Abstract
Obese older adults are particularly susceptible to sarcopenia and have a higher prevalence of disability than their peers of normal weight. Interventions to improve body composition in late life are crucial to maintaining independence. The main mechanisms underlying sarcopenia have not been determined conclusively, but chronic inflammation, apoptosis, and impaired mitochondrial function are believed to play important roles. It has yet to be determined whether impaired cellular quality control mechanisms contribute to this process. The objective of this study was to assess the effects of a 6-month weight loss program combined with moderate-intensity exercise on the cellular quality control mechanisms autophagy and ubiquitin-proteasome, as well as on inflammation, apoptosis, and mitochondrial function, in the skeletal muscle of older obese women. The intervention resulted in significant weight loss (8.0 ± 3.9 % vs. 0.4 ± 3.1% of baseline weight, p = 0.002) and improvements in walking speed (reduced time to walk 400 meters, − 20.4 ± 16% vs. − 2.5 ± 12%, p = 0.03). In the intervention group, we observed a three-fold increase in messenger RNA (mRNA) levels of the autophagy regulators LC3B, Atg7, and lysosome-associated membrane protein-2 (LAMP-2) compared to controls. Changes in mRNA levels of FoxO3A and its targets MuRF1, MAFBx, and BNIP3 were on average seven-fold higher in the intervention group compared to controls, but these differences were not statistically significant. Tumor necrosis factor-α (TNF-α) mRNA levels were elevated after the intervention, but we did not detect significant changes in the downstream apoptosis markers caspase 8 and 3. Mitochondrial biogenesis markers (PGC1α and TFAm) were increased by the intervention, but this was not accompanied by significant changes in mitochondrial complex content and activity. In conclusion, although exploratory in nature, this study is among the first to report the stimulation of cellular quality control mechanisms elicited by a weight loss and exercise program in older obese women.
Introduction
Over the past decade, the prevalence of obesity in older adults in the United States has doubled.1 Recent estimates indicate that more than 35% of Americans over the age of 60 are obese,1 and therefore are at increased risk for a number of health conditions, including cardiovascular disease (CVD), diabetes, hypertension, dyslipidemia, asthma, osteoarthritis, and several cancers.2–6 In addition, obese elderly are particularly susceptible to sarcopenia (the involuntary loss of muscle mass and function), and the combination of muscle loss and fat gain may act synergistically to increase the risk of adverse health outcomes, including disability7 and mortality.8 The pathogenesis of sarcopenic obesity is probably linked to the progressive decline in total energy expenditure resulting from the “physiologic” age-related muscle mass decline, decreased physical activity, and reduced basal metabolic rate, in the face of increased or stable caloric intake.9 The accumulation of adipose tissue, in turn, aggravates muscle wasting via increased secretion of catabolic cytokines (e.g., tumor necrosis factor-α [TNF-α] and interleukin-6 [IL-6]), reduced insulin sensitivity and decreased incretion of anabolic hormones (e.g., growth hormone [GH], insulin-like growth factor-1 [IGF-1], and testosterone). In addition, intermuscular fat infiltration may distort muscle architecture, further contributing to the loss of muscle strength.10
Lifestyle-based interventions may attenuate muscle loss and improve body composition in obese, older adults, but an optimal method has yet to be established. Caloric restriction (i.e., a caloric deficit of 500–1,000 kcal/day below the steady state) is generally required to achieve significant weight loss. Diet-induced weight loss, however, typically leads to a decrease of fat-free mass, a significant concern for older adults. In contrast, exercise interventions may increase muscle mass, improve muscle quality,11,12 and increase muscle protein synthesis,13 but rarely produce significant weight loss unless individuals engage in very high amounts of moderate or vigorous activity.14 Therefore, the challenge for clinicians and researchers working with overweight elderly is to design lifestyle-based interventions that can produce significant weight loss while avoiding (or limiting) the loss of fat-free mass.15
It is generally believed that weight loss improves physical function by indirectly benefiting the musculoskeletal system.2,16 Additional biological processes, however, may influence the relationship between weight loss and improvements in physical function in late life. The importance of cellular quality control, accomplished by the interplay between autophagy and the ubiquitin–proteasome system (UPS), has recently been reviewed.17,18 The decline in autophagic and proteasomal activity observed during aging has been proposed to contribute to different aspects of age-related phenotypes, such as neurodegeneration, osteoarthritis, declining liver and T cell function, as well as age-related muscle loss.19–23 It is noteworthy that caloric restriction and exercise increase the activity and effectiveness of autophagy and the UPS.24,25 Recently, we conducted a preclinical study in which autophagy was assessed in the skeletal muscle of young and old rats that were either fed ad libitum and sedentary or mildly calorie restricted and exercised. Markers of autophagy were decreased in the old, sedentary, ad libitum-fed rats, but were upregulated in the old, calorie-restricted, and exercising rats. This increase was accompanied by an attenuation of skeletal muscle apoptosis.22 However, the effects of a moderate weight loss plus exercise intervention in overweight older adults on cellular quality control mechanisms in skeletal muscle have yet to be elucidated.
