Abstract
Although adult motoneurons do not die if their axons are injured at some distance from the cell body, they are unable to survive injury caused by ventral root avulsion. Some of the injured motoneurons can be rescued if the ventral root is re-inserted into the spinal cord. Brachial plexus injuries that involve the complete or partial avulsion of one or more cervical ventral roots can be treated successfully only if satisfactory numbers of motoneurons remain alive following such an injury at the time of reconstructive surgery. Here we investigated the various strategies that could be used to rescue injured rat cervical motoneurons. The seventh cervical ventral root (C7) was avulsed and various therapeutic approaches were applied to induce motoneuronal survival and regeneration. Avulsion of the root without reimplantation resulted in very low numbers of surviving motoneurons (65 ± 8 SEM), while treatment of the injured motoneurons with riluzole resulted in high numbers of surviving motoneurons (637 ± 26 SEM). When the C7 ventral root was reimplanted or a peripheral nerve implant was used to guide the regenerating axons to a muscle, considerable numbers of motoneurons regenerated their axons (211 ± 15 SEM and 274 ± 28 SEM, respectively). Much greater numbers of axons regenerated when reimplantation was followed by riluzole treatment (573 ± 9 SEM). These results show that injured adult motoneurons can be rescued by riluzole treatment, even if they cannot regenerate their axons. Reinnervation of the peripheral targets can also be further improved with riluzole treatment.
Key words: avulsion, cell death, motoneuron, regeneration, riluzole, spinal cord
Introduction
Axonal injury inflicted upon adult motoneurons at some distance from their cell bodies causes minimal loss of these neurons. On the other hand, axotomy close to the cell body, especially that occurring at the spinal cord-ventral root interface, induces the vast majority of the affected motoneurons to die (Koliatsos et al., 1994; Nógrádi and Vrbová, 1996). This latter mechanism, when the ventral root is detached by harsh forces from the spinal cord, is called avulsion, and is a typical component of severe brachial plexus injuries (Carlstedt, 2008, 2009). Recently several attempts have been made to rescue adult motoneurons following avulsion injury, including therapy with neurotrophic factors (Blits et al., 2004; Haninec et al., 2003; Novikov et al., 1995; Wu et al., 2003), and progenitor and stem cell therapy (Hell et al., 2009; Su et al., 2009). Since the injured motoneurons are thought to die as result of their increased sensitivity to excitatory influences, and/or the lack of availability of a target they can reinnervate, it can be argued that rescue of significant numbers of motoneurons can be achieved by reducing excitatory effects and providing them with a favorable conduit to regenerate their axons (Greensmith and Vrbová, 1996; Mentis et al., 1993).
Riluzole (2-amino-6-trifluoromethoxybenzothiazole) is a compound that acts to block voltage-activated Na+ and Ca++ channels, to activate K+ channels, and to inhibit presynaptic glutamate release (Doble, 1996; Duprat et al., 2000). Riluzole was able to effectively reduce ischemic neuronal damage in the spinal cord (Lang-Lazdunski et al., 1999), and prevent motoneuron death in vitro after exposure to glutamate agonists (Estevez et al., 1995). Moreover, clinical trials have proven that riluzole increased survival of a subset of amyotrophic lateral sclerosis (ALS) patients with bulbar onset, and it is one of the most promising drugs for the treatment of ALS (Bensimon et al., 1994; Gordon, 2005; Meininger et al., 1997). We have shown in our previous studies that systemic administration of riluzole in animals that had their lumbar ventral root avulsed and reimplanted prevented the death of motoneurons (Nógrádi and Vrbová, 2001), even if onset of treatment was delayed by 10 days (Nógrádi et al., 2007).
Most of the studies focusing on motoneuron survival after ventral root avulsion use the lumbar spinal cord as a well-described model. Little is known about the events following ventral root injury in the lower cervical spinal cord, which is typically affected by human brachial plexus injuries. It is thus important to establish from both a clinical and a theoretical point of view whether it is possible: (1) to rescue the injured cervical motoneurons by providing them a favorable conduit, including an autologous sensory nerve, to regenerate their axons; and (2) to prevent cell death with riluzole treatment started early after injury, even if the damaged motoneurons have no opportunity to regenerate their axons. The aim of our study was to reveal whether any of these strategies can rescue significant amounts of motoneurons that can be used for the reinnervation of the brachial plexus and the denervated forelimb muscles.
Methods
Surgery
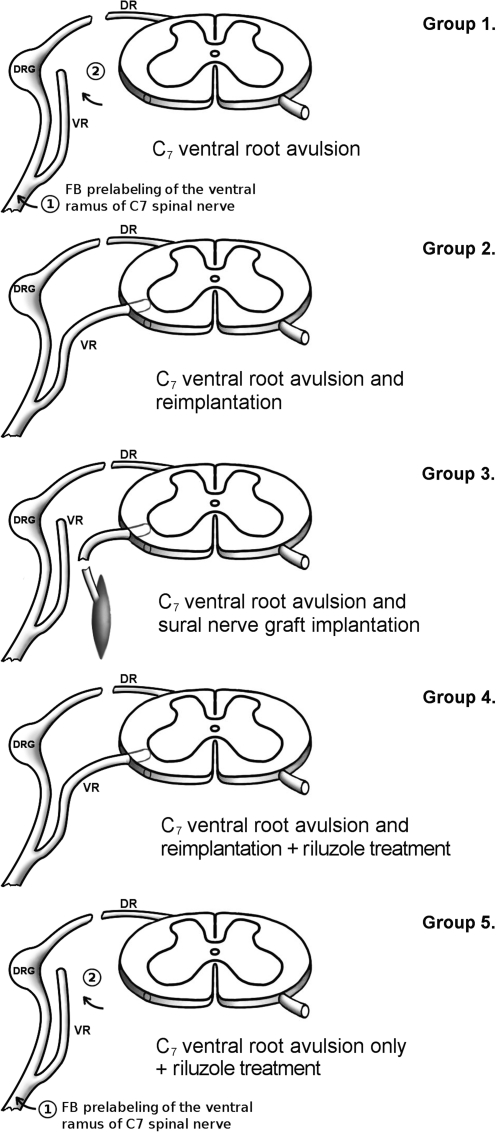
In all, 30 female Sprague-Dawley rats (weight at time of surgery: 180–200 g; Biological Services, University of Szeged, Szeged, Hungary) were used in this study. Five intact animals were used for counting the C7 motoneuron pool. In 25 animals the right C7 ventral root was avulsed from the cord, and these animals were used to set up five experimental groups, each group consisting of five animals (Fig. 1).
