Abstract
Tea prepared from the aerial parts of Antigonon leptopus is used as a remedy for cold and pain relief in many countries. In this study, A. leptopus tea, prepared from the dried aerial parts, was evaluated for lipid peroxidation (LPO) and cyclooxygenase (COX-1 and COX-2) enzyme inhibitory activities. The tea as a dried extract inhibited LPO, COX-1 and COX-2 enzymes by 78%, 38% and 89%, respectively, at 100 μg/mL. Bioassay-guided fractionation of the extract yielded a selective COX-2 enzyme inhibitory phenolic aldehyde, 2,3,4-trihydroxy benzaldehyde. Also, it showed LPO inhibitory activity by 68.3% at 6.25 μg/mL. Therefore, we have studied other hydroxy benzaldehydes and their methoxy analogs for LPO, COX-1 and COX-2 enzymes inhibitory activities and found that compound 1 gave the highest COX-2 enzyme inhibitory activity as indicated by a 50% inhibitory concentration (IC50) at 9.7 μg/mL. The analogs showed only marginal LPO activity at 6.25 μg/mL. The hydroxy analogs 6, 7 and 9 showed 55%, 61% and 43% of COX-2 inhibition at 100 μg/mL. However, hydroxy benzaldehydes 3 and 12 showed selective COX-1 inhibition while compounds 4 and 10 gave little or no COX-2 enzyme inhibition at 100 μg/mL. At the same concentration, compounds 14, 21 and 22 inhibited COX-1 by 83, 85 and 70%, respectively. Similarly, compounds 18, 19 and 23 inhibited COX-2 by 68%, 72% and 70%, at 100 μg/mL. This is the first report on the isolation of compound 1 from A. leptopus tea with selective COX-2 enzyme and LPO inhibitory activities.
1. Introduction
The plant Antigonon leptopus is native to Mexico and commonly found in tropical Asia, Africa, the Caribbean and the Americas [1], and is one of the medicinal plants used in Jamaica. The hot tea prepared from the aerial portion of this plant is used traditionally for the prevention and treatment of cough and flu-related pain (2Mitchell and Ahmad, 2006 S. A. Mitchell and M. H. Ahmad, A review of medicinal plant research at the University of the West Indies, Jamaica, West Indian Medicinal Journal 55 [2] (2006), pages 243–253.). Studies have also shown that A. leptopus plant extracts exhibited anti-thrombin, analgesic, anti-inflammatory, anti-diabetic and lipid peroxidation inhibitory activities [2–5].
Phenolic compounds are widely distributed secondary metabolites in the plant kingdom and play an important role in their physiological and morphological functions [6]. These compounds are diverse group of phytochemicals derived from the shikimate and phenylpropanoid pathways in plants. Phenolic compounds are known as strong antioxidants and might prevent antioxidative damage to biomolecules such as DNA, lipids and proteins [7]. A number of epidemiological studies have shown that phenolic compounds can reduce the risk of chronic disorders such as cardiovascular disease and cancer [8]. Also, the phenolic compounds are reported to inhibit several stages of carcinogenesis in vivo [9]. In addition, they possess significant anti-inflammatory activity as suggested by both in vitro and in vivo studies [10].
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most popular products used for pain management. The NSAIDs act as inhibitors of prostaglandin synthesis by cyclooxygenase (COX) enzymes. The COX enzymes catalyze the conversion of arachidonic acid to prostaglandin endoperoxide, the immediate substrate, for a series of cell-specific prostaglandins, which play critical roles in various biological functions [8]. The two isoforms of COX differ mainly in their pattern of expression. COX-1 is expressed in most tissues and COX-2 is an inducible enzyme that is expressed in response to pro-inflammatory agents. Naturally occurring selective COX-2 inhibitors are significant since they can be consumed as supplements, reducing inflammation and potentially preventing cancer [11–14]. In the present study, we report the bioassay-guided isolation of a selective COX-2 inhibitor, a polyhydroxy benzaldehyde, from the tea extract and LPO and COX assay results for the tea extract of A. leptopus, the isolate and a number of its analogs.