Energy production by healthy mitochondria is equally as important as cellular quality control processes for upholding cellular function. However, mitochondrial function declines with age and dysfunctional mitochondria accumulate, causing further cellular damage (for review, see refs. 26 and 27). Therefore, autophagic removal and degradation of damaged mitochondria balanced with mitochondrial biogenesis are essential to prevent or attenuate the age-related decline in cellular function. In younger adults, exercise and caloric restriction stimulate mitochondrial biogenesis in the skeletal muscle.11,12 In addition, the number of cristae per mitochondrion as well as components of the mitochondrial electron transport chain increase.14 However, whether these same effects are observed in older adults following an exercise and diet intervention has yet to be determined. If similar biological changes occur in older adults, then improvements in mitochondrial function may be another mechanism through which weight loss plus exercise interventions may ameliorate physical function and reduce functional decline in the overweight elderly.
Therefore, the purpose of the present study was to assess in the skeletal muscle of older, overweight women the impact of a weight loss plus exercise intervention on markers of autophagy, components of the UPS, as well as markers of inflammation, apoptosis, and mitochondrial biogenesis and function. We hypothesized that the combination of dietary restriction and exercise would stimulate cellular quality control processes (specifically autophagy and components of the ubiquitin proteasome pathway), attenuate inflammation and apoptosis, and improve mitochondrial function. We also hypothesized that the physical performance of individuals participating in the weight loss plus exercise intervention would be improved.
Materials and Methods
Participants
This study was approved by the University of Florida's Institutional Review Board. Participants selected for this exploratory study were a subset of a larger-scale, randomized, controlled trial. Participants of this larger randomized controlled trial were community-dwelling, sedentary, overweight, African-American and Caucasian women with mild to moderate functional limitations. Eligibility requirements included: age between 55 and 79 years, body mass index (BMI) >29 kg/m2, sedentary lifestyle (defined as <20 min/week of aerobic exercise), and mild to moderate functional impairment, as defined by a Short Physical Performance Battery (SPPB) summary score of 4–10.28 Eligible individuals were excluded if their medical history, clinical examination, or laboratory results revealed any of the following conditions: Weight >300 pounds (136 kg), weight loss >10 pounds (4.5 kg) in the past 6 months, history of surgery for weight loss, hospitalization within the past 6 months, significant diseases likely to limit life span and/or increase risk of intervention (i.e., coronary heart disease; chronic or recurrent respiratory or gastrointestinal conditions; cancer [except nonmelanoma skin cancer] within 5 years or active cancer treatment, fasting blood glucose >110 mg/dL, resting blood pressure >160/90 mmHg), or musculoskeletal or neurological conditions that would preclude walking on a regular basis. Potential participants were also excluded if they reported taking any of the following medications: Antipsychotic agents, monoamine oxidase inhibitors, systemic corticosteroids, medications for human immunodeficiency virus (HIV) or tuberculosis (TB), or weight-loss drugs.
For the present exploratory study, a total of 13 participants were included who agreed to undergo a percutaneous muscle biopsy before (pre) and after (post) intervention. Of these participants, 7 had been randomized to the educational control group and 6 to the weight loss plus exercise (WL + E) group. Note that the muscle biopsies did not yield sufficient amounts of tissue to perform all analyses on all participants. The number of participants included in each of the analyses conducted is indicated in the respective graphs or tables.
Study design and interventions
The larger randomized controlled study, from which the subset for this exploratory study was drawn, comprised a single-blinded study, in which personnel responsible for testing were blinded to participant's assigned intervention group. Eligible participants were randomized to either the WL + E intervention or an educational control group. SAS's PROC PLAN was used for computerized randomization.29
The WL + E intervention targeted a 6% or greater weight loss through moderate reductions in energy intake (i.e., a reduction of 500–1,000 kcal/day) coupled with exercise sessions during which participants engaged in both aerobic activities (i.e., walking) and lower-body moderate intensity resistance training. Participants attended a weekly, group-based weight management session, led by a registered dietician and a doctoral student with training in behavior science. Each participant's caloric assignment represented an ∼750 kcal/day deficit from her estimated energy intake at baseline, determined through analysis of food records. Such caloric restriction was intended to promote weight loss at a rate of 1.5 pounds (0.7 kg)/week. In line with the American Heart Association's dietary recommendations,30 the diet contained 55%, 30%, and 15% of energy intake from carbohydrates, fats, and proteins, respectively. Food was self-selected under the supervision of a registered dietitian. Participants were instructed to complete daily food records, which they brought to each group session. During these group sessions, each participant's food record was reviewed by the registered dietitian, who provided specific suggestions about dietary changes to help participants achieve their calorie goal.