FIG. 1.
Schematic drawing of the surgical procedures applied in this study. Note the lack of possible reinnervation in groups 1 and 5, as in these animals fast blue (FB) was first applied to the ventral ramus of the C7 spinal nerve (1), and 3 days later the C7 ventral root was avulsed without reimplantation (2). To get access to the ventral root, the C7 dorsal root was transected in every surgical paradigm. The muscle shown in group 3 refers to the spinocervical muscle, and in that case there was also no reinnervation of the originally-innervated muscles (VR, ventral root; DR, dorsal root; DRG, dorsal root ganglion).
All the operations were carried out under deep chloral hydrate anesthesia (4%; 1 mL/100 g body weight) and sterile conditions. In the experimental groups in which avulsion of the C7 ventral root was followed by the reimplantation of the root, laminectomy was performed at the level of the C5–C6 vertebrae, the dura was opened, and the right C7 ventral root was pulled out after cutting the dorsal root (Fig. 1). The C7 ventral root was subsequently laterally reimplanted in the spinal cord just above its original entry zone. The spinal cord was covered with the remaining dura, the wound was closed, and the animals were allowed to recover. In group 1 the ventral ramus of the C7 spinal nerve was cut, and the proximal stump was prelabeled with the fluorescent dye fast blue (FB; Illing Plastics GmbH, Breuberg, Germany). Three days later the C7 ventral root was avulsed and placed beside the cord far from the ventral root exit zone to avoid regeneration of motor axons into the root (Fig. 1). In group 2 the C7 ventral root was avulsed, and then the free end of the ventral root was inserted into the ventrolateral part of the spinal cord (Fig. 1). To avoid damage to the cord, a small hole was created on the ventrolateral surface of the cord, and the avulsed root was inserted into the hole using a watchmaker's forceps. Special care was taken to avoid damage to the cord, including its motoneuron pool, or to the reimplanted root. In group 3 animals the ventral root was avulsed, and the C7 motor pool was connected to the spinocervicalis muscle by a sural nerve graft removed from the same animal. The nerve graft was implanted at the same position as the ventral root in group 2 animals. Group 4 animals underwent the same operation as the animals in group 2 (avulsion and reimplantation), but the animals received riluzole treatment for 3 weeks. The animals in group 5 were treated the same as those in group 1 (FB prelabeling of the C7 motor pool and avulsion), but in addition they also received riluzole therapy. Group 1 and 5 animals survived for 5 weeks, while the rest of the animals were sacrificed after 3 months of survival. In groups 1 and 5 shorter survival times were used, as motoneuron death was definitely completed by this time (Koliatsos et al., 1994; Nógrádi et al., 2007), and the retrograde tracer fast blue was still detectable in the surviving motoneurons (Novikova et al., 1997). In groups 2–4 the sectioned ventral ramus of the right C7 spinal nerve or the nerve graft (group 3) was labeled with FB at the end of the survival period.
The experiments were carried out with the approval of the Committee for Animal Experiments, University of Szeged, and rules regarding the care and use of animals for experimental procedures were followed. All the procedures were carried out according to the Helsinki Declaration on Animal Rights. Adequate care was taken to minimize pain and discomfort.
Riluzole treatment
The animals were treated with riluzole for 3 weeks (4 mg/kg; a kind gift of Tocris Cookson Ltd., Langford, U.K.). Riluzole treatment started immediately on the day of surgery, and the drug was injected IP daily for 1 week, and every second day for the next 2 weeks. This treatment protocol was based on the successful riluzole treatment described in our earlier articles (Nógrádi and Vrbová, 2001; Nógrádi et al., 2007). The dose of riluzole was established from data obtained from our earlier and other researchers' experiments (Lang-Lazdunski et al., 1999; Nógrádi and Vrbová, 2001; Schwartz and Fehlings, 2001, 2002; Wahl et al., 1993). It has also been reported that 5 mg/kg riluzole administered IP in rats produces a significant riluzole level in the brain (Maltese et al., 2005), suggesting that this dose is able to produce therapeutic effects.
Retrograde labeling and immunohistochemistry
Three months after surgery, the group 2, 3, and 4 animals were deeply anesthetized with chloral hydrate. On the operated side the ventral ramus of the C7 spinal nerve (groups 2 and 4), or the nerve graft (group 3), was sectioned and the proximal stump of the nerve was covered with a few crystals of fast blue. Three days after the application of fluorescent dye, the animals were reanesthetized and perfused transcardially with 4% paraformaldehyde in 0.1 mol/L phosphate buffer. The C7 motoneuron pool of intact animals was labeled as described above, and these animals were allowed to survive for 3 days. The animals in groups 1 and 5 were only processed for perfusion after 5 weeks of survival. The C7 motoneuron pool of intact animals labeled with fast blue was used as a control pool for both groups 1 and 5, and groups 2–4, as it was reported by Novikova and associates (1997) that motoneuron counts after fast blue labeling remain unchanged at least for 12 weeks after application of the dye. The cervical part of the spinal cords, with the reimplanted ventral root (if reimplantation was performed), was removed and kept in fixative for 4 h. The tissues were then immersed in 30% sucrose. Serial 25-μm-thick cryostat sections were cut, mounted on gelatinized slides, and examined with an Olympus BX50 fluorescence microscope (Olympus Ltd., Tokyo, Japan). The number of retrogradely-labeled cells was determined. To avoid double counting the same neuron present in two consecutive sections, the retrogradely-labeled neurons were mapped with the aid of an Olympus drawing tube, and their locations were compared to those of labeled neurons in the previous section. All sections from the C7 motoneuron pool were used.