2. Methods
2.1. Plant Material and Reagents
Aerial parts of A. leptopus were collected in Jamaica during 2005 and authenticated by Mr Patrick Lewis at University of the West Indies, Mona, Kingston, Jamaica. A voucher specimen (UWI 35294) has been deposited at the herbarium of the University of the West Indies, Mona, Kingston, Jamaica. The hydroxy benzaldehydes, 2-hydroxy benzaldehyde; 3-hydroxy benzaldehyde; 4-hydroxy benzaldehyde, 2,3-dihydroxy benzaldehyde; 2,4-dihydroxy benzaldehyde, 2,5-dihydroxy benzaldehyde, 3,4-dihydroxy benzaldehyde, 3,5-dihydroxy benzaldehyde, 2,4,5-trihydroxy benzaldehyde, 2,4,6-trihydroxy benzaldehyde and 3,4,5-trihydroxy benzaldehyde, were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO).
2.2. Extraction and Isolation
Antigonon leptopus tea was prepared from the dried aerial parts of the plant (5 g) by soaking it with boiled water (50 mL) for 6 h. The resulting tea was evaporated under reduced pressure to yield a dark brown residue (606 mg). This residue was assayed LPO and COX enzyme inhibitory activities at 100 μg/mL. This bioactive extract was then analyzed by TLC using CHCl3 and MeOH (4 : 1) as the mobile phase along with the methanolic extract prepared earlier in our laboratory [5]. The TLC profiles of tea residue and methanolic extract were similar except a new band observed under UV (R f. 0.63). In order to isolate this UV-active compound, an aliquot of the water extract (200 mg) was purified by preparative TLC with CHCl3 and MeOH (4 : 1) as the developing solvents afforded compound 1 (0.7 mg). The structure of this compound was confirmed by 1H and 13C NMR spectral methods.
Compound 1. 1H NMR (d 6-DMSO): δ 9.83 (1H, s, CHO), 7.107 (1H, d, J = 8.4 Hz, H-6), 6.47 (1H, d, J = 8.4 Hz, H-5), 10.67, 10.32, 8.77 (each 1H, OH); 13C NMR (d 6-DMSO): δ 193.1 (CHO), 153.2 (C-4), 150.9 (C-2), 132.2 (C-3), 123.7 (C-6), 115.4 (C-1), 108.3 (C-5). Therefore, the structure of compound 1 was established as 2,3,4-trihydroxy benzaldehyde and further confirmed by comparison of its NMR chemical shifts with its published spectral data [15].
2.3. Methylation of Hydroxy Benzaldehydes
Methylation of hydroxy benzaldehydes was performed according to published procedure [16]. The monohydroxy benzaldehyde (0.5 mmol) was dissolved in 5 mL of acetone in a round-bottomed flask. To this solution, anhydrous potassium carbonate (1 mmol) was added, sealed with septa and stirred for 10 min. Dimethyl sulfate (1 mmol) was then added to the reaction mixture by injection, stirred at room temperature for 6 h while monitoring the reaction by TLC for every 30 min. The reaction mixture was evaporated under vacuum and the residue dissolved in 25 mL of RO water, transferred to a separating funnel and extracted with ethyl acetate (25 mL), the ethyl acetate layer treated with saturated sodium bicarbonate solution and evaporated under vacuum. The product thus obtained was purified by preparative TLC using chloroform : methanol (80 : 20) as the mobile phase. The methylation of di- and trihydroxy benzaldehydes was carried out by using a similar procedure but with 2 and 3 mmols of potassium carbonate and dimethyl sulfate, respectively. The reaction times were 12 and 18 h for dihydroxy and trihydroxy benzaldehydes, respectively. The yields of mono-, di- and trimethoxy benzaldehydes were 90%, 86%, 75%, respectively. The compounds were characterized by NMR spectral methods. The NMR spectra of hydroxy benzaldehydes purchased from Sigma-Aldrich are not presented in this manuscript but confirmed that the compounds were pure.
Compound 13. 1H NMR (d 6-DMSO): δ 10.19 (1H, s, CHO), 7.51 (1H, d, J = 9.0 Hz, H-6), 7.00 (1H, d, J = 9.0 Hz, H-5), 3.98, 3.89, 3.89 (each 3H, s, OMe).