The exercise intervention consisted of aerobic, strength training, and flexibility exercises. Walking was the primary aerobic activity encouraged, but other forms of activity (e.g., stationary cycling) were also employed if participants were unable to walk due to injury. After the third week, participants were encouraged to meet a weekly walking goal of 150 min. Throughout the intervention, three supervised exercise sessions were provided each week. Blood pressure and heart rate were monitored before and after each exercise session, which was preceded by a brief warm up and followed by a cool-down period. Participants completed two 15-min bouts of walking during each session. Following the first walking bout, participants were guided to complete a set of five lower body exercises (i.e., wide leg squat, standing leg curl, knee extension, side hip raise, and toe stand) during a 15-min strength training routine. For each exercise, participants were encouraged to perform one set of 10 repetitions, rest for 1 min, and then perform a second set. Ankle weights were used to provide increasing levels of resistance. Following their second walking bout, participants engaged in a 5-min cool-down period, during which they completed a series of flexibility exercises.
Participants were gradually introduced to the intervention exercises, starting with lighter-intensity exercise and gradually increasing the intensity level over the first 2–3 weeks of the intervention. Following the initial adaptation phase, participants were instructed to begin walking at a moderate intensity level, as determined by the Borg Perceived Exertion scale.31 Participants were asked to walk at an intensity level of 13 (activity perception “somewhat hard”), and they were discouraged from exercising at levels above 15 (“hard”) or at level 11 (“fairly hard”) and below. For the strength-training component, participants were encouraged to complete each exercise at an intensity level of 15–16 (“hard”).
Participants in the educational control group were asked to maintain their usual eating and physical activity patterns and not to engage in any intentional effort aimed at losing weight for 6 months. During the intervention, participants in this group attended monthly health education lectures on topics relevant to older adults that were not related to weight loss, diet, or physical activity (e.g., skin protection, sleep hygiene). Following their 6-month assessments, participants in this group were offered the opportunity to receive the full 24-week WL + E intervention.
Anthropometric and physical function outcome measures
Body weight was measured in a fasting state and after voiding in the morning before the intervention began and again following its completion. Physical function was assessed by determining walking speed over 400 meters. Participants were asked to complete a standard walking course at their usual pace. During the walk, participants were permitted to stop but were not allowed to sit or receive help from others and were required to complete the course within 15 min.
Muscle biopsy
Biopsies were obtained from the vastus lateralis muscle percutaneously, before (pre) and after 6 months (post) of intervention. Muscle specimens were cleaned of blood and fat and immediately snap-frozen in liquid nitrogen for biochemical analyses. A separate piece of tissue was immersed in RNAlater (Ambion, Austin, TX) and frozen in liquid nitrogen for gene expression studies. The number of participants per group in the respective analysis is noted in the graphs or tables. Note that we could not perform all analyses on all samples due to tissue limitations from some participants.
Protein expression: Subcellular fractionation and immunoblotting
Subcellular fractionation of muscle samples was performed as previously described.32 Cytosolic fractions were used to detect cleaved caspase 3 (Millipore, Temecula, CA), and active caspase 8 (Abcam, Cambridge, MA). Cytochrome c oxidase subunit 4 (Cox4; Cell Signaling, Danvers, MA), Cytochrome c oxidase subunit 1 (Cox1; Mitosciences, Eugene, OR), and mitochondrial transcription factor A (TFAm; Santa Cruz Biotechnology, Santa Cruz, CA) were measured in the mitochondrial fraction, while endonuclease G (EndoG; Abcam, Cambridge, MA) and apoptosis-inducing factor (AIF; BD Biosciences, San Jose, CA) were assayed in both the mitochondrial and nuclear fraction. Electrophoresis and immunoblotting were performed as detailed elsewhere.32 Digital images were captured with an Alpha Innotech Fluorchem SP imager (Alpha Innotech, San Leandro, CA) and analyzed using the AlphaEase FC software (Alpha Innotech). Spot density of the target bands (arbitrary optical density [OD] units) was normalized to the total amount of protein loaded in each lane, as determined by densitometric analysis of the corresponding Ponceau S–stained membranes.33
Gene expression: Quantitative real-time PCR
Relative gene expression assessment was performed as previously described.22 TaqMan Universal PCR Master Mix (2x) (Roche, Branchburg, NJ), as well as 0.2 μM primers and TaqMan probe mix (Applied Biosystems, Foster City, CA) were used for each 25-μL reaction. Amplification of the following targets was achieved using the ABI 7300 real-time PCR system and universal cycling conditions: Forkhead box O3 (FOXO3; NM_201559), Atrogin1/MAFbx or muscle-specific F-box protein (MAFbx; NM_148177), Muscle RING-finger protein-1 (MuRF1; NM_032588), BCL2/adenovirus E1B 19 kDa. protein-interacting protein 3 (BNIP3; NM_004052), Microtubule-associated proteins 1A/1B light chain 3B (LC3B; NM_022818), lysosome-associated membrane protein-2 (LAMP-2); NM_013995), Atg7 (NM_006395), Tumor Necrosis Factor alpha (TNFα; NM_000594), TFAM (NM_003201), peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC1α; NM_013261), and 18S (X03205) as the housekeeping gene. All samples were examined in triplicate and negative controls (i.e., no template and no reverse transcriptase) included. Differences in expression of the target genes are determined by the 2−ΔΔCT method34 with 18S as the housekeeping gene.