Three spinal cords from groups 2–5 were then further processed for choline acetyltransferase (ChAT) immunohistochemistry. Sections from group 1 animals were not used for ChAT immunohistochemistry because of the relatively low number of retrogradely-labeled cells. Sections processed for ChAT immunohistochemistry were preincubated in 3% normal goat serum for 1 h, then incubated with a polyclonal goat anti-ChAT antibody (1:200; Chemicon, Hofheim, Germany) overnight at 4°C. The immune reaction was completed by using the avidin-biotin technique (reagents were purchased from Vector Laboratories, Burlingame, CA), and finally were tyramide-amplified with the Cyanine3 TSA kit (Tyramide Signal Amplification; Perkin Elmer, Waltham, MA). The number of ChAT-stained motoneurons in the pools where retrogradely-labeled cells were found was also determined, both on the operated and control sides. Some sections were stained with cresyl violet to assess the morphology of the spinal cord. Sections were photographed using an Olympus DP70 digital camera mounted on the microscope. Digital images were resized and their contrast and brightness adjusted.
Functional analysis
The forelimb movements of the operated animals were monitored every week. The degree of dorsiflexion in the wrist joint, and the extent of flexure contraction developed in the same joint were observed. As the forelimb muscles of animals in groups 1, 3, and 5 were not reinnervated, and therefore complete wrist joint contraction developed in these animals, none of the sophisticated tests such as pellet reaching or detailed forelimb movement analysis could be performed. The grading system used to determine the extent of dorsiflexion and flexure contraction are shown in Table 1.
Table 1.
Grading System for Evaluation of Wrist Joint Dorsiflexion and Contraction
| Grading | Extent of dorsiflexion (wrist joint) | Degree of flexure contraction (wrist joint) |
|---|---|---|
| 0 | No dorsiflexion | No contraction is present |
| 1 | Minimal dorsiflexion (<30°) | Minimal contraction, wrist joint is moveable |
| 2 | Dorsiflexion ∼ 30° | Medium degree of contraction, wrist joint is moved with difficulty |
| 3 | Dorsiflexion > 30° | Severe contraction |
Statistical analysis
The non-parametric Mann-Whitney U test, and analysis of variance (ANOVA) computed using Tukey's all pair-wise multiple comparison procedures, were used to compare the group data. The tests were used according to the parametric or non-parametric nature of the data.
Results
Observations of the movement pattern of operated animals
All of the animals survived the surgery and the subsequent riluzole treatment, and no side effects of riluzole treatment were observed.
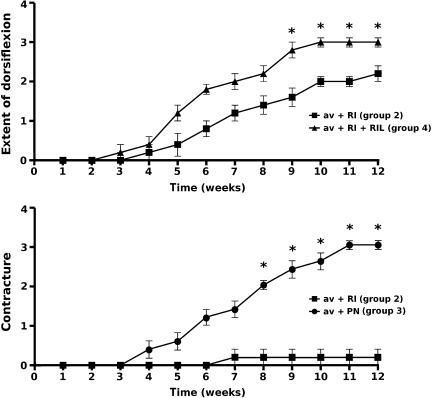
Initially all animals developed partial paralysis in the operated forelimb. Animals whose C7 ventral root was avulsed without further surgical treatment or peripheral nerve implantation (groups 1, 3, and 5) developed marked atrophy in the extensor musculature of the upper limb, thus the wrist joint and toes were permanently fixed in a flexion contracture by 4–5 weeks after surgery (grade 3 of contraction). They were unable to dorsiflex the wrist joint or perform a gripping function at any time point (grade 0 of dorsiflexion). In contrast, all the animals that had their C7 motoneuron pool connected to the target muscles by ventral root reimplantation (groups 2 and 4) started to recover from paralysis during the third week following surgery, but complete recovery took a few more weeks (Fig. 2). By the end of the survival period they were able to walk without major deficits, and during locomotion they extensively dorsiflexed their wrist joints. Animals that had the C7 ventral root reimplanted without riluzole treatment developed about grade 2 dorsiflexion in their wrist joint (group 2; dorsiflexion ∼ 30°), while better functional results were seen in the animals treated with riluzole following reimplantation (group 4; grade of dorsiflexion = 3). None of these treated rats developed significant contractures, and only one animal in group 2 showed minimal contraction of the wrist joint (grade 1), with satisfactory wrist dorsiflexion (Fig. 2).
FIG. 2.
Functional evaluation of experimental animals during their 12-week survival period. The upper panel shows the extent of dorsiflexion produced by animals in groups 2 and 4 (group 3 animals were not included, as their forelimb muscles were not reinnervated). Animals that received riluzole treatment regained their ability to dorsiflex the wrist joint earlier and to a greater extent than animals without riluzole treatment. Significant differences were seen from week 9 after surgery onward (p ≤ 0.05 by Mann-Whitney U test). The lower panel displays the degree of contracture in the wrist joint as a function of time in experimental animals belonging to groups 2 and 3. Group 4 animals are not included, as they developed no contracture, and only one animal from the avulsion and reimplantation group (group 2) developed minimal contracture. Significant differences were observed from week 8 after surgery onward (p ≤ 0.01 by Mann-Whitney U test; values are shown as mean ± standard error of the mean; asterisks indicate significant differences between data at individual time points; av, avulsion; RI, reinnervation; RIL, riluzole; PN, peripheral nerve graft).
General observations on the morphology of spinal cords
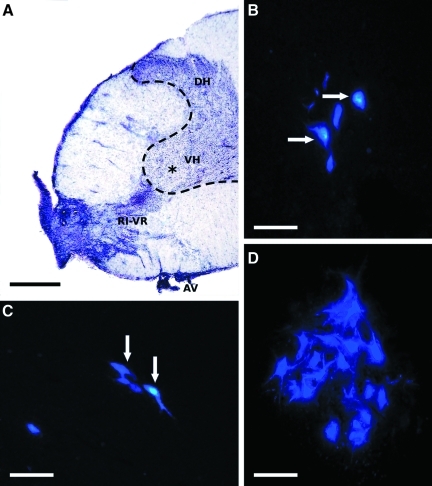
In cresyl violet–stained specimens the postoperative morphology of the spinal cords could be studied. In all experimental groups fewer motoneurons were present in the operated ventral horn of the C7 segment, and some gliosis could be seen at the site of root avulsion (Fig. 3A). This finding was also seen during localization of reinnervating fast blue–labeled motoneurons in the cord. In some cords of the animals in groups 2–4, the reimplanted ventral root could be clearly recognized close to the lateral motoneuron pool (Fig. 3A).