Compound 14. 1H NMR (d 6-DMSO): δ 10.35 (1H, s, CHO), 7.67 (1H, m, H-6), 7.22 (1H, d, J = 7.5 Hz, H-4), 7.09 (2, d, J = 7.5 Hz, H-3, 5), 3.90 (3H, s, OMe).
Compound 15. 1H NMR (d 6-DMSO): δ 9.98 (1H, s, CHO), 7.52 (2H, m, H-6, 5), 7.42 (1H, d, J = 1.5 Hz, H-2), 7.29 (1H, m, H-4), 3.83 (3H, s, OMe).
Compound 16. 1H NMR (d 6-DMSO): δ 9.87 (1H, s, CHO), 7.87 (2H, d, J = 7.0 Hz), 7.13 (2H, d, J = 7.0 Hz), 3.86 (3H, s, OMe).
Compound 17. 1H NMR (d 6-DMSO): δ 10.30 (1H, s, CHO), 7.40 (1H, dd, J = 1.8, 7.8 Hz, H-6), 7.26 (1H, dd, 1H, dd, J = 1.8, 7.8 Hz, H-4), 7.02 (1H, d, J = 7.8 Hz, H-5), 3.89, 3.98 (each 3H, s, OMe).
Compound 18. 1H NMR (d 6-DMSO): δ 10.17 (1H, s, CHO), 7.65 (1H, d, J = 8.7 Hz, H-6), 6.69 (1H, d, J = 2.4 Hz, H-3), 6.32 (1H, dd, J = 8.7, 2.4 Hz, H-5), 3.87, 3.90 (each 3H, s, OMe).
Compound 19. 1H NMR (d 6-DMSO): δ 10.34 (1H, s, CHO), 7.25 (1H, dd, J = 1.8, 9.0 Hz, H-6), 7.21 (1H, brs, H-5), 7.16 (1H, d, J = 9.0 Hz, H-4), 3.87, 3.98 (each 3H, s, OMe).
Compound 20. 1H NMR (d 6-DMSO): δ 9.9 (1H, s, CHO), 7.65 (1H, s, H-6), 7.55 (1H, s, H-2), 7.08 (1H, s, H-5), 3.8 (each 3H, s, 3, 4-OMe).
Compound 21. 1H NMR (d 6-DMSO): δ 9.91 (1H, s, CHO), 7.06 (2H, d, J = 2.4 Hz, H-2, 6), Compound 22. 1H NMR (d 6-DMSO): δ 10.19 (1H, s, CHO), 7.15 (1H, s, 6-H), 6.79 (1H, s, 3-H), 3.92, 3.91, 3.73 (each 3H, s, OMe).
Compound 23. 1H NMR (d 6-DMSO): δ 10.01 (1H, s, CHO), 6.14 (1H, s, H-3, 5), 3.85 (3H, s, 4-OMe), 3.98 (6H, s, 2.6-OMe).
Compound 24. 1H NMR (d 6-DMSO): δ 9.88 (1H, s, CHO), 7.26 (2H, s, H-2, 6), 3.86 (6H, s, 3, 5-OMe), 3.76 (3H, s, 4-OMe).
2.4. Cyclooxygenase Enzyme Inhibitory Assay
Cyclooxygenase enzyme inhibitory assay was carried out according to the published procedure [17]. The COX-1 enzyme was prepared from ram seminal vesicles purchased from Oxford Biomedical Research, Inc. (Oxford, MI). COX-2 enzyme was prepared from insect cell lysate diluted with Tris buffer (pH 7) to yield an approximate final concentration of 1.5 mg protein/mL. Activities of phenolic compounds were assessed by monitoring the initial rate of O2 uptake using a micro-oxygen chamber and electrode (Instech Laboratories, Plymouth Meetings, PA) attached to a YSI model 5300 biological oxygen monitor (Yellow springs Instrument, Inc., Yellow Springs, OH) at 37°C. Each assay mixture contained Tris buffer (0.6 mL, 0.1 M, pH 7), phenol (1 mM), hemoglobin (85 μg) and DMSO or test samples (10 μL). Cyclooxygenase enzymes (COX-1 or COX-2, 20 μL) were added to the chamber and incubated for 3 min and the reaction was initiated by the addition of arachidonic acid (10 μL of a 1 mg/mL solution). Analysis was performed in duplicate for each sample and the standard deviation was calculated for n = 2. The data were recorded using QuickLog for windows data acquisition and control software (Strawberry Tree, Inc., Sunnyvale, CA). The compounds 2–24 were tested at 100 μg/mL concentration. The percent inhibition was calculated with respect to DMSO control. Compound 1 was tested at 25 μg/mL and serial dilutions were made in order to obtain dose—response curve. Non-steroidal anti-inflammatory drugs, Aspirin (60 μM), Celebrex (26 nM) and Vioxx (32 nM) were used as positive controls. Aspirin was purchased from Sigma-Aldrich Co. (St. Louis, MO) and Celebrex and Vioxx were physician samples kindly provided by Dr Subash Gupta, Sparrow Pain Center, MI, USA. We have used Vioxx as a positive control only in our in vitro assays. We continue to use it as a positive control in in vitro assays because Vioxx is a specific inhibitor of COX-2 enzyme among the non-steroidal anti-inflammatory agents (NSAIDs) produced and marketed. The use of Vioxx as a positive control in our in vitro assay is only for comparison purposes and not intended as a treatment. It is only used to study the mechanism of action of test compounds.
2.5. Lipid Peroxidation Inhibitory Assay
In vitro lipid peroxidation assay was carried out using large unilamellar vesicles (LUVs) using fluorescence spectroscopy according to the published procedure [17]. The phospholipid, 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphatidylcholine (SLPC), in CHCl3 and fluorescence probe, 3-[p-(6-phenyl)-1,3,5-hexatrienyl] phenylpropionic acid (DPH-PA), in DMF (mg/mL) were homogenized and dried under reduced pressure. The LUVs were produced by suspension of the lipid-probe mixture (0.15 M NaCl, 0.1 mM EDTA and 0.01 M MOPS buffer maintained over Chelex resin) followed by ten freeze-thaw cycles in a dry ice-EtOH bath and extrusion (29 times) through a 100 nm pore-size membrane (Avestin Inc., Ottawa, Canada). The final assay volume was 2 mL, consisting of 100 μL HEPES buffer (50 mM HEPES and 50 mM TRIS), 200 μL 1 M NaCl, 1.64 mL of N2-purged water, 20 μL of test sample or DMSO and 20 μL of liposome suspension. Lipid peroxidation was initiated by the addition of 20 μL of FeCl2 · 4H2O (0.5 mM) and the fluorescence was monitored at 0 min, 1 min, 3 min and every 3 min up to 21 min using a Turner Model 450 Digital Fluorometer. The decrease of relative fluorescence intensity over the time was used to determine the rate of peroxidation. The percentage of inhibition was calculated with respect to DMSO control. Extracts were tested at 100 μg/mL. Pure compounds were tested at 6.25 μg/mL since the activity of compound 1 was >50%. Commercial antioxidants BHA (butylated hydroxyanisol), BHT (butylated hydroxytoluene) and TBHQ (t-butyl hydroquinone) were tested at 1 μg/mL.
3. Results
3.1. Bioactive Constituents in A. leptopus Tea
The crude water extract obtained after evaporating the tea gave strong LPO, COX-1 and COX-2 enzyme inhibitory activities as indicated by 78%, 38.3% and 89%, respectively, at 100 μg/mL. The bioassay-guided isolation of this extract gave a phenolic compound with selective COX-2 enzyme inhibitory activity. The structure of compound 1 was identified to be 2,3,4-trihydroxy benzaldehyde [1] by using NMR spectral data and further confirmed by TLC comparison with an authentic sample (Figure 1). It inhibited COX-2 enzyme by 90% and was inactive against COX-1 enzyme at 25 μg/mL (Figure 2(a)). Serial dilutions of compound 1 were made to determine the 50% inhibitory concentration. Therefore, the dose-dependent evaluation of the inhibitory activity of compound 1 against COX-2 enzyme gave the 50% (IC50) inhibitory concentration as 9.7 μg/mL. Interestingly, compound 1 did not show any activity against COX-1 enzyme at 500 μg/mL, the highest concentration tested.
Figure 1.
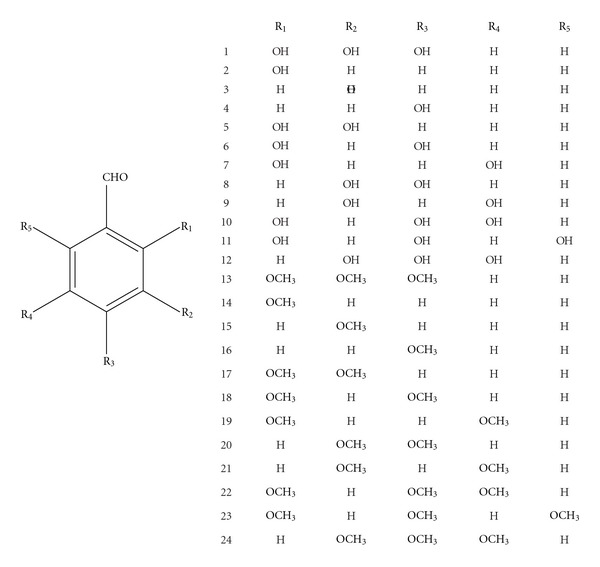
Structures of hydroxy and methoxy benzaldehydes.
Figure 2.
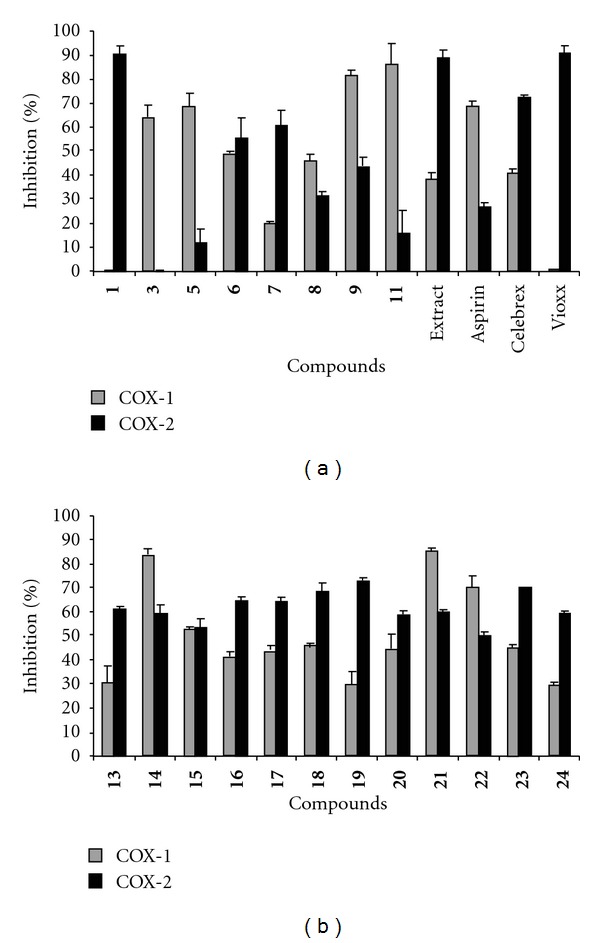
In vitro COX-1 and COX-2 enzyme inhibitory activities of: (a) hydroxyl benzaldehydes and (b) methoxy benzaldehydes. The concentration of compound 1 tested in this assay was 25 and the tea extract and compounds 2–24 at 100 μg/mL. Positive controls used in the assay were Aspirin (60 μM), and Celebrex (26 nM) and Vioxx (32 nM). DMSO was used as solvent control and the percent inhibition was calculated with respect to DMSO control. Vertical bars represent average of two experiments ± SD.
3.2. Activity of Methoxylated Benzaldehydes
Although compound 1 was isolated from A. leptopus tea as a natural product, the synthetic version of it is available in the market along with a number of other substituted hydroxyl benzaldehydes. Studies on antioxidant and anti-inflammatory activity of some of the hydroxy benzaldehydes and their analogs have been reported in the literature [18, 19]. Therefore, we have compared the structure-activity relationship of some of the commercially available hydroxy benzaldehydes (2–12) with compound 1 along with their methoxy derivatives (13–24) using in vitro LPO and COX enzyme inhibitory assays. The hydroxyl benzaldehydes were methylated in our laboratory to yield corresponding methoxy derivatives [15]. All compounds were characterized by proton and carbon NMR experiments (Figure 1).
Among the monohydroxy tested, compound 3 selectively inhibited COX-1 by 64% at 100 μg/mL. The inhibitory values of compounds 14 and 15 against COX-1 and COX-2 enzymes were 83% and 53% and 59% and 53%, respectively at 100 μg/mL (Figure 2(b)). Although compound 4 showed little or no activity against COX enzymes, the methylated product 16 inhibited COX-1 and COX-2 enzymes by 41% and 65%, respectively, at 100 μg/mL (Figure 2(a)).
Among the dihydroxy benzaldehydes, compound 7 was the most active against COX-2 enzyme with an inhibition of 61% at 100 μg/mL. Similarly, compounds 6 and 9 showed moderate inhibition against COX-2 enzyme by 55% and 43%, respectively. However, compounds 5 and 9 inhibited COX-1 enzyme by 68% and 82%, respectively, at the same concentration (Figure 2(a)). The COX-2 enzyme inhibitory activity of compound 5 was increased to 64% after methylation as shown in Figure 2(a). Other methylated compounds, 18–21, showed little or no variation in the activity profile. The inhibitory values observed for compounds 18, 19, 20 and 21 against COX-1 and COX-2 enzymes were 46%, 30%, 44%, 85% and 68%, 72%, 59%, 60%, respectively, at 100 μg/mL (Figure 2(b)).
Among the trihydroxy benzaldehydes tested, compound 10 was not inhibitory to COX-1 and COX-2 enzymes when tested at 100 μg/mL. However, its methylated product 22 inhibited COX-1 and COX-2 enzymes by 70% and 50%, respectively, at the same concentration (Figure 2(b)). This is evident from the activity profile of hydroxy benzaldehyde 11, a reversal of COX-1 and COX-2 activities due to methylation from 86 and 16% at 100 μg/mL to 45% and 70%. Commercial standards Vioxx, Celebrex and aspirin were tested at 32 nM, 26 nM and 60 μM concentrations. The inhibition values of Aspirin and Celebrex against COX-1 annd COX-2 enzymes were 69%, 41%, 27% and 72%, respectively. Vioxx is inactive to COX-1 and inhibited COX-2 enzyme by 91% (Figure 2(a)). The varying concentrations of positive controls used were necessary to keep the COX enzyme inhibition between 50% and 100%.
The LPO assay revealed that compounds 1 and 5 were the most active and inhibited LPO by 68% and 64%, respectively, at 6.25 μg/mL (Figure 3). The LPO inhibition observed for compounds 2, 6 and 8 was in the range of 40%–45% at the same concentration (data not shown). The methoxy benzaldehydes showed little or no activity against LPO when tested at 6.25 μg/mL. This is in agreement with the general understanding that hydroxy group is essential for antioxidant activity and methylation may result in lower activity [15]. Standard antioxidants BHA, BHT and TBHQ were used as positive controls in the assay and the inhibition values were in the range of 84%–93% for these compounds at 1 μg/mL (Figure 3).
Figure 3.

Lipid peroxidation inhibitory activities of tea prepared from A. leptopus and compounds 1 and 5. Positive controls, antioxidants BHA (butylated hydroxyanisol), BHT (butylated hydroxytoluene) and TBHQ (t-butyl hydroquinone) were tested at 1 μg/mL. Extract and compounds were tested 100, 6.25 μg/mL, respectively. Water or DMSO was used as solvent control and the percent inhibition was calculated with respect to water or DMSO control. Vertical bars represent the average of two experiments ± SD.
4. Discussion
Traditional use of the tea prepared from the aerial parts of A. leptopus has been implicated to the alleviation of swelling and cold. Studies have shown that the anti-inflammatory activity of A. leptopus plant material was due to the presence of phenolic compounds present in the organic extract [4, 5]. However, the constituents of the tea, the form consumed as a remedy, and its biological activity have not been studied till now. The results from the current study suggest that several compounds present in the tea extract also exist in the organic extract. The tea contained the phenolic compound 2,3,4-trihydroxy benzaldehyde with selective COX-2 enzyme inhibitory activity.
Phenolic compounds have attracted considerable interest in recent years due to their potential health benefits such as anti-oxidant, antimicrobial, anti-inflammatory and cardioprotective activities [21–24]. The inhibition of prostaglandin synthesis by phenolic compounds has also been reported [25–27] and their COX inhibitory activity was correlated to the antioxidant properties [27]. This observation is evident from our COX assay results that benzaldehyde is inactive but its hydroxylated analogs showed varying degrees of COX-1 and COX-2 enzyme inhibitory activities, although a trend in activity related to the number of hydroxy and methoxy substitutions were not evident.
The amount of aerial portions of A. leptopus used in the preparation of tea and the frequency of its consumption by folklore is unclear. Although the amount of A. leptopus plant material used in the preparation of tea in our study was primarily to yield adequate amount of extract for bioassay and the isolation of bioactive principles, it is evident that a tea prepared from 5 g of dried plant material deliver about 2.1 mg of COX-2 active hydroxy benzaldehyde, compound 1. This is in comparison to the efficacy of Vioxx observed in our in vitro bioassay. Therefore, a 50% inhibitory concentration (IC50) of 9.7 μg/mL observed for compound 1 is in par with the 32 nM concentration of Vioxx. Hypothetically, the consumption of A. leptopus tea containing 650–700 mg of extract containing compound 1 per day could yield COX-2-related pain relief similar to the daily dose of an NSAID to a person with an average body weight of 70 kg. In conclusion, our efficacy results of the tea and active components present in it support the use of A. leptopus tea as an ethnomedicine to ameliorate inflammatory pain and may be beneficial to improve the quality of life.
Funding
Michigan State University Agricultural Experiment Station; Natural Therapeutics, LLC, Ann Arbor, MI, USA.
References
- 1.Raju AJS, Raju VK, Victor P, Naidu SA. Floral ecology, breeding system and pollination in Antigonon leptopus L. (Polygonaceae) Plant Species Biology. 2001;16(2):159–164. [Google Scholar]
- 2.Lans CA. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. Journal of Ethnobiology and Ethnomedicine. 2006;2:1–11. doi: 10.1186/1746-4269-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chistokhodova N, Nguyen C, Calvino T, Kachirskaia I, Cunningham G, Miles DH. Antithrombin activity of medicinal plants from central Florida. Journal of Ethnopharmacology. 2002;81(2):277–280. doi: 10.1016/s0378-8741(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 4.Mamidipalli WC, Nimmagadda VR, Bobbala RK, Gottumukkala KM. Preliminary studies of analgesic and anti-inflammatory properties of Antigonon leptopus Hook. et Arn roots in experimental models. Journal of Health Science. 2008;54(3):281–286. [Google Scholar]
- 5.Vanisree M, Alexander-Lindo RL, DeWitt DL, Nair MG. Functional food components of Antigonon leptopus tea. Food Chemistry. 2008;106(2):487–492. [Google Scholar]
- 6.Makoi JHJR, Ndakidemi PA. Biological, ecological and agronomic significance of plant phenolic compounds in rhizosphere of the symbiotic legumes. African Journal of Biotechnology. 2007;6(12):1358–1368. [Google Scholar]
- 7.Liu J, Mori A. Antioxidant and pro-oxidant activities of p-hydroxybenzyl alcohol and vanillin: effects of free radicals, brain peroxidation and degradation of benzoate, deoxyribose, amino acids and DNA. Neuropharmacology. 1993;32(7):659–669. doi: 10.1016/0028-3908(93)90079-i. [DOI] [PubMed] [Google Scholar]
- 8.Jiang F, Dusting GJ. Natural phenolic compounds as cardiovascular therapeutics: potential role of their antiinflammatory effects. Current Vascular Pharmacology. 2003;1(2):135–156. doi: 10.2174/1570161033476736. [DOI] [PubMed] [Google Scholar]
- 9.Knekt P, Järvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. British Medical Journal. 1996;312(7029):478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newmark HL. Dietary Phytochemicals in Cancer Prevention and Treatment. New York, NY, USA: Plenum Press; 1996. Plant phenolics as potential cancer prevention agents; pp. 25–34. [DOI] [PubMed] [Google Scholar]
- 11.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacological Reviews. 2004;56(3):387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 12.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18(55):7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 13.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Seminars in Oncology. 2004;31:22–29. doi: 10.1053/j.seminoncol.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 14.Senior K. COX-2 inhibitors: cancer prevention or cardiovascular risk? Lancet Oncology. 2005;6(2):p. 68. doi: 10.1016/s1470-2045(05)01720-1. [DOI] [PubMed] [Google Scholar]
- 15.Lide DR, Milne GWA. Handbook of Data on Organic Compounds. Boca Raton, Fla, USA: CRC Press; 1994. [Google Scholar]
- 16.Deng D, Zhnag J, Cooney JM, et al. Ethylated polyphenols are poor chemical antioxidants but can still effectively protect cells from hydrogen peroxide-induced cytotoxicity. FEBS Letters. 2006;580:5247–5250. doi: 10.1016/j.febslet.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Mills GL, Nair MG. Cyclooxygenase inhibitory and antioxidant compounds from the fruiting body of an edible mushroom, Agrocybe aegerita . Phytomedicine. 2003;10(5):386–390. doi: 10.1078/0944-7113-00272. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Jang YW, Kang HS, Moon H, Sim SS, Kim CJ. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Archives of Pharmacal Research. 2006;29(10):849–858. doi: 10.1007/BF02973905. [DOI] [PubMed] [Google Scholar]
- 19.Shyamala BNS, Naidu M, Sulochanamma GS, Srinivas P. Studies on the antioxidant activities of natural vanilla extract and its constituent compounds through in vitro models. Journal of Agricultural and Food Chemistry. 2007;55:7738–7743. doi: 10.1021/jf071349+. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell SA, Ahmad MH. A review of medicinal plant research at the University of the West Indies, Jamaica. West Indian Medical Journal. 2006;55:243–253. doi: 10.1590/s0043-31442006000400008. [DOI] [PubMed] [Google Scholar]
- 21.Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Current Opinion in Lipidology. 2005;16(1):77–84. doi: 10.1097/00041433-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Pereira JA, Pereira APG, Ferreira ICFR, et al. Table olives from Portugal: phenolic compounds, antioxidant potential, and antimicrobial activity. Journal of Agricultural and Food Chemistry. 2006;54(22):8425–8431. doi: 10.1021/jf061769j. [DOI] [PubMed] [Google Scholar]
- 23.Jiang F, Dusting GJ. Natural phenolic compounds as cardiovascular therapeutics: potential role of their antiinflammatory effects. Current Vascular Pharmacology. 2003;1(2):135–156. doi: 10.2174/1570161033476736. [DOI] [PubMed] [Google Scholar]
- 24.Lands WEM, Hanel AM. Phenolic anticyclooxygenase agents in antiinflammatory and analgesic therapy. Prostaglandins. 1982;24(2):271–277. doi: 10.1016/0090-6980(82)90153-8. [DOI] [PubMed] [Google Scholar]
- 25.Azuma Y, Ozasa N, Ueda Y, Takagi N. Pharmacological studies on the anti-inflammatory action of phenolic compounds. Journal of Dental Research. 1986;65(1):53–56. doi: 10.1177/00220345860650010901. [DOI] [PubMed] [Google Scholar]
- 26.Egan RW, Gale PH, Beveridge GC, Marnett LJ, Kuehl FA., Jr. Direct and indirect involvement of radical scavengers during prostaglandin biosynthesis. Advances in Prostaglandin and Thromboxane Research. 1980;6:153–155. [PubMed] [Google Scholar]
- 27.Dewhirst FE. Structure-activity relationships for inhibition of prostaglandin cyclooxygenase by phenolic compounds. Prostaglandins. 1980;20(2):209–222. doi: 10.1016/s0090-6980(80)80040-2. [DOI] [PubMed] [Google Scholar]