Activity and content of mitochondrial respiratory complexes: Blue Native polyacrylamide gel electrophoresis
Mitochondrial extraction
Approximately 40 mg of muscle was homogenized in 1 mL of extraction buffer (20 mM 3-(N-morpholino)propanesulfonic acid [MOPS], 440 mM sucrose, 1 mM EDTA, and 0.5 mM phenylmethylsulfonyl fluoride [PMSF], pH 7.2 at 4°C) and centrifuged at 500 × g for 10 min. The supernatant was collected and centrifuged at 20,000 × g for 20 min. The resulting pellet was resuspended in 30 μL of buffer (1 M aminocaproic acid, 50 mM Bis-Tris and 0.2 mM PMSF, pH 7.0 at 4°C), and solubilization of the membranes was achieved with 30% n-dodecylmaltoside. The suspension was incubated on ice for 30 min with vortexing every 5 min and then centrifuged at 100,000 × g for 30 min (Beckmann Optima LE-80K ultracentrifuge, rotor 50.2 Ti, 28,300 rpm). The resulting supernatant was collected and stored at −80°C.
Native electrophoresis
Samples were prepared with 10% wt/vol Coomassie Brilliant Blue G-250 in aminocaproic acid (1 M) and glycerol. Approximately 40 μg of sample protein and 15 μg of control protein (bovine heart mitochondria, Mitosciences) were loaded onto 3–12% gradient precast gels (Invitrogen, Carlsbad, CA). Running buffers included ice-cold cathode buffer (50 mM Tricine, 15 mM Bis-Tris, pH 7.0 at 4°C) and anode buffer (50 mM Bis-Tris, pH 7.0 at 4°C). Finally 10 mL of cathode buffer containing 0.5% Coomassie Brilliant Blue G-250 was added and mixed with the cathode buffer in the gel apparatus. Electrophoresis was performed at 4°C as follows: 70 V for 30 min, then 170 V for approximately 1 hr. The cathode buffer was removed and replaced with cathode buffer without Coomassie stain. The gel was then run at 170 V for about 2 hr.
In-gel activity assay
Immediately following electrophoresis, in-gel enzymatic colorimetric reactions were performed for complexes I, II, IV, and V, according to Zerbetto et al.35 Activity buffers were as follows: complex I (nicotinamide adenine dinucleotide [NADH] dehydrogenase), 2 mM Tris · HCl, 0.1 mg/mL NADH and 2.5 mg/mL NBT (nitro blue tetrazolium), diluted 1:10 (wt/vol); complex II (succinate dehydrogenase), 1.5 mM phosphate buffer containing 4.5 mM EDTA, 10 mM potassium cyanide, 0.2 mM phenazine methosulfate, 84 mM sodium succinate, 10 mM nitroblue tetrazolium chloride; complex IV (cytochrome c oxidase, COX), 5 mg of 3.3 diaminobenzidine dissolved in 9 mL 50 mM phosphate buffer, 10 mg cytochrome c, 0.05 mL catalase (20 μg/mL), and 0.75 mg sucrose; complex V (adenosine triphosphate [ATP] synthase), 35 mM Tris, 270 mM glycine, 14 mM MgSO4, 0.2% Pb(NO3)2, and 8 mM ATP. All buffers were pH 7.4. Enzymatic reactions were allowed to continue until optimum color was observed. Images were captured with an Alpha Innotech Fluorchem SP imager (Alpha Innotech) and quantitation performed using the AlphaEase FC software (Alpha Innotech). Spot density of target bands (arbitrary OD units) was normalized to complex content.
Complex content
Western blot analysis was performed to determine complex content. Mitochondrial extracts were electrophoresed as described above. Membranes were then incubated overnight with primary antibodies against each of the complexes (1:200) (Mitosciences). Secondary antibody incubation, generation of the chemoluminescent signal, digital acquisition, and densitometry of the bands were performed as described above. The total amount of protein loaded in each lane was determined via Ponceau S staining and used as the loading control.
Statistical analysis
All data are reported as group mean ± standard error of the mean (SEM) (or ± standard deviation [SD], as noted in Tables 1–3). Protein and gene expression data are expressed as fold change from pre- to posttreatment. Statistical analysis was performed using SigmaPlot vs. 11.0 (Systat Software, Inc., Chicago, IL). Nonparametric Mann–Whitney Rank Sum Tests were used to compare groups, and the exact p value is reported. For all tests the significance level was set at p < 0.05.
Table 1.
Baseline Characteristics of Study Participants
| Educational control group (n = 7)Mean ± SD | WL + E group (n = 6) Mean ± SD | p valuea | |
|---|---|---|---|
| Age (years) | 67.1 ± 8.8 | 65.8 ± 6.2 | 0.765 |
| Weight (kg) | 90.5 ± 18.9 | 93.6 ± 14.5 | 0.753 |
| SPPB Score | 9.0 ± 1.2 | 9.2 ± 0.8 | 0.768 |
| 400-meter walk (sec) | 407.1 ± 77.8 | 449.3 ± 106.9 | 0.428 |
| Body mass index (kg/m2) | 37 ± 7.8 | 36.1 ± 4.7 | 0.80 |
p value based on group comparison: WL + E vs. educational control group.
WL + E, Weight loss plus exercise; SD, standard deviation; SPPB, Short Physical Performance Battery.28
Table 3.
Changes in Mitochondrial Complex (I, II, IV and V) Content (cont), Activity (act), and Activity/Content (a/c) Determined by BN-PAGE in Muscle Biopsy Samples from Subjects in the Weight Loss plus Exercise Intervention Group (I) and the Educational Control Group (C)
| |
Complex I |
Complex II |
Complex IV |
Complex V |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SEM) | p | Pa | Sa | Mean (SEM) | p | Pa | Sa | Mean (SEM) | p | Pa | Sa | Mean (SEM) | p | Pa | Sa | |
| cont (C) | 0.87 (0.11) | 0.97 (0.15) | 0.85 (0.13) | 1.04 (0.16) | ||||||||||||
| cont (I) | 1.42 (0.40) | 0.548 | 0.14 | 41 | 1.00 (0.12) | 1.0 | 0.05 | 1380 | 0.80 (0.18) | 1.0 | 0.05 | 717 | 1.42 (0.16) | 0.095 | 0.31 | 16 |
| act (C) | 0.87 (0.18) | 0.69 (0.18) | 0.69 (0.25) | 0.84 (0.18) | ||||||||||||
| act (I) | 0.85 (0.10) | 0.841 | 0.05 | 4254 | 0.97 (0.10) | 0.229 | 0.17 | 20 | 1.16 (0.34) | 0.40 | 0.11 | 35 | 2.60 (0.78) | 0.056 | 0.29 | 17 |
| a/c (C) | 1.04 (0.21) | 0.72 (0.16) | 0.77 (0.15) | 0.71 (0.09) | ||||||||||||
| a/c (I) | 0.86 (0.23) | 0.690 | 0.08 | 127 | 1.04 (0.22) | 0.229 | 0.12 | 32 | 1.84 (0.59) | 0.40 | 0.16 | 21 | 1.98 (0.66) | 0.056 | 0.21 | 24 |
| n(C;I) | 5;5 | 3;4 | 3;4 | 5;5 | ||||||||||||
Data represent fold-change of content, activity, and activity/content, respectively, between samples taken before and after 6 months of intervention. Data are displayed as mean, standard error of the mean (SEM), and p value. None of the changes between pre- and posttreatment differed significantly between the WL + E and the control group with the given sample size (significance level was set at p < 0.05).
Additionally listed are power (P) with α set at 0.05, and sample size (S) for power set at 0.8.
SEM, Standard error of the mean; P, power; S, sample size with P set at 0.8; C, educational control group; I, exercise intervention group; WL + E: weight loss plus exercise group.
Results
Descriptive characteristics of the study sample
Baseline characteristics of participants included in this study are depicted in Table 1. The 13 participants included in this exploratory study did not differ from the larger study population (N = 34; data not shown) on any demographic characteristic. Differences between controls and WL + E participants at baseline were not statistically significant for age, body weight, BMI, SPPB score, and time to walk 400 meters (Table 1).
Body weight and physical performance
After 6 months of treatment, participants in the WL + E group lost significantly more weight than those in the educational control group (8.0 ± 1.6% vs. 0.3 ± 1.3%; or 7.4 ± 1.4 kg vs. 0.6 ± 1.2 kg; p < 0.002). Participants in the WL + E intervention experienced an improvement in physical performance as determined by time to walk 400 meters (change in WL + E group: −20.4 ± 6.6% or −103 ± 44.4 sec, versus change in control group: −3.2 ± 4.9% or −16.0 ± 17.6 sec; p = 0.05).
Cellular quality control
Autophagy
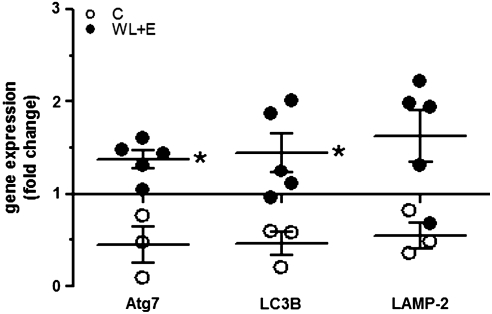
Transcript levels of the autophagy regulatory proteins Atg7 and LC3B, both involved in formation and expansion of the autophagosome, were both significantly increased by three-fold in the WL + E group compared to controls (Fig. 1; p = 0.036 for both). We also analyzed the transcript levels of LAMP-2, a protein that is essential for the fusion of the autophagosome with the lysosome. LAMP-2 transcript level was also upregulated by three-fold, but this change did not reach statistical significance (Fig. 1; p = 0.07).
FIG. 1.
Changes in gene expression of Atg7, LC3B, and LAMP-2 determined by quantitative real-time PCR in muscle biopsy samples from participants in the weight loss plus exercise (WL + E) intervention (WL + E, black circles) and the educational control group (open circles). Data represent the fold change of gene expression between samples from the same subject taken before and after 6 months of intervention. The line at y = 1 represents baseline. Individual data, group mean, and standard error of the mean (SEM) are displayed. (*) Significant difference of change between groups. Number of participants: Controls, 3; WL + E, 5. LAMP-2, lysosome-associated membrane protein-2.
FoxO3A transcription factor and its downstream targets
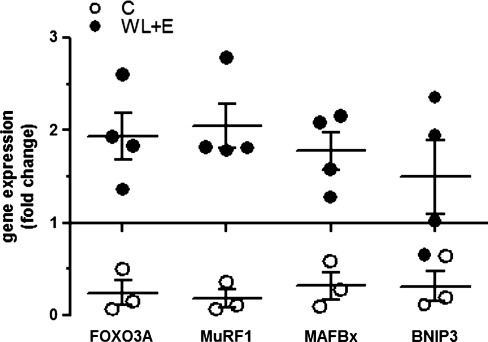
mRNA content of the transcription factor FoxO3A was eight-fold higher in the WL + E group after 6 months of intervention compared to the educational control group, but this difference did not reach statistical significance (Fig. 2; p = 0.057). Concomitantly, gene expression of FoxO3A downstream targets MuRF1, atrogin-1/MAFBx, and BNIP3 showed the same tendency (Fig. 2; 11-, 5.6-, 4.7-fold difference, respectively, between the WL + E and control group with p = 0.057 for MuRF1, MAFBx, and BNIP3).
FIG. 2.
Changes in gene expression of FoxO3A and its targets MuRF1, MAFbx, and BNIP3 determined by quantitative real-time PCR in muscle biopsy samples from participants in the weight loss plus exercise (WL + E) intervention (WL + E, black circles) and the educational control group (open circles). Data represent the fold change of gene expression between samples from the same subject taken before and after 6 months of intervention. The line at y = 1 represents baseline. Individual data, group mean and standard error of the mean (SEM) are displayed. (*) Significant difference of change between groups. Number of participants: Controls, 3; WL + E, 4–5.
Inflammation and apoptosis
The expression of TNF-α mRNA in the skeletal muscle was elevated after 6 months of WL + E treatment compared with controls (fold-change in WL + E group: 1.43 ± 0.37, vs. fold-change in control group: 0.40 ± 0.02; p = 0.036). We observed no statistically significant differences between groups in changes in protein expression levels of active caspase-8 and cleaved caspase-3 (Table 2). Protein contents of AIF and EndoG in either the mitochondrial or nuclear fraction were also not significantly affected by the WL + E intervention (Table 2).
Table 2.
Apoptosisa
| Marker | Mean (SEM) | n | p value | Pb | Sb |
|---|---|---|---|---|---|
| cl casp-3 (C) | 0.88 (0.20) | 7 | |||
| cl casp-3 (I) | 0.81 (0.10) | 7 | 0.902 | 0.054 | 1792 |
| cl casp-8 (C) | 1.15 (0.53) | 6 | |||
| cl casp-8 (I) | 0.39 (0.08) | 7 | 0.234 | 0.161 | 57 |
| mtAIF (C) | 1.75 (1.67) | 5 | |||
| mtAIF (I) | 0.79 (0.17) | 7 | 0.432 | 0.09 | 157 |
| nucAIF (C) | 0.87 (0.19) | 6 | |||
| nucAIF (I) | 0.54 (0.15) | 6 | 0.310 | 0.13 | 81 |
| mtEndoG (C) | 1.11 (0.22) | 6 | |||
| mtEndoG (I) | 1.26 (0.47) | 11 | 0.628 | 0.17 | 47 |
| nucEndoG (C) | 0.78 (0.17) | 6 | |||
| nucEndoG (I) | 1.09 (0.27) | 8 | 0.282 | 0.096 | 125 |
Data represent fold-change in expression between before and after 6 months of intervention. Data are displayed as mean, standard error of the mean (SEM), and p value. None of the changes between pre- and posttreatment differed significantly between the WL + E and the control group with the given sample size (significance level was set at p < 0.05).
Changes in protein expression of cleaved caspase 3 (cl casp3), activated caspase 8 (cl casp8) in the cytosolic fraction, and apoptosis-inducing factor (AIF) and endonuclease G (EndoG) in mitochondrial (mt) and nuclear (nuc) fractions in muscle biopsy samples from subjects in the weight loss plus exercise (WL + E) intervention group (I) and the educational control group (C).
Additionally listed are power (P) with α set at 0.05, and sample size (S) for power set at 0.8.
Mitochondrial biogenesis and function
Expression of PGC1α gene and TFAm gene and protein
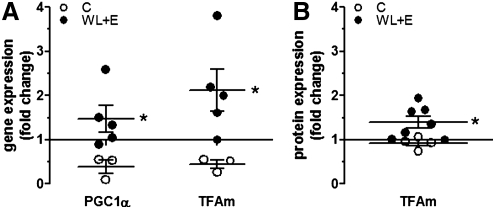
The weight loss plus exercise (WL + E) treatment significantly increased the gene expression of PGC-1α and TFAm (Fig. 3A; p = 0.036 for both), and protein levels of TFAm (Fig. 3B; p = 0.024).
FIG. 3.
Changes in gene expression of PGC1α (peroxisome proliferator-activated receptor gamma co-activator 1 alpha and TFAm (mitochondrial transcription factor A)) (A) and protein expression of TFAm (B) determined by quantitative real-time PCR and immunoblotting, respectively, in muscle biopsy samples from participants in the weight loss plus exercise (WL + E) intervention (WL + E, black circles) and the educational control group (open circles). Data represent the fold change of expression between samples from the same subject taken before and after 6 months of intervention. The line at y = 1 represents baseline. Individual data, group mean and standard error of the mean (SEM) are displayed. (*) Significant difference of change between groups. Number of participants for gene expression: Controls, 3; WL + E, 5. Number of participants for protein expression: controls, 4; WL + E, 7.
Mitochondrial respiratory complex content and activity
Blue-Native polyacrymamide gel electrophoresis (BN-PAGE) and in-gel enzyme activity assays were used to assess the content and activity of isolated mitochondrial respiratory complexes I, II, IV, and V. Results are summarized in Table 3. Neither content nor activity of any of the complexes was significantly affected by the WL + E intervention (for p values, see Table 3). We also measured the protein expression of subunits 1 and 4 of COX in the mitochondrial fraction, but we did not observe statistically significant changes following the WL + E treatment (COX-IV subunit 1, fold-change in WL + E group, 1.65 ± 0.59, vs. fold-change in control group, 1.21 ± 0.0.31; p = 0.54; COX-IV subunit 4, fold-change in WL + E group, 1.35 ± 0.47, vs. fold-change in control group, 2.07 ± 0.4, p = 0.18).
Discussion
The major findings of the present study are that 6-month WL + E intervention, which produced significant weight loss and improvements in physical performance, stimulated pathways of cellular quality control (autophagy, FOXO3A and its targets), increased myocyte TNF-α gene expression, and enhanced markers of mitochondrial biogenesis (PGC1α and TFAm). However, we did not observe a statistically significant effect of the WL + E intervention on function or content of mitochondrial complexes, or the expression of several apoptosis markers. Thus, our findings suggest that weight loss plus exercise regimens may stimulate specific cellular quality control mechanisms (i.e., autophagy, components of the UPS), without affecting other cellular components and pathways to the same degree (mitochondrial respiratory complexes, apoptosis).
Autophagy
Our data suggest that weight loss combined with exercise stimulated gene expression of autophagy-associated genes Atg7 and LC3B in skeletal muscle. Gene expression levels of BNIP3 and LAMP-2 were higher in the WL + E group when compared to the control group, but the differences between groups were not statistically significant. However, all observations for BNIP3 and all but one observation for LAMP-2 in the WL + E group were higher (i.e., greater change in gene expression) than the observations in the control group. The autophagic markers assessed in this study are essential for the autophagic process. Specifically, BCL2/adenovirus E1B 19-kDa protein-interacting protein 3 (BNIP3) and LC3B (microtubule-associated protein light chain) control early autophagosome formation36,37; autophagy protein 7 (Atg7) is required for the formation and expansion of the autophagosome,38,39 and lysosome-associated membrane protein 2 (LAMP-2) is essential for the efficient fusion of autophagosomes and lysosomes.40 Deficiencies in Atg7 or LAMP-2 are lethal or lead to tissue degeneration, emphasizing the importance of these regulators and the process of autophagy itself for cellular homeostasis.38,41–43
Our findings suggest that autophagy may play an important part in WL + E–induced improvement of physical performance through enhanced cellular quality control. Indeed, mice deficient in muscle-specific Atg7 demonstrate profound skeletal muscle atrophy, reduced muscle strength, and accumulation of abnormal mitochondria, protein aggregates, as well as increased oxidative stress and apoptosis.44 Our results are in agreement with our earlier findings,22 in which we observed increased expression of autophagy-regulatory proteins (Atg7 and LAMP-2) in the skeletal muscle of aged, calorie-restricted, and exercising rats compared with sedentary, ad libitum–fed controls. Importantly, the increase in these autophagy markers was concurrent with a mitigation of skeletal muscle apoptosis and oxidative damage.22
FoxO3A and downstream targets
Concomitant with the stimulation of the autophagic pathway, the WL + E treatment also enhanced the gene expression of the forkhead transcription factor FoxO3A and its downstream targets, the ubiquitin ligases muscle-specific RING dinger protein 1 (MuRF1) and atrogin-1, or muscle-specific F-box protein (MAFBx). The differences observed in the present study between groups did not reach statistical significance using the Mann–Whitney rank sum test. However, for all four response types (FoxO3A, MuRF1, MAFBx, BNIP3), all of the observations in the WL + E group were higher (i.e., greater change in gene expression) than in the control group. This is the most extreme case for the Mann–Whitney test, which is based on ranks; but given the small sample size, it was not possible to obtain an exact p value below 0.05. However, the distribution of data suggests that the observed difference might be real. The FoxO3A targets MuRF1 and MAFBx are part of the UPS and have been implicated as sensitive markers of muscle atrophy.45 However, Leger and co-authors46 proposed that an increase in the level of UPS components, as observed here in the WL + E group, could be a mechanism serving exercise-induced elevated muscle protein turnover. Interestingly, FoxO3A is also required for lysosomal-dependent protein breakdown in cell culture and in vivo, possibly through induction of BNIP3.37,47 Therefore, the observed increase in FoxO3A transcript levels, may be associated with the increase in autophagy markers.
Inflammation
The expression of the ubiquitin ligases MuRF1 and MAFbx can also be induced by the inflammatory marker TNF-α.48 We detected a modest but significant increase in skeletal muscle TNF-α transcript level in the WL + E group compared with the control group. This low level of local inflammation in response to exercise might have supported the elevated expression of the ubiquitin ligases. Plasma TNF-α levels were unaltered by the intervention (data not shown), suggesting local rather than systemic inflammation.
Apoptosis
TNF-α is also a central mediator orchestrating apoptotic signaling pathways.49 However, we did not observe a significant activation of apoptotic markers downstream of TNF-α, such as activated caspase 3 and 8. Moreover, no statistically significant differences in markers of mitochondria-mediated caspase-independent apoptosis (AIF and EndoG) were detected between groups. Therefore, it is unlikely that the observed increased muscle TNF-α expression initiated apoptotic signaling in the WL + E participants.
Mitochondria
We observed a significant increase in two markers of mitochondrial biogenesis, PGC-1α and TFAm, in the muscle samples from WL + E participants. Similarly, previous studies also found an exercise-induced stimulation of mitochondrial biogenesis in skeletal muscle.50,51 Interestingly, we did not observe a concomitant statistically significant increase in the content of mitochondrial electron transport chain complexes in the WL + E group. Similarly, Menshikova et al.52 found no change in mitochondrial (mt) DNA copy number, indicative of mitochondrial content, in the vastus lateralis muscle of middle-aged, overweight participants after a 4-month weight loss and walking intervention. One explanation for the seemingly unaltered levels of mitochondrial content after the WL + E treatment could be an increased mitochondrial turnover concomitant with balanced biogenesis and autophagic removal. This may have provided a mitochondrial quality check and resulted in a healthier population of mitochondria. Indeed, Menshikova and co-authors52 found an increase in electron transport chain activity as well as in cardiolipin content, which suggested an increase in both oxidative capacity and respiratory complex content per mitochondrion. On the contrary, when we assessed activity of individual, isolated mitochondrial complexes, we did not detect any statistically significant differences between the control and WL + E group.
Strengths and limitations
To the best of our knowledge, this is the first study to report the effect of a weight loss plus exercise intervention on cellular quality control mechanisms, namely autophagy and the UPS, in elderly, overweight women. All exercise sessions were conducted under direct supervision to ensure that participants exercised at the appropriate intensity and used proper exercise techniques. The results presented here provide preliminary insights into the potential cellular mechanisms driving the improvements in skeletal muscle function through diet-induced weight loss combined with exercise. These data can be used as a foundation for larger-scale studies to further explore the mechanisms influencing skeletal muscle function in aged human participants.
Some aspects of our study deserve further discussion. First and foremost, our study is of exploratory nature, evident by the small sample size. From the larger, randomized controlled trial population (N = 34), only 13 participants consented to a pre- and postintervention muscle biopsy for this exploratory study. Although the baseline characteristics of this subpopulation did not differ from the study population as a whole, the randomization might have been compromised. However, this subset was similar to the entire sample at baseline and demonstrated similar responses to the intervention with regard to weight loss and physical function. In addition, we were not able to obtain sufficient muscle tissue from all of the participants, which limited the scale of the analyses performed. The small sample size likely affected our ability to detect significant differences between groups for certain outcomes. Thus, we may not have observed statistically significant differences for some outcomes solely due to the sample size of the present study. Therefore, we calculated the number of participants needed to detect significant differences between groups with alpha set at 0.05 for all study parameters (see Tables 2 and 3). As displayed in Tables 2 and 3, a larger-scale study would more adequately test the effect of a WL + E intervention on some of our study parameters, including mitochondrial respiratory complexes and their content and activity. Importantly, by uncovering limitations with regard to sample and specimen size for biochemical analyses of human muscle, this exploratory study provides data that will facilitate the development and conduct of larger-scale trials investigating muscle biology in older human participants.
In summary, our pilot data suggest that the WL + E intervention stimulated cellular quality control mechanisms, which could have contributed to improved overall muscle health and function. The present exploratory investigation was not designed to unravel the more proximate causes of improved cellular quality control mechanisms in the skeletal muscle. Further in-depth investigations on a larger scale are warranted to substantiate or refute our findings and identify cellular mechanisms that are affected by lifestyle interventions aimed at improving physical function in the elderly.
Acknowledgments
The authors would like to express their appreciation to the participants, research associates, and administrative support staff that made it possible to complete this study. This research was supported by the National Institutes of Health–funded Claude D. Pepper Center (P30AG028740). Stephen Anton is supported by a K23 AT004251-01A2, an Early Career Investigator Award from the American Heart Association (09CRP