FIG. 3.
Composite figure shows the morphological appearance of an avulsed and reimplanted spinal cord, and retrogradely-labeled cells in the C7 pool following various experimental procedures. (A) Transverse section taken from a spinal cord that had its C7 ventral root (AV) avulsed and reimplanted (RI-VR; reimplanted ventral root in an animal from group 2 with cresyl violet staining). The lateral part of the ventral horn in this section does not contain motoneurons; only some glial scar is present (asterisk indicates glial scar; VH, ventral horn; DH, dorsal horn). (B–D) Retrogradely-labeled (fast blue) surviving motoneurons are shown in spinal cords of group 2–4 animals (B: group 3, avulsion and peripheral nerve graft; C: group 2, avulsion and reimplantation; D: group 4, avulsion, reimplantation, and riluzole treatment). Note the greater number of reinnervating motoneurons following riluzole treatment (in D), compared to that seen in C (arrows indicate clearly identifiable surviving cells; scale bars in A = 500 μm, and in B–D = 100 μm). Color image is available online at www.liebertonline.com/neu.
Survival of cervical motoneurons following C7 ventral root avulsion
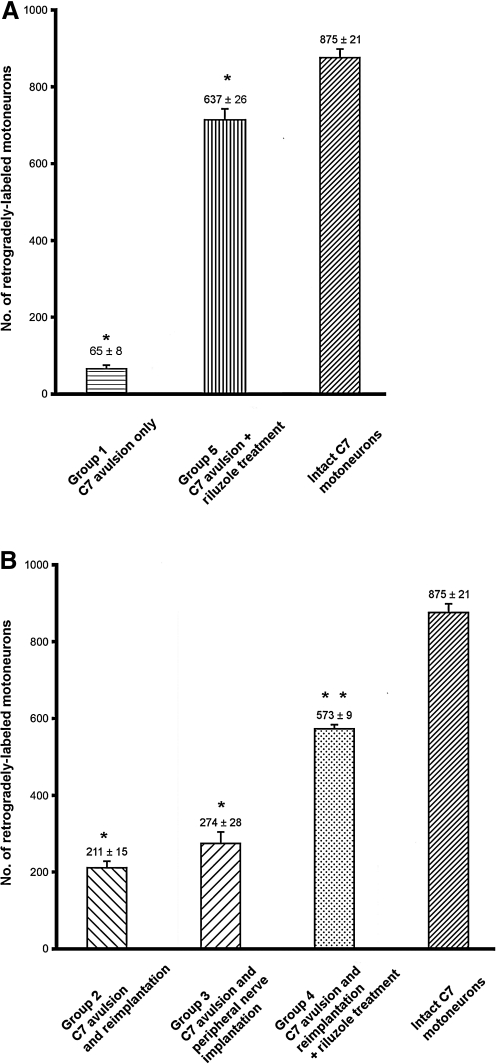
In the first set of experiments the number of resident motoneurons in the C7 motoneuron pool was assessed by retrograde labeling of the ventral ramus of the C7 spinal nerve. We found the average number of retrogradely-labeled motoneurons to be 875 ± 21 (SEM; Fig. 4A and B). This number of C7 motoneurons correlated with the motoneuron numbers calculated by other researchers (Jivan et al., 2006; Watabe et al., 2000). The surviving motoneurons were localized mainly in the lateral motoneuron column of the C7 spinal segment (Fig. 3A–D). When the motoneuron pool was prelabeled with FB 3 days before avulsion of the root, the avulsion of the C7 ventral root resulted in a dramatic decrease in surviving motoneuron numbers 5 weeks following avulsion, with only 65 ± 8 SEM surviving (group 1; Fig. 4A).
FIG. 4.
Bar graphs show the number of retrogradely-labeled neurons in the experimental groups. In panel A, the results of experiments with ventral root avulsion only (without reimplantation, 5 weeks survival only) are compared. Avulsion alone without any treatment induced a dramatic drop in the survival of the prelabeled motoneuron pool (group 1); however, riluzole treatment without a reimplantation strategy rescued the vast majority of these injured motoneurons (73% of the intact C7 motoneuron pool; *p ≤ 0.001 indicates significant differences among group 1, group 5, and intact animals, by analysis of variance [ANOVA] computed using Tukey's all pair-wise multiple comparison procedures for each group). Panel B summarizes the results of the avulsion and reimplantation or peripheral nerve implantation experiments (groups 2–4). Note that approximately 24% of the motoneurons found in the intact C7 motoneuron pool were able to regenerate their axons following C7 ventral root avulsion and reimplantation, compared with the reinnervation capacity of the injured motoneuron pool connected to a nearby muscle via a peripheral nerve graft (31%). Increased survival and reinnervation was found when riluzole treatment was applied after avulsion injury. *Groups 2 and 3 were significantly different from group 4 and intact animals, but no difference was found between groups 2 and 3. **Group 4 animals were significantly different from all other groups (p ≤ 0.001 by ANOVA, computed using Tukey's all pair-wise multiple comparison procedures; values are shown as mean ±standard error of the mean).
These motoneurons were found throughout the length of the C7 spinal segment, and marked autofluorescence indicated the presence of gliotic scar tissue at the place of motoneuron loss.
We wanted to test the neuroprotective effect of riluzole on the injured motoneurons, and whether they can be rescued when they have no possibility to regenerate their axons and reach a muscle. The same experiment was carried out as on group 1 animals, but the animals received riluzole treatment for 3 weeks following surgery. It was found that the vast majority of the prelabeled motoneurons survived for 5 weeks following avulsion (637 ± 26 SEM), and that these motoneuorons had more developed dendritic trees than those in group 1 (data not shown).
Regeneration of the axons of injured motoneurons through a peripheral nerve guide
The effect of various surgical procedures on the regeneration of injured axons of motoneurons following avulsion and reimplantation of the C7 ventral root was studied in the next series of experiments. In animals whose avulsed C7 ventral root was reimplanted into the ventrolateral part of the cord (group 2), 211 ± 15 SEM retrogradely-labeled motoneurons were found, indicating that nearly one-quarter of the total population of C7 motoneurons survived and was able to grow axons into the reimplanted C7 ventral root (Figs. 3B and 4B). In the experiments in which the C7 ventral root was avulsed, and the motor pool of the affected segment and the spinocervical muscle was connected with a sural nerve graft, the procedure resulted in similar numbers of retrogradely-labeled motoneurons (274 ± 28 SEM; Figs. 3C and 4B). Although the number of retrogradely-labeled motoneurons appeared to be somewhat higher in group 3, there was no significant difference in reinnervating motoneuron numbers between group 2 and 3 animals. In contrast, a significant increase in the number of retrogradely-labeled motoneurons was seen when riluzole treatment was applied following C7 avulsion and reimplantation for 3 weeks (Figs. 3D and 4B). In these animals, much larger numbers of retrogradely-labeled motoneurons were found (573 ± 9 SEM) than in group 2 and 3 animals.
Next we investigated whether there were some motoneurons that were unable to extend their axons into the ventral root or peripheral nerve graft (groups 2–4), if they were just left in the appropriate part of the spinal cord after avulsion and reimplantation of the ventral root.
Expression of choline acetyltransferase in injured and regenerating motoneurons
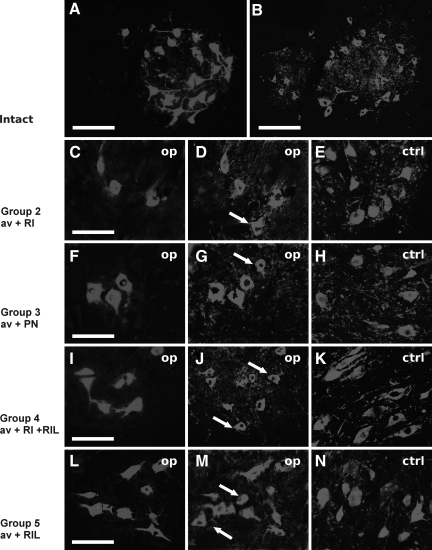
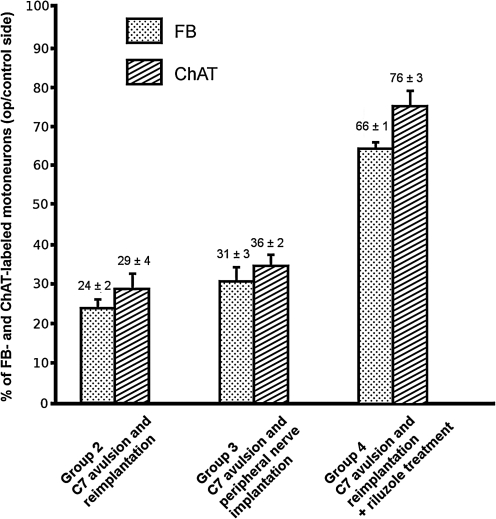
We compared the localization of ChAT with that of fast blue–labeled reinnervating and surviving cells. In control rats, all the retrogradely-labeled motoneurons in the lateral motoneuron pools were ChAT-immunoreactive, but only a few ChAT-immunoreactive motoneurons in the ventromedial pool were found not to be labeled with fast blue (Fig. 5A and B). Strong co-localization was found in spinal cords of group 2 and 3 animals, in which the ventral root was avulsed and reimplanted, or a peripheral nerve was implanted into the cord, respectively. However, in these spinal cords there were some ChAT-immunoreactive cells that were not retrogradely-labeled. Accordingly, in these animals the proportions of fast blue–labeled motoneurons on the operated side and those of the ipsilateral ChAT-immunoreactive motoneurons were 84 ± 12% SEM in group 2 (Figs. 5C–E and 6), and 88 ± 7% SEM in group 3 (Figs. 5F–H and 6), respectively. Similarly, in animals that had the C7 ventral root avulsed and reimplanted, and that also received riluzole treatment (group 4), 86 ± 4% SEM of the ChAT-positive motoneurons was retrogradely-labeled (Figs. 5I–K and 6). None of these three groups were significantly different from each other with regard to the proportion of FB-positive/ChAT-positive neurons.
FIG. 5.
Transverse sections of spinal cord taken from (A and B): an intact C7 spinal cord segment with retrogradely-labeled motoneurons and ChAT-immunoreactive neurons, respectively. (C–E) Spinal cord with C7 ventral root avulsion and reimplantation 3 months after surgery. (F–H) Spinal cord with ventral root avulsion and peripheral nerve graft implantation. (I–K) Spinal cord with ventral root avulsion and reimplantation followed by riluzole treatment for 3 weeks after surgery. (L–N) Surviving motoneurons following prelabeling with fast blue (FB) and ventral root avulsion (without reimplantation). Note the large numbers of surviving cells in the ventral horn in I and J. Surviving and reinnervating motoneurons were retrogradely labeled with FB, and the same sections were processed for ChAT immunohistochemistry. The arrows in D, G, J, and M indicate ChAT-positive/FB-positive cells. In E, H, K, and N, ChAT-immunoreactive neurons on the control side are shown to demonstrate the difference between the operated and intact sides of the same section (scale bars in A and B = 200 μm, those in C–N = 100 μm; av, avulsion; RI, reinnervation; RIL, riluzole; PN, peripheral nerve graft; ChAT, choline acetyltransferase).
FIG. 6.
Bar graph showing the percentages of regenerating (FB-labeled), and surviving (ChAT-positive) motoneurons compared to intact and contralateral motoneuron numbers. Note that in groups 2–4 there were visible differences between the numbers of surviving and reinnervating motoneurons. When the pooled data of the differences between reinnervating and surviving motoneurons were computed using Tukey's all pair-wise multiple comparison procedure, no groups were significantly different from each other (values are shown as mean ± standard error of the mean; FB, fast blue; ChAT, choline acetyltransferase).
Discussion
The results of this study confirm and expand our previous findings in the lumbar spinal cord (Nógrádi and Vrbová, 2001; Nógrádi et al., 2007), that injured adult motoneurons destined to die following avulsion of their axons in the ventral root can be rescued. Herewith we present evidence that large numbers of injured cervical motoneurons can be rescued with riluzole, a potent neuroprotective molecule. The cell death of these damaged motoneurons was also prevented by riluzole when the avulsed ventral root was not reimplanted, and in addition, these motoneurons were able to regenerate their axons into the vacated endoneural sheaths of the ventral root following reimplantation. However, significantly fewer, but still considerable, numbers of motoneurons could be rescued without riluzole treatment, when the avulsed root was reimplanted or a peripheral nerve graft was used to connect the injured motoneurons to a target muscle.
Riluzole rescues neurons unable to regenerate and supports the regeneration of surviving motoneurons
The present findings show that riluzole is a potent neuroprotective drug that is widely used in experimental ischemic and traumatic conditions to improve functional recovery following such insults to the central nervous system (Lang-Lazdunski et al., 1999; Schwartz and Fehlings, 2001, 2002). Its protective action might be due to the fact that riluzole inhibits presynaptic glutamate release, blocks Na+ and Ca++ channels, and transiently activates K+ channels. These actions all point toward the considerable reduction of the excitability of injured neurons. This may be the pharmacological background for our findings and those of others, that riluzole is able to rescue large proportions of injured motoneurons in vivo (Bergerot et al., 2004; Nógrádi and Vrbová, 2001; Nógrádi et al., 2007). In our earlier study we suggested that treatment with riluzole not only rescues the injured motoneurons from cell death, but maintains these cells in a condition that enables them to regenerate their axons given the right conditions (Nógrádi et al., 2007). The present study also provides evidence that the vast majority of motoneurons with avulsed axons die if they do not have the chance to regenerate their axons, but that these motoneurons can be rescued by riluzole, even if their axons cannot regenerate. When the riluzole-treated motoneurons had the opportunity to extend their axons into the reimplanted C7 ventral root, large numbers of the surviving motoneurons regenerated. Moreover, the results have shown that equal numbers of motoneurons survived (i.e., expressed ChAT in both experimental groups in which the animals received riluzole treatment), no matter whether they had a chance to regenerate or not (76 ± 3% and 76 ± 5% SEM for groups 4 and 5, respectively, expressed as percentages of the intact motoneuron pool; Fig. 6). However, it should be noted that these observations were made in experiments with different survival times (5 weeks versus 3 months), although motoneurons with avulsed axons die by the end of the second week after injury (Koliatsos et al., 1994; Nógrádi et al., 2007). We observed a minor (3%) difference in the number of fast blue- and ChAT-labeled motoneurons in group 5 animals, in which the animals with C7 avulsion injury received riluzole treatment. As both markers should be present in each surviving motoneuron in the C7 pool, it can be argued that in some neurons the tracer fast blue did not accumulate to an extent that it could be detected. On the other hand, our data suggest that riluzole is able to prevent cell death of injured motoneurons, and restores their cellular metabolic activity to an extent that they can readily regenerate if an appropriate target is provided. The present finding, that great numbers of surviving cholinergic cells are found in the ventral horn even 5 weeks after injury, suggest that given the appropriate stimulus such as having access to a fresh, recently axotomized nerve conduit, that these dormant motoneurons can regenerate. It is therefore clinically likely that immediate riluzole treatment, combined with a fresh conduit applied several weeks after the avulsion injury, may be effective in guiding considerable numbers of motor axons to denervated muscles after longer periods of time. The results of functional testing confirmed our morphological findings, indicating that morphologically-proven survival and regeneration of injured motoneurons resulted in functional reinnervation of forelimb muscles. The animals in groups 1 and 5 were not able to produce reinnervation, as targets were not available for surviving motoneurons. Animals that had their avulsed root reimplanted with or without riluzole treatment (groups 2 and 4) showed functional reinnervation of the forelimb to differing extents, indicating that riluzole treatment significantly improved the reinnervation of forelimb muscles by rescuing greater numbers of motoneurons. Moreover, the rapid and successful reinnervation induced by riluzole treatment (group 4) prevented contracture formation, while minimal contracture developed in some animals without riluzole treatment (group 2). However, in group 3 animals, in which reinnervating motoneurons were provided with an ectopic target, this kind of reinnervation did not allow functional reinnervation of the forelimb, and led to significant contracture formation. These data suggest that riluzole treatment is required to produce significant improvement in reinnervation of denervated muscles, and to prevent the long-term negative effects of denervation.
Peripheral nerve grafts and reimplanted ventral roots are equally efficient guides for reinnervating motoneurons
In this study two experimental models were used to determine the number of surviving and regenerating motoneurons into various conduits. While reimplantation of the avulsed ventral root (group 2) itself induced sufficient numbers of motoneurons to grow their axons into this conduit, the use of a sensory peripheral nerve (group 3) also achieved the same effect. Although more motoneurons sent their axons into the peripheral nerve implant, there was no significant difference between these two groups. These data suggest that if ventral root reimplantation is not possible, an autologous peripheral nerve can be used to guide the regenerating axons of motoneurons to the peripheral targets.
Another question that remains to be answered is why relatively more motoneurons in the present cervical reimplantation model regenerated into the reimplanted ventral root than in our earlier studies on the lumbar cord (L4 avulsion and reimplantation; Nógrádi and Vrbová, 1996, 2001; Nógrádi et al., 2007). One important difference between the two models lies in the reimplantation site of the avulsed ventral root. In the lumbar model, the implantation of the avulsed L4 ventral root was only possible into the caudal part of the L4 segment on the dorsolateral surface, due to the steep course of the root. On the other hand, cervical root 7 takes a nearly perpendicular course to the spinal cord, and the ventral part of the cord was not lying as deep in the vertebral canal as were the lumbar segments. Thus we could reimplant the avulsed root in a more ventral position into the affected segment than for the lumbar spinal cord, without compromising the integrity of the lateral motoneuron pools. It can be argued that the proximity of the reimplanted ventral root to the injured cervical motoneurons promoted their survival and regeneration. Accordingly, greater numbers of motoneurons had the chance to survive and repopulate the vacated C7 ventral root with their axons. Another possible explanation is that the regenerating cervical motor axons can reach the target muscles much earlier than those emerging from the lumbar cord, as postulated by Eggers and associates (2010). According to their hypothesis, which is supported by an elegant series of experiments, the regenerating lumbar motor axons of recovering motoneurons travel too long in the denervated peripheral nerves to reach their targets, and during this process the axons enter pre-degenerated nerves that prevent significant reinnervation of denervated hindlimb muscles (Gordon et al., 2008; Sulaiman and Gordon, 2000). These findings coincide with ours and those of others, namely that good reinnervation of denervated muscles is only possible when the regeneration of motoneurons is supported by neuroprotective molecules, such as riluzole (Bergerot et al., 2004; Nógrádi and Vrbová, 2001; Nógrádi et al., 2007).
Clinical aspects
The results reported here can be interpreted in the context of present therapeutic strategies for brachial plexus injuries. Patients with brachial plexus injuries are usually polytraumatized, and it takes several weeks or months after injury before the series of surgical interventions needed to restore the lost function of the plexus can be begun. During this time the majority of the motoneurons appear to die, and if the re-establishment of the original anatomical pathways is chosen as the preferred surgical strategy (ventral root reimplantation), only limited numbers of dormant motoneurons are available to reinnervate the target muscles. Therefore, in cases of severe brachial plexus injury, when reconnection of the cervical motoneurons with their original targets is the chosen surgical strategy (Carlstedt, 2008, 2009; Carlstedt et al., 1995, 2000), neuroprotective treatment with riluzole could be useful. The therapeutic advantages of riluzole are its relatively few side effects, and its potential for delayed treatment with the same efficacy as that seen with immediate application of the drug (Nógrádi et al., 2007). Although in the present and our earlier studies we used a considerably higher dose of riluzole than that used in clinical practice, we did not observe any side effects. This study provides evidence that injured motoneurons destined to die can be rescued by riluzole, even if they do not have a target available to reinnervate. When ventral root reconnection is chosen to reconstruct the integrity of the brachial plexus, it requires the implantation of lengthy peripheral nerves into the spinal cord close to the remaining motoneuron pools, followed by the coaptation of the distal end of the nerve to the ventral root that was avulsed during the traumatic event (Carlstedt, 2008, 2009). The data presented here also support the viability of this surgical strategy, as peripheral nerves implanted into the damaged cord were as effective as conduits as the reimplanted ventral root itself.
The results detailed here indicate that treatment with riluzole induces damaged cervical motoneurons to survive, even in the absence of the opportunity to regenerate into a reimplanted root. This appears to be a promising new treatment, and with refinements of the technique, one day it may be possible to restore function after various peripheral nerve injuries, even after long delays between injury and surgical intervention.
Acknowledgments
The authors are indebted to Mrs. I. Kovács for her technical assistance. We are grateful to the Sir Samuel Scott of Yews Trust, and The Wellcome Trust (grant no. 054131 and 069652), for their support, and to Tocris Cookson for kindly providing us with riluzole.
Author Disclosure Statement
No competing financial interests exist.
References
- Bensimon G. Lacomblez L. Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. A.L.S. Riluzole Study Group. N. Engl. J. Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Bergerot A. Shortland P.J. Anand P. Hunt S.P. Carlstedt T. Co-treatment with riluzole and GDNF is necessary for functional recovery after ventral root avulsion injury. Exp. Neurol. 2004;187:359–366. doi: 10.1016/j.expneurol.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Blits B. Carlstedt T.P. Ruitenberg M.J. De Winter F. Hermens W.T. Dijkhuizen P.A. Claasens J.W. Eggers R. van der Sluis R. Tenenbaum L. Boer G.J. Verhaagen J. Rescue and sprouting of motoneurons following ventral root avulsion and reimplantation combined with intraspinal adeno-associated viral vector-mediated expression of glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor. Exp. Neurol. 2004;189:303–316. doi: 10.1016/j.expneurol.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. Anand P. Hallin R. Misra P.V. Norén G. Seferlis T. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J. Neurosurg. (Spine 2) 2000;93:237–247. doi: 10.3171/spi.2000.93.2.0237. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. Grane P. Hallin R.G. Noren G. Return of function after spinal cord implantation of avulsed spinal nerve roots. Lancet. 1995;346:1323–1325. doi: 10.1016/s0140-6736(95)92342-x. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. Nerve root replantation. Neurosurg. Clin. N. Am. 2009;20:39–50. doi: 10.1016/j.nec.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. Root repair review: Basic science background and clinical outcome. Rest. Neurol. Neurosci. 2008;26:225–241. [PubMed] [Google Scholar]
- Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- Duprat F. Lesage F. Patel A.J. Fink M. Romey G. Lazdunski M. The Neuroprotective agent riluzole activates the two p domain K+ channels TREK-1 and TRAAK. Mol. Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- Eggers R. Tannemaat M.R. Ehlert E.M. Verhaagen J. A spatio-temporal analysis of motoneuron survival, axonal regeneration and neurotrophic factor expression after lumbar ventral root avulsion and implantation. Exp. Neurol. 2010;223:207–220. doi: 10.1016/j.expneurol.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Estevez A.G. Stutzmann J.-M. Barbeito L. Protective effect of riluzole on excitatory amino acid-mediated neurotoxicity in motoneuron-enriched cultures. Eur. J. Pharmacol. 1995;280:47–53. doi: 10.1016/0014-2999(95)00186-o. [DOI] [PubMed] [Google Scholar]
- Gordon P.H. Advances in clinical trials for amyotrophic lateral sclerosis. Curr. Neurol. Neurosci. Rep. 2005;5:48–54. doi: 10.1007/s11910-005-0023-2. [DOI] [PubMed] [Google Scholar]
- Gordon T. Brushart T.M. Chan K.M. Augmenting nerve regeneration with electrical stimulation. Neurol. Res. 2008;30:1012–1022. doi: 10.1179/174313208X362488. [DOI] [PubMed] [Google Scholar]
- Greensmith L. Vrbová G. Motoneuron survival: A functional approach. Trends Neurosci. 1996;19:450–455. doi: 10.1016/s0166-2236(96)20034-7. [DOI] [PubMed] [Google Scholar]
- Haninec P. Houstava L. Stejskal L. Dubovy P. Rescue of rat spinal motoneurons from avulsion-induced cell death by intrathecal administration of IGF-I and cerebrolysin. Ann. Anat. 2003;185:233–238. doi: 10.1016/S0940-9602(03)80030-4. [DOI] [PubMed] [Google Scholar]
- Hell R.C.R. Costa M.M.S. Goes A.M. Oliveira A.L.R. Local injection of BDNF producing mesenchymal stem cells increases neuronal survival and synaptic stability following ventral root avulsion. Neurobiol. Dis. 2009;33:290–300. doi: 10.1016/j.nbd.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Jivan S. Novikova L.N. Wiberg M. Novikov L.N. The effects of delayed nerve repair on neuronal survival and axonal regeneration after seventh cervical spinal nerve axotomy in adult rats. Exp. Brain. Res. 2006;170:245–254. doi: 10.1007/s00221-005-0207-7. [DOI] [PubMed] [Google Scholar]
- Koliatsos V.E. Price W.L. Pardo C.E. Price D.L. Ventral root avulsion: An experimental model of death of adult motor neurons. J. Comp. Neurol. 1994;342:35–44. doi: 10.1002/cne.903420105. [DOI] [PubMed] [Google Scholar]
- Lang-Lazdunski L. Heurteaux C. Vaillant N. Widmann C. Lazdunski M. Riluzole prevents ischemic spinal cord injury caused by aortic crossclamping. J. Thorac. Cardiovasc. Surg. 1999;117:881–889. doi: 10.1016/S0022-5223(99)70367-3. [DOI] [PubMed] [Google Scholar]
- Maltese A. Maugeri F. Drago F. Bucolo C. Simple determination of riluzole in rat brain by high-performance liquid chromatography and spectrophotometric detection. J. Chromatography B. 2005;817:331–334. doi: 10.1016/j.jchromb.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Meininger V. Dib M. Aubin F. Jourdain G. Zeisser P. The riluzole early access programme: Descriptive analysis of 844 patients in France. ALS/Riluzole Study Group III. J. Neurol. 1997;244S2:S22–S25. doi: 10.1007/BF03160577. [DOI] [PubMed] [Google Scholar]
- Mentis G.Z. Greensmith L. Vrbová G. Motoneurons destined to die are rescued by blocking N-methyl-D-aspartate receptors by MK-801. Neuroscience. 1993;54:283–285. doi: 10.1016/0306-4522(93)90253-c. [DOI] [PubMed] [Google Scholar]
- Nógrádi A. Vrbová G. Improved motor function of denervated rat hindlimb muscles by embryonic spinal cord grafts. Eur. J. Neurosci. 1996;8:2198–2203. doi: 10.1111/j.1460-9568.1996.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Nógrádi A. Vrbová G. The effect of riluzole treatment in rats on the survival of injured adult and grafted embryonic motoneurons. Eur. J. Neurosci. 2001;13:113–118. doi: 10.1046/j.0953-816x.2000.01362.x. [DOI] [PubMed] [Google Scholar]
- Nógrádi A. Szabó A. Pintér S. Vrbová G. Delayed riluzole treatment is able to rescue injured rat spinal motoneurons. Neuroscience. 2007;144:431–438. doi: 10.1016/j.neuroscience.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Novikova L. Novikov L. Kellerth J.O. Persistent neuronal labeling by retrograde fluorescent tracers: A comparison between fast blue, fluoro-gold and various dextran conjugates. J. Neurosci. Meth. 1997;74:9–15. doi: 10.1016/s0165-0270(97)02227-9. [DOI] [PubMed] [Google Scholar]
- Novikov L. Novikova L. Kellerth J.O. Brain-derived neurotrophic factor promotes survival and blocks nitric oxide synthase expression in adult rat spinal motoneurons after ventral root avulsion. Neurosci. Lett. 1995;200:45–48. doi: 10.1016/0304-3940(95)12078-i. [DOI] [PubMed] [Google Scholar]
- Schwartz G. Fehlings M.G. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: Improved behavioral and neuroanatomical recovery with riluzole. J. Neurosurg. (Spine 2) 2001;94:245–256. doi: 10.3171/spi.2001.94.2.0245. [DOI] [PubMed] [Google Scholar]
- Schwartz G. Fehlings M.G. Secondary injury mechanisms of spinal cord trauma: A novel therapeutic approach for the management of secondary pathophysiology with the sodium channel blocker riluzole. Prog. Brain Res. 2002;137:177–190. doi: 10.1016/s0079-6123(02)37016-x. [DOI] [PubMed] [Google Scholar]
- Su H. Zhang W. Guo J. Guo A. Yuan Q. Wu W. Neural progenitor cells enhance the survival and axonal regeneration of injured motoneurons after transplantation into the avulsed ventral horn of adult rats. J. Neurotrauma. 2009;26:67–80. doi: 10.1089/neu.2008.0656. [DOI] [PubMed] [Google Scholar]
- Sulaiman O.A. Gordon T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination and size. Glia. 2000;32:234–246. doi: 10.1002/1098-1136(200012)32:3<234::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Wahl F. Allix M. Plotkine M. Boulu R.G. Effect of riluzole on focal cerebral ischemia in rats. Eur. J. Pharmacol. 1993;230:209–214. doi: 10.1016/0014-2999(93)90804-q. [DOI] [PubMed] [Google Scholar]
- Watabe K. Ohashi T. Sakamoto T. Kawazoe Y. Takeshima T. Oyanagi K. Inoue K. Eto Y. Kim S.U. Rescue of lesioned adult rat spinal motoneurons by adenoviral gene transfer of glial cell line-derived neurotrophic factors. J. Neurosci. Res. 2000;60:511–519. doi: 10.1002/(SICI)1097-4547(20000515)60:4<511::AID-JNR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wu W. Li L. Yick L.W. Xie Y. Yang Y. Prevette D.M. Oppenheim R.W. GDNF and BDNF alter the expression of neuronal NOS, c-Jun, and p75 and prevent motoneuron death following spinal root avulsion in adult rats. J. Neurotrauma. 2003;20:603–612. doi: 10.1089/089771503767168528. [DOI] [PubMed] [Google Scholar]