords: Oviduct, Uterus, Pimonidazole, Hypoxia, GLUT, Carbonic anhydrase
Introduction
Many studies have examined the relationship between oxygen availability and reproductive success, and the occurrence of hypoxia in normal physiological situations within the female reproductive tract is a well established phenomenon; for example, hypoxic stimuli are thought to be critical for ovulation to occur (Kim et al. 2009) and both embryogenesis and organogenesis depend on hypoxic environments (reviewed in Webster and Abela 2007). A variety of approaches to investigate the oxygen environment of the reproductive tract in various species (for example, rhesus monkeys, rabbits and rodents) have been employed. Oxygen probe recordings have been utilised to examine the oviductal lumen and intrauterine environments with low pO2 concentrations consistently reported in both (albeit with some menstrual/oestrous cycle stage variations in certain instances) and, particularly, in the uterus (Mastroianni and Jones 1965; Maas et al. 1976; Fischer and Bavister 1993; Kaufman and Mitchell 1994).
More recent studies have focussed on oxygen-sensitive molecular mechanisms to determine oxygen status within the oviduct and uterus as responses to hypoxia are mediated via alterations in the expression of a number of genes controlled by the hypoxia inducible factor (HIF) family of proteins. These act as transcription factors for genes with hypoxia response elements (HREs) via formation of a heterodimer of HIFα and HIFβ subunits (HIFα subunits have three isoforms, HIF1α, 2α and 3α: HIF3α is not as well studied as the former isoforms). HIF1α usually stabilises in instances of hypoxia [reviewed in Semenza (2004)] and rapidly degrades in a normoxic environment (although not always: for example, Fukushima et al. 2008), while HIF1β is constitutively expressed. Some major physiological functions that HIF regulates include angiogenesis, cell migration, hormonal regulation and energy metabolism, while examples of the target genes it controls these processes by are vascular endothelial growth factor (VEGF), some glucose transporter (GLUT) subtypes, some carbonic anhydrases (CAs) and erythropoietin (EPO) [reviewed in Semenza (2004)].
The presence of HIF and some of its major target genes has been demonstrated in the uterus. Critchley et al. (2006) have shown that both HIF1α and HIF1β are expressed in the human endometrium, and expression levels vary throughout the menstrual cycle. As mentioned, some GLUT subtypes are regulated by HIF, such as GLUT1 and 3, and an adequate supply of glucose to provide cells with nutrition for processes of differentiation is essential for normal endometrial function. GLUT1 is essential for maintaining basal levels of cellular glucose uptake and storage in all cells (Mückler 1990) and plays a pivotal role in many aspects of reproduction. GLUT1 is involved in transporting glucose from the oviductal epithelium into the fluid-filled lumen to maintain an appropriate glucose environment for embryonic development (Tadokoro et al. 1995). Transfer of glucose from the maternal to fetal circulation is vital for proper fetal development as inferred from the correlation of increases in mouse placental and decidual GLUT1 mRNA expression with advancing day of gestation (Yamaguchi et al. 1996). Von Wolff et al. (2003) also discuss the crucial role of glucose metabolism in the successful establishment of pregnancy, which accordingly is particularly intensive in stromal cell decidualisation (their results imply GLUT1 is central to this process) and endometrial cell proliferation and differentiation, which take place around the time of implantation/early pregnancy. Positive immunohistochemical staining of GLUT1 and 3 (but not GLUT2, 4 and 8) in human endometrium during the secretory phase has been reported (Von Wolff et al. 2003; Goldman et al. 2006) and GLUT1 is also present in uterine adenocarcinomas (Goldman et al. 2006). Several CA subtypes are present in the uterus of mice, including CAIX which has an HRE (Hynninen et al. 2004). Hypoxia (as determined by expression of an exogenous hypoxic marker, see below) correlates strongly with VEGF expression within the mouse endometrium during phases of neovascularisation (Fan et al. 2008). By contrast, studies investigating the role (if any) of HIF in the oviduct are lacking, although the roles of HIF target genes have been examined, such as Masuda and colleagues (2000) mimicking of oestrogen (E2) induced EPO production that occurs in the oviduct (as well as the uterus and ovaries) of mice via exposure to hypoxia. Similarly, Friedley and Rosen (1975) demonstrated CA activity in the oviduct and uterus of several mammalian species, although they did not focus on any specific CA subtype.
In addition to the examination of expression of HIF/HIF target genes, investigations of tissue hypoxia have been facilitated through the use of pimonidazole, a 2-nitroimidazole that following administration to humans and other animals, forms highly reactive adducts which accumulate in living cells experiencing concentrations of pO2 less than 10 mmHg (Arteel et al. 1995; Raleigh et al. 1999). These adducts can be detected by immunohistochemistry [for example, Ljunkvist et al. (2000)]. Pimonidazole has been used in a variety of different tissues and species to demonstrate the presence of a hypoxic environment: for example, during various phases of mammalian and non-mammalian embryological development [reviewed in Webster and Abela (2007)]; in colocalisation studies with HIF and its target genes (Jankovic et al. 2006); and to study hypoxia in various tumours including carcinomas of the oviduct and uterus (Olive et al. 2010). Thus, pimonidazole is a powerful investigative tool for examining the degree and spatial distribution of hypoxia in different tissues across a wide range of species in vivo.
The aim of this study was to characterise pimonidazole adduct formation in the rat oviduct and uterus and assess to what degree that intrinsic markers of hypoxia (HIF1α, HIF2α, GLUT1, GLUT4 and CAIX) co-compartmentalise with pimonidazole adduct formation within those same regions. We conducted this study as a descriptive study in order to provide a basis for formulating hypotheses for future studies about the causative physiological role(s) region-specific associations of hypoxia and hypoxia-regulated gene products may play in reproductive function.
Methods
Animals
Ten adult female Ludwig Wistar rats were used in this study. Rats were housed in individual cages in a room kept on a 12 h/12 h light/dark cycle. They were given ad libitum access to food and water. Experimental procedures were approved by a local ethical review committee and carried out in accordance with the Animals (Scientific Procedures) Act (1986).
Experimental procedures
Pimonidazole [HPI Inc., Burlington, MA (US)] was administered (60 mg/kg, i.p. injection) 1 h prior to sacrificing the rats by cervical dislocation. A vaginal smear was taken before the reproductive tract was removed and the oestrous cycle of each rat was staged using a protocol described by Marcondes et al. (2002). The reproductive organs were then rapidly excised and fixed in 4% formalin prior to being prepared for sectioning. Once fixed, the reproductive tracts were hemisected, dehydrated through an alcohol gradient, cleared with xylene, incubated in infiltrating wax (1:1 paraffin wax/xylene) then embedded in paraffin wax and allowed to set before being stored at 4°C until ready for use. Sections were cut from the wax blocks using a microtome at a thickness of 6–7 μm and placed on gelatine coated glass slides. The sections were thoroughly dried before being stored at room temperature (RT) prior to being processed for immunohistochemistry.
Antibodies
Six different primary antibodies were used in this study for immunohistochemical staining. A monoclonal hypoxyprobe-1 antibody against pimonidazole adducts was purchased from Chemicon International and used at a concentration of 1:100. HIF1α (1:100, monoclonal), HIF2α (1:200, polyclonal) and CAIX (1:1,000, polyclonal) were purchased from Novus Biologicals. GLUT1 antibody (1:1,000, polyclonal) was purchased from Abcam and GLUT4 antibody (1:200, monoclonal) was purchased from AbD Serotec. Each antibody was optimised for use in this study.
Immunohistochemistry
Sections were dewaxed with 2 × 5 min rinses in xylene then rehydrated through an alcohol gradient followed by 3 × 5 min washes in Phosphate Buffered Saline (PBS). Sections were then treated with 3% H2O2 in methanol for 10 min at RT to quench endogenous peroxidases. Antigen retrieval was done using proteinase K treatment (20 ng/ml, Fisher Scientific) for 20 min at 37°C followed by 10 min at RT. After washing 3 × 5 min in PBS, the sections were placed in 10% normal serum in 0.1% Triton X-100 (TX-100) PBS for 30 min at RT. Sections were then placed in a humidified chamber and incubated in the relevant primary antibody for 1 h at RT followed by an overnight incubation at 4°C. The following morning sections were washed 3 × 5 min in PBS then incubated for 1 h at RT in the appropriate biotinylated secondary antibody (all obtained from Vector Laboratories, UK). Sections were then washed 3 × 5 min in PBS followed by a 1 h RT incubation in Vector A + B solution (Vector Laboratories, UK). Following this, sections were washed in PBS for 2 × 5 min followed by 5 min in Tris buffer (10 mM, pH 7.4) then incubated in 3,3-diaminobenzidine (DAB) solution (Vector Laboratories, UK) for 8 min to visualise the antigen–antibody complex. Sections were washed in Tris buffer, counterstained with Gill’s hematoxylin (Vector Laboratories UK) before being run through an alcohol dehydration gradient, cleared with xylene then mounted using DPX mounting media with glass coverslips.
Two sections from each hemisection for each animal were immunostained for each antigen: this was done for the ampulla, isthmus and uterus (a total of 72 sections per animal excluding control sections). Sections immunostained in the absence of primary antibodies were also done for each hemisection of each animal and were incubated in TX-100 PBS containing either horse or goat (depending on which primary antibody was being controlled against) non-immune serum (1%) under the same conditions as the experimental sections: these were the negative controls.
Analyses
Processed sections were visualised using a Leica DM2000 light microscope with a Leica DFC420 camera attachment. Positive or absent DAB staining was determined for the mucosal layer, lamina propria and muscularis of the ampulla and isthmus regions of the oviduct as well as in the uterine endometrial, lamina propria and myometrial layers. Staining was assessed as − (no staining), ± (weak-moderate and/or sporadic staining) and + (strong-very strong and widespread staining). Pictures were captured using Leica Application Suite software.
Results
Positive immunohistochemical staining for pimonidazole, HIF1α, HIF2α, GLUT1, GLUT4 and CAIX was detected in the rat oviduct (both ampulla and isthmus) and uterus. Example micrographs for each antigen for the ampulla, isthmus and uterus are presented in Figs. 1, 2 and 3 (please note, although within each figure the micrographs are taken from the same individual, the 3 figures are from different individuals). Examples of immunohistochemical staining in the glandular epithelia of the uterus of one rat are presented in Fig. 4. A summary of the main observations is also presented in Table 1. Comparisons were not made between different stages of the oestrous cycle because of the low number of animals at some stages.
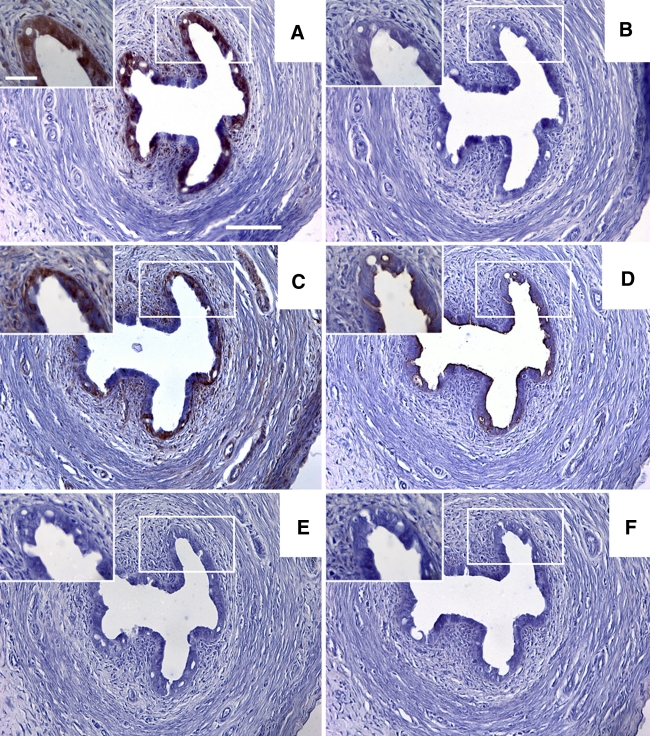
Fig. 1.
a–f Sections taken from a representative rat showing the results for immunohistochemical staining in the ampulla region of the oviduct for pimonidazole, HIF1α, HIF2α, GLUT1, GLUT4 and CAIX, respectively. The boxed diagrams in a–f show a higher magnification detail for each stain. Scale bars 100 μm (a–f) or 30 μm (detail pictures in a–f)
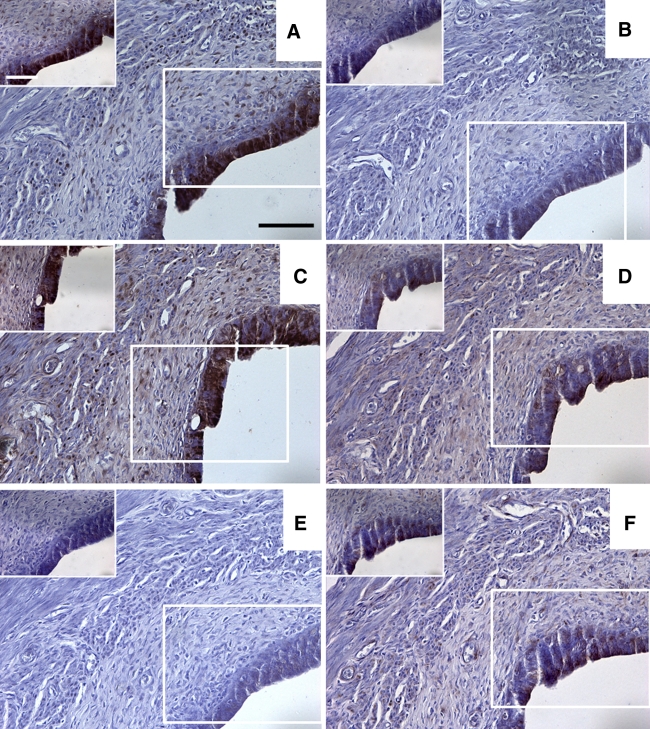
Fig. 2.
a–f Sections taken from a representative rat showing the results for immunohistochemical staining in the isthmus region of the oviduct for pimonidazole, HIF1α, HIF2α, GLUT1, GLUT4 and CAIX, respectively. The boxed diagrams in a–f show a higher magnification detail for each stain. Scale bars 100 μm (a–f) or 30 μm (detail pictures in a–f)
Fig. 3.
a–f Sections taken from a representative rat showing the results for immunohistochemical staining in the uterus for pimonidazole, HIF1α, HIF2α, GLUT1, GLUT4 and CAIX, respectively. The boxed diagrams in a–f show a higher magnification detail for each stain. Scale bars 100 μm (a–f) or 30 μm (detail in a–f)
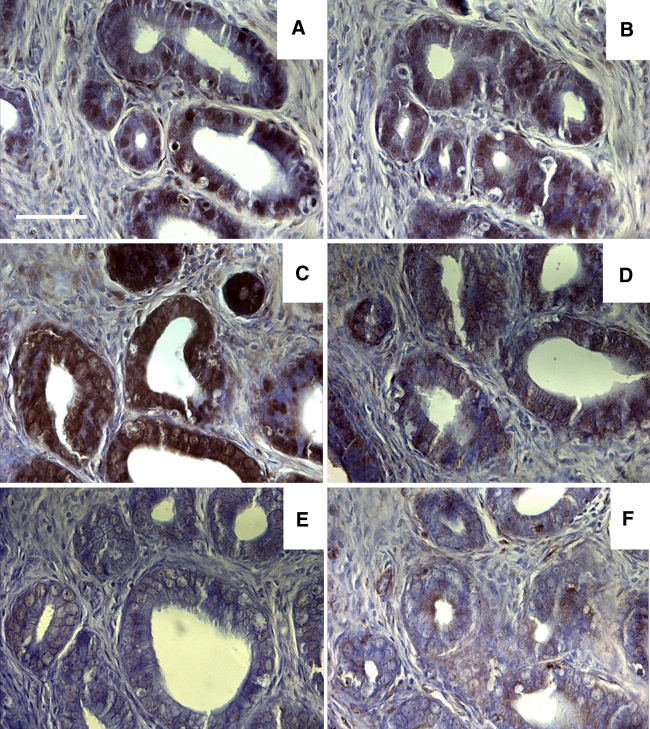
Fig. 4.
a–f Sections taken from a representative rat showing examples of immunohistochemical staining in glandular epithelia for pimonidazole, HIF1α, HIF2α, GLUT1, GLUT4 and CAIX, respectively. Scale bar 50 μm
Table 1.
Summary of the major findings for the 3 subcompartments of each reproductive tract region
| Reproductive tract region | Compartment | Antigen present (>50%) | Co-compartmentalised with pimonidazole (>50%) |
|---|---|---|---|
| Oviduct (ampulla) | Mucosa | Pimo, HIF2α | None |
| Lamina propria | None | None | |
| Muscularis | None | None | |
| Oviduct (isthmus) | Mucosa | Pimo, HIF2α, GLUT1, CAIX | HIF2α, GLUT1, CAIX |
| Lamina propria | Pimo, HIF2α | HIF2α | |
| Muscularis | HIF2α, GLUT1, CAIX | None | |
| Uterus | Mucosa | Pimo, HIF1α, HIF2α, GLUT1, CAIX | HIF1α, HIF2α, GLUT1, CAIX |
| Lamina propria | HIF2α, GLUT1 | None | |
| Myometrium | None | None |
Column 3 highlights gene products that were present (scored as either ± or +) in >50% of sections for each of the respective compartments. Column 4 highlights endogenous gene products that showed a co-compartmentalisation with pimonidazole in >50% of sections in the respective reproductive tract compartments
Mucosa
Within the tunica mucosa of the ampulla, isthmus and uterus, the epithelial cell layer was the most immunoreactive for all 6 antigens (see all figures). The epithelia of the ampulla, isthmus and uterus were, in the majority of animals, immunopositive for pimonidazole (Fig. 5a, b, c). In both the ampulla and the isthmus positive immunostaining for HIF1α and GLUT4 was absent in the majority of animals (Fig. 5a, b, c). HIF2α was expressed in a majority of sections in both the ampulla and isthmus. Of these two regions only the isthmus was positive for the majority of sections for GLUT1 and CAIX. In the uterine mucosa, immunopositive staining was observed in 50% or greater sections for all 6 antigens.
Fig. 5.
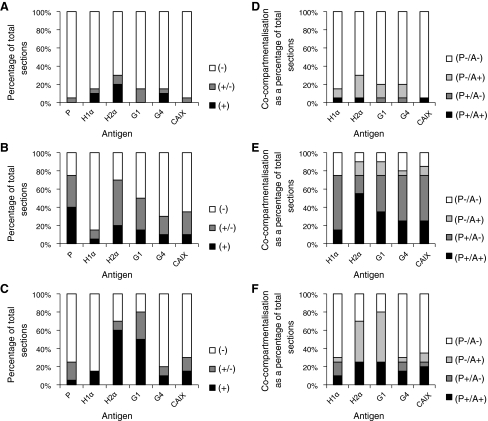
Charts showing the staining intensity of the 6 hypoxia-related antigens in the mucosa of a the ampulla, b the isthmus and c the endometrium as a percentage of the total number of sections examined (n = 20). (+) denotes strong-very strong positive staining, (±) denotes weak/moderate or sporadic staining and (−) denotes an absence of staining. Charts d–f show the proportions of co-compartmentalisation of the 5 endogenous hypoxia-related antigens with pimonidazole in the mucosa of the ampulla, isthmus and endometrium, respectively. Percentages are relative to the total number of sections examined (n = 20). (P+/A+) denotes the presence of both pimonidazole and the respective endogenous antigen within the same compartment in a section: (P+/A−) is as before, but with the absence of the respective antigen; (P−/A+) is as for the 1st criterion, but with the absence of pimonidazole and (P−/A−) denotes that both pimonidazole and the respective antigen were absent from the same compartment. X axis labels: P pimonidazole, H1α HIF1α, H2α HIF2α, G1 GLUT1 and G4 GLUT4
Co-compartmentalisation of the 5 endogenous antigens with pimonidazole was also examined within the mucosal layer of the ampulla, isthmus and uterus. In the ampulla, the number of instances of co-compartmentalisation of most antigens with pimonidazole is very low, particularly for HIF1α (Fig. 5d). In contrast, HIF2α co-compartmentalises with pimonidazole in 45% of cases (Fig. 5d). In the isthmus there were a very high proportion of sections in which pimonidazole was expressed in the same cell types as HIF2α and GLUT1, as well as, but to a slightly lesser extent, CAIX. The epithelium of the endometrial layer of the uterus displays a high degree of co-compartmentalisation with pimonidazole for all 5 endogenous antigens, with values of at least 50% in all cases (Fig. 5f).
Lamina propria
Within the lamina propria of the ampulla, isthmus and uterus, instances of immunopositive staining were observed for all 6 antigens. Whilst the vast majority of sections showed an absence of pimonidazole in the ampulla and uterus, the majority of sections for the isthmus were immunopositive for pimonidazole (Fig. 6a, b, c). In almost all sections HIF1α and GLUT4 were absent in the lamina propria of all 3 reproductive tract regions. In the isthmus and uterus the majority of sections were positive for HIF2α and GLUT1 expression (Fig. 6a, b, c).
Fig. 6.
Charts showing the staining intensity of the 6 hypoxia-related antigens in the lamina propria of a the ampulla, b the isthmus and c the endometrium as a percentage of the total number of sections examined (n = 20). (+) denotes strong-very strong positive staining, (±) denotes weak/moderate or sporadic staining and (−) denotes an absence of staining. Charts d–f show the proportions of co-compartmentalisation of the 5 endogenous hypoxia-related antigens with pimonidazole in the lamina propria of the ampulla, isthmus and endometrium, respectively. Percentages are relative to the total number of sections examined (n = 20). (P+/A+) denotes the presence of both pimonidazole and the respective endogenous antigen within the same compartment in a section: (P+/A−) is as before, but with the absence of the respective antigen; (P−/A+) is as for the 1st criterion, but with the absence of pimonidazole and (P−/A−) denotes that both pimonidazole and the respective antigen were absent from the same compartment. X axis labels: P pimonidazole, H1α HIF1α, H2α HIF2α, G1 GLUT1 and G4 GLUT4
In regards to co-compartmentalisation of the 5 endogenous antigens with pimonidazole in the ampulla, isthmus and uterus, there were either no or virtually no instances of this for all 5 endogenous antigens in the ampulla (Fig. 6d). Co-compartmentalisation was also limited in the lamina propria of the isthmus, with only HIF2α co-compartmentalising in a majority of sections, while in the lamina propria in the uterus co-compartmentalisation occurred in 25% of sections or fewer for all 5 endogenous antigens.
Muscularis/myometrium
Examination of the muscular layers of the ampulla, isthmus and uterus showed pimonidazole to be absent from the majority of sections in all 3 regions (Fig. 7a, b, c). Of the endogenous antigens, HIF1α and GLUT4 were virtually absent in the ampulla, isthmus and uterus and the isthmus was again the only region to show a majority of immunopositive sections for any of the remaining endogenous antigens, with HIF2α, GLUT1 and CAIX all showing an expression rate of 50% of section or greater (Fig. 7a, b, c).
Fig. 7.
Charts showing the staining intensity of the 6 hypoxia-related antigens in the muscularis/myometrium of a the ampulla, b the isthmus and c the endometrium as a percentage of the total number of sections examined (n = 20). (+) denotes strong-very strong positive staining, (±) denotes weak/moderate or sporadic staining and (−) denotes an absence of staining. Charts d–f show the proportions of co-compartmentalisation of the 5 endogenous hypoxia-related antigens with pimonidazole in the muscularis/myometrium of the ampulla, isthmus and endometrium, respectively. Percentages are relative to the total number of sections examined (n = 20). (P+/A+) denotes the presence of both pimonidazole and the respective endogenous antigen within the same compartment in a section: (P+/A−) is as before, but with the absence of the respective antigen; (P−/A+) is as for the 1st criterion, but with the absence of pimonidazole and (P−/A−) denotes that both pimonidazole and the respective antigen were absent from the same compartment. X axis labels: P pimonidazole, H1α HIF1α, H2α HIF2α, G1 GLUT1 and G4 GLUT4
In terms of co-compartmentalisation of the endogenous antigens with pimonidazole, none of the muscular regions of the ampulla, isthmus and uterus showed a majority of sections where this was observed to occur and in both the ampulla and uterus no instances of this happening were observed for some of the antigens (Fig. 7d, e, f).
Glandular epithelium
The glands of the uterus were also examined, with instances of staining observed for all 6 antigens. Staining intensity was variable both within and between antigens in individual animals, although the examples given in Fig. 4 show robust pimonidazole, HIF1α, HIF2α and GLUT1 staining and weak/sporadic GLUT4 and CAIX staining. Staining was primarily restricted to the glandular epithelia, although both GLUT1 and HIF2α showed evidence of staining in the surrounding uterine stroma too.
Discussion
The epithelial cell layers of the ampulla, isthmus and uterus frequently experience severe hypoxia as part of their normal physiology as demonstrated by the detection of pimonidazole adducts in these cells. A second major set of findings of this study are the high frequency of expression of HIF1α within the uterus, but not the ampulla and isthmus, the high prevalence of HIF2α immunostaining within the mucosal layers of the ampulla, isthmus and uterus, as well as a high frequency of GLUT1 and CAIX expression within the same layer of the isthmus and uterus only. Finally, our data also highlight that, although co-compartmentalisation of the 5 endogenous antigens with pimonidazole is limited within the mucosa of the ampulla, HIF2α, GLUT1 and CAIX extensively co-compartmentalise with pimonidazole in the isthmus mucosa, while the same is true for HIF1α, HIF2α, GLUT1 and CAIX in the uterine mucosa. Taken together, these data suggest potential mechanisms by which portions of the reproductive system respond to severe hypoxia as well as putative physiological roles hypoxia may be involved in. As highlighted in the introduction, hypoxia is known to be involved in ovulation (Kim et al. 2009), but it is also involved in other localised events in the reproductive tract of various species, such as corpus luteum formation (Nishimura and Okuda 2010), initiation of endometrial cell apoptosis and menstruation via ischaemia–reperfusion cycles (Okazaki et al. 2005), driving post-menstrual endometrial neoangiogenesis (Fan et al. 2008) and stimulation of EPO in the oviduct and uterus, which controls E(2)-dependent angiogenesis (Masuda et al. 2000).
Combining antibodies against exogenous and endogenous antigens, and targetting more than one HIF isoform, has overcome some of the limitations of previous studies with a similar format to this one. Although endogenous hypoxia markers such as HIF are frequently associated with physiological responses to low O2 environments, there are instances where they are also associated with normoxic environments and processes (e.g. Fukushima et al. 2008; Chae et al. 2011). Similarly, many studies focus on one member of the HIFα family (e.g. Tomes et al. 2003; Nico et al. 2007) despite the fact that other isoforms can potentially be active within the same tissue (e.g. Rosenberger et al. 2002) and can have unique targets and expression patterns (Elvert et al. 2003; Hu et al. 2003). Therefore, pimonidazole adduct formation provides a more direct confirmation of the severe hypoxic status of the regions examined and, unlike HIF, its expression is cumulative and is not degraded following the cessation of the hypoxic stimulus. Additionally, since HIF1α is usually thought to degrade in states of normoxia, it is also worth noting that the phenomenon of episodic endometrial ischaemia–reperfusion occurs in primates prior to menstruation, resulting in elevations in TNFα and apoptosis of endometrial cells, and similar effects have been seen in a rodent model of uterine ischaemia–reperfusion (Okazaki et al. 2005). Transient changes in perfusion may also result in rapid degradation of HIF1α expression; however, since pimonidazole is not degraded by reoxygenation, it is less affected by any intermittent blood flow. Due to adduct formation being induced only by severe hypoxic stimuli, pimonidazole is not suitable as a marker for regions experiencing more moderate hypoxia (hypoxia where pO2 > 10 mmHg).
It is probably unsurprising that we have observed indications of severe hypoxia within the epithelial cell layer of the mucosa as, in addition to the studies cited in the introduction, it has been seen that hypoxia in cultured human oviductal epithelial cells induces VEGF expression, a process that may result in increased vascular permeability of the oviductal epithelium (Itoh et al. 2006), allowing movement of proteins into the oviductal lumen, enabling changes in the composition of the oviductal fluid to occur (this is important due to the variety of processes that the oviductal fluid is thought to help regulate, including final oocyte maturation, fertilisation and early embryonic development). Additionally, in the bovine oviduct, VEGF expression is relatively stable throughout the oestrous cycle, although expression of its receptors varies with cycle phase as does its release into the oviductal lumen (Gabler et al. 1999). This may explain why we observed consistent indications of hypoxia regardless of oestrous cycle stage in certain regions as it may be essential in maintaining a constant production of VEGF to allow for regulation of vascular permeability.
Our results suggest there may also be a degree of HIF isoform functional specificity, as (for example) the isthmus oviductal mucosa compartment shows a high proportion of instances of HIF2α expression, whereas HIF1α expression is very limited. As mentioned, it is known that tissues and cells can differ in respect to which isoform is dominant. Our results suggest that HIF2α is the primary means of mediating hypoxia responses within this compartment of the oviduct. Conversely, both HIF1α and 2α were prevalent in the endometrial epithelium. This may suggest that either isoform is capable of mediating responses to hypoxia within this region. Alternatively they may activate different target genes within the endometrium or work in conjunction with each other to control responses to hypoxia, which has also been observed in other organs such as the kidney (Rosenberger et al. 2002). Finally, another plausible explanation for the presence of HIF2α is that, rather than (or in addition to) being hypoxic, the mucosal regions are hypoglycaemic. Brusselmans et al. (2001) have shown that HIF2α regulates apoptotic responses to low glucose concentrations, but is not involved in hypoxia-linked apoptosis. This may potentially mean that within the epithelial cells of the mucosa HIF2α drives responses to meet glucose demands.
It is notable that pimonidazole expression seems to be rather limited in the lamina propria and muscular compartments of the oviduct and uterus. Despite this, in the case of both the uterus and the isthmus there are still a majority of sections that show positive HIF2α expression within the lamina propria. This perhaps means that these regions do experience hypoxia but possibly not to the same extent or severity as the mucosa does. Alternatively, as discussed above, the connection between HIF2α and hypoglycaemia may mean that HIF2α is involved in the delivery of glucose to these regions. In contrast, HIF1α expression was again limited in both segments of the oviduct and in the uterus for the respective lamina propria and muscular layers. This would again point to scenarios such as target gene specificity being of importance in the physiological regulation of these regions.
Two of the HIF target genes we examined, GLUT1 and CAIX, were both expressed within the endometrium and isthmus mucosa although not in the mucosa of the ampulla. Glucose is a major oviductal fluid component and is secreted by the oviductal epithelium. The consistent presence of GLUT1 staining in the mucosa of the isthmus in our study complements previous work by Tadokoro et al. (1995), although in contrast to their results we detected GLUT1 expression in the mucosa of the ampulla in comparatively few sections. In the endometrium, previous studies have shown the presence of GLUT1 in endometria from human hysterectomy patients, although its expression is higher in cases of endometrial cancer than in the normal endometrium (Goldman et al. 2006). However, Wang et al. (2000) were unable to detect GLUT1 in non-cancerous endometrial tissue. The differences in results can plausibly be explained by protocol differences between the studies. We also examined GLUT4 expression, as although some studies have shown that GLUT4 can be responsive to hypoxic stimuli [for example, Royer et al. (2000)], there is no evidence to suggest that it possesses an HRE. Only the endometrium and isthmus mucosa layers displayed a substantial number of samples with positive GLUT4 expression while regions such as the lamina propria of the ampulla showed a near absence of GLUT4 expression.
CAIX may help with maintaining high bicarbonate content in tubal and uterine fluid to facilitate processes such as increasing the capacitance of sperm and the separation of the corona radiata from the oocyte, and is maintained by the tubal epithelial cells. We believe that this is the first time CAIX’s expression has been demonstrated in the rat endometrium and in the oviduct of any species (Ge and Spicer (1988) had demonstrated the presence of other CA isoforms within the oviduct), which is consistent with its presence in the endometrium of mice (Hynninen et al. 2004), although samples from human patients showed an absence of CAIX (and GLUT1) in non-metaplastic inactive endometrium (Horree et al. 2007). This difference can again be explained by protocol differences, although it is also feasible that there are species differences between rodents and humans. As very little work on the role of CAIX within the endometrium and oviduct has been conducted, it presents an interesting target for further research.
It may also be of further interest to examine expression patterns relative to oestrous cycle stage. However, having also determined the cycle stage of each of our rats, it appears that severe hypoxia occurs within the reproductive tract irrespective of cycle stage. Additionally, some studies have shown hypoxia-regulated genes like VEGF to maintain a relatively stable expression profile throughout the oestrous cycle (Gabler et al.1999), and others have reported no cyclical variation in intrauterine pO2 in certain species (Fischer and Bavister 1993). Conversely, as mentioned in the introduction, other studies have shown variation in oviductal lumen pO2 in some species with cycle phase, so this may be worth investigating further. As mentioned earlier, transient ischaemia–reperfusion is involved in certain critical stages of the reproductive cycle, so it would also be interesting to examine the effect of time of reperfusion on HIF1α expression to assess if perfusion-dependent variation occurs. This experiment could either be done by arterial clamping or by incubation of the reproductive tract ex vivo in a hypoxia chamber. Comparisons of pregnant with non-pregnant animals may also be of future research interest as it has also been shown that HIF target genes (e.g. VEGF) facilitate fertilisation and maturation in embryos developed in culture (Luo et al. 2002). Finally, although the rat does provide a good model for human reproduction given the basic tissue organisation of the reproductive tract (i.e. the muscular, lamina propria and mucosal layers), and hypoxia in the lumen in both species impacts reproduction and fertility, we would also be interested in extending our methods to species other than rats for comparative purposes.
Conclusions
It has been seen that many subcompartments of the oviduct and uterus experience severe hypoxia, as supported by the presence of pimonidazole adducts. In particular, it appears that a severe hypoxic state is common in the mucosal layers and as these regions are vital for secretion of proteins and other relevant molecules into the oviductal and uterine lumens in order to facilitate a variety of reproductive processes, it may be that hypoxia plays a key role in facilitating this, perhaps via controlling changes in vascular permeability. Additionally, hypoxia may contribute to the regulation of the oviductal and uterine fluid environments via production of proteins such as GLUT1 and CAIX within the mucosal layers. Our results suggest that the key mediator of these responses within the oviduct is HIF2α, while both HIF1α and HIF2α appear to be involved within the endometrium.
Acknowledgments
We gratefully acknowledge the support of The Wellcome Trust (project grant #84363) and Cancer Research UK (project grant C16412/A6269) in funding this work, and the help of the staff of the BRF, St George’s University of London.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer. 1995;72(4):889–895. doi: 10.1038/bjc.1995.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusselmans K, Bono F, Maxwell P, Dor Y, Dewerchin M, Collen D, Herbert JM, Carmeliet P. Hypoxia-inducible factor-2 alpha (HIF-2 alpha) is involved in the apoptotic response to hypoglycemia but not to hypoxia. J Biol Chem. 2001;276(42):39192–39196. doi: 10.1074/jbc.C100428200. [DOI] [PubMed] [Google Scholar]
- Chae KS, Kang MJ, Lee JH, Ryu BK, Lee MG, Her NG, Ha TK, Han J, Kim YK, Chi SG (2011) Opposite functions of HIF-alpha isoforms in VEGF induction by TGF-beta1 under non-hypoxic conditions. Oncogene 20(10):1213–1228 [DOI] [PubMed]
- Critchley HO, Osei J, Henderson TA, Boswell L, Sales KJ, Jabbour HN, Hirani N. Hypoxia-inducible factor-1alpha expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2 (EP2) Endocrinology. 2006;147(2):744–753. doi: 10.1210/en.2005-1153. [DOI] [PubMed] [Google Scholar]
- Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1) J Biol Chem. 2003;278(9):7520–7530. doi: 10.1074/jbc.M211298200. [DOI] [PubMed] [Google Scholar]
- Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, Brenner RM, Giudice LC, Nayak NR. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008;22(10):3571–3580. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99(2):673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- Friedley NJ, Rosen S. Carbonic anhydrase activity in the mammalian ovary, fallopian tube, and uterus: histochemical and biochemical studies. Biol Reprod. 1975;12(2):293–304. doi: 10.1095/biolreprod12.2.293. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Murata M, Hachisuga M, Tsukimori K, Seki H, Takeda S, Asanoma K, Wake N. Hypoxia inducible factor 1 alpha regulates matrigel-induced endovascular differentiation under normoxia in a human extravillous trophoblast cell line. Placenta. 2008;29(4):324–331. doi: 10.1016/j.placenta.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Gabler C, Einspanier A, Schams D, Einspanier R. Expression of vascular endothelial growth factor (VEGF) and its corresponding receptors (flt-1 and flk-1) in the bovine oviduct. Mol Reprod Dev. 1999;53(4):376–383. doi: 10.1002/(SICI)1098-2795(199908)53:4<376::AID-MRD2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ge ZH, Spicer SS. Immunocytochemistry of ion transport mediators in the genital tract of female rodents. Biol Reprod. 1988;38(2):439–452. doi: 10.1095/biolreprod38.2.439. [DOI] [PubMed] [Google Scholar]
- Goldman NA, Katz EB, Glenn AS, Weldon RH, Jones JG, Lynch U, Fezzari MJ, Runowicz CD, Goldberg GL, Charron MJ. GLUT1 and GLUT8 in endometrium and endometrial adenocarcinoma. Mod Pathol. 2006;19(11):1429–1436. doi: 10.1038/modpathol.3800656. [DOI] [PubMed] [Google Scholar]
- Horree N, Heintz AP, Sie-Go DM, van Diest PJ. p16 is consistently expressed in endometrial tubal metaplasia. Cell Oncol. 2007;29(1):37–45. doi: 10.1155/2007/868952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23(24):9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynninen P, Hamalainen JM, Pastorekova S, Pastorek J, Waheed A, Sly WS, Tomas E, Kirkinen P, Parkkila S. Transmembrane carbonic anhydrase isozymes IX and XII in the female mouse reproductive organs. Reprod Biol Endocrinol. 2004;2:73. doi: 10.1186/1477-7827-2-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Nasu K, Matsumoto H, Kawano Y, Yoshimatsu J, Narahara H. Hypoxia regulates vascular endothelial growth factor and soluble fms-like tyrosine kinase-1 secretion by human oviductal epithelial cells and stromal fibroblasts. Fertil Steril. 2006;85(Suppl 1):1097–1102. doi: 10.1016/j.fertnstert.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Jankovic B, Aquino-Parsons C, Raleigh JA, Stanbridge EJ, Durand RE, Banath JP, MacPhail SH, Olive PL. Comparison between pimonidazole binding, oxygen electrode measurements, and expression of endogenous hypoxia markers in cancer of the uterine cervix. Cytometry B Clin Cytom. 2006;70(2):45–55. doi: 10.1002/cyto.b.20086. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Mitchell JA. Intrauterine oxygen tension during the oestrous cycle in the hamster: patterns of change. Comp Biochem Physiol Comp Physiol. 1994;107(4):673–678. doi: 10.1016/0300-9629(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology. 2009;150(7):3392–3400. doi: 10.1210/en.2008-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungkvist AS, Bussink J, Rijken PF, Raleigh JA, Denekamp J, Van Der Kogel AJ. Changes in tumor hypoxia measured with a double hypoxic marker technique. Int J Radiat Oncol Biol Phys. 2000;48(5):1529–1538. doi: 10.1016/S0360-3016(00)00787-2. [DOI] [PubMed] [Google Scholar]
- Luo H, Kimura K, Aoki M, Hirako M. Effect of vascular endothelial growth factor on maturation, fertilization and developmental competence of bovine oocytes. J Vet Med Sci. 2002;64(9):803–806. doi: 10.1292/jvms.64.803. [DOI] [PubMed] [Google Scholar]
- Maas DH, Storey BT, Mastroianni L., Jr Oxygen tension in the oviduct of the rhesus monkey (Macaca mulatta) Fertil Steril. 1976;27(11):1312–1317. doi: 10.1016/s0015-0282(16)42201-6. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62(4A):609–614 [DOI] [PubMed]
- Mastroianni L, Jr, Jones R. Oxygen tension within the rabbit fallopian tube. J Reprod Fertil. 1965;9:99–102. doi: 10.1530/jrf.0.0090099. [DOI] [PubMed] [Google Scholar]
- Masuda S, Kobayashi T, Chikuma M, Nagao M, Sasaki R. The oviduct produces erythropoietin in an estrogen- and oxygen-dependent manner. Am J Physiol Endocrinol Metab. 2000;278(6):E1038–E1044. doi: 10.1152/ajpendo.2000.278.6.E1038. [DOI] [PubMed] [Google Scholar]
- Mückler M (1990) Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes 39(1):6–11 [DOI] [PubMed]
- Nico B, Mangieri D, Crivellato E, Longo V, De Giorgis M, Capobianco C, Corsi P, Benagiano V, Roncali L, Ribatti D. HIF activation and VEGF overexpression are coupled with ZO-1 up-phosphorylation in the brain of dystrophic mdx mouse. Brain Pathol. 2007;17(4):399–406. doi: 10.1111/j.1750-3639.2007.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Okuda K. Hypoxia is important for establishing vascularization during corpus luteum formation in cattle. J Reprod Dev. 2010;56(1):110–116. doi: 10.1262/jrd.09-162E. [DOI] [PubMed] [Google Scholar]
- Okazaki M, Matsuyama T, Kohno T, Shindo H, Koji T, Morimoto Y, Ishimaru T. Induction of epithelial cell apoptosis in the uterus by a mouse uterine ischemia-reperfusion model: possible involvement of tumor necrosis factor-alpha. Biol Reprod. 2005;72(5):1282–1288. doi: 10.1095/biolreprod.104.035840. [DOI] [PubMed] [Google Scholar]
- Olive PL, Banuelos CA, Durand RE, Kim JY, Aquino-Parsons C. Endogenous and radiation-induced expression of gammaH2AX in biopsies from patients treated for carcinoma of the uterine cervix. Radiother Oncol. 2010;94(1):82–89. doi: 10.1016/j.radonc.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Raleigh JA, Chou SC, Arteel GE, Horsman MR. Comparisons among pimonidazole binding, oxygen electrode measurements, and radiation response in C3H mouse tumours. Radiat Res. 1999;151(5):580–589. doi: 10.2307/3580034. [DOI] [PubMed] [Google Scholar]
- Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13(7):1721–1732. doi: 10.1097/01.ASN.0000017223.49823.2A. [DOI] [PubMed] [Google Scholar]
- Royer C, Lachuer J, Crouzoulon G, Roux J, Peyronnet J, Mamet J, Pequignot J, Dalmaz Y. Effects of gestational hypoxia on mRNA levels of Glut3 and Glut4 transporters, hypoxia inducible factor-1 and thyroid hormone receptors in developing rat brain. Brain Res. 2000;856(1–2):119–128. doi: 10.1016/S0006-8993(99)02365-3. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- Tadokoro C, Yoshimoto Y, Sakata M, Imai T, Yamaguchi M, Kurachi H, Oka Y, Maeda T, Miyake A. Expression and localization of glucose transporter 1 (GLUT1) in the rat oviduct: a possible supplier of glucose to embryo during early embryonic development. Biochem Biophys Res Commun. 1995;214(3):1211–1218. doi: 10.1006/bbrc.1995.2415. [DOI] [PubMed] [Google Scholar]
- Tomes L, Emberley E, Niu Y, Troup S, Pastorek J, Strange K, Harris A, Watson PH. Necrosis and hypoxia in invasive breast carcinoma. Breast Cancer Res Treat. 2003;81(1):61–69. doi: 10.1023/A:1025476722493. [DOI] [PubMed] [Google Scholar]
- von Wolff M, Ursel S, Hahn U, Steldinger R, Strowitzki T. Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2003;88(8):3885–3892. doi: 10.1210/jc.2002-021890. [DOI] [PubMed] [Google Scholar]
- Wang BY, Kalir T, Sabo E, Sherman DE, Cohen C, Burstein DE. Immunohistochemical staining of GLUT1 in benign, hyperplastic, and malignant endometrial epithelia. Cancer. 2000;88(12):2774–2781. doi: 10.1002/1097-0142(20000615)88:12<2774::AID-CNCR16>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Webster WS, Abela D. The effect of hypoxia in development. Birth Defects Res C Embryo Today. 2007;81(3):215–228. doi: 10.1002/bdrc.20102. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Sakata M, Ogura K, Miyake A. Gestational changes of glucose transporter gene expression in the mouse placenta and decidua. J Endocrinol Invest. 1996;19(8):567–569. doi: 10.1007/BF03349018. [DOI] [PubMed] [Google Scholar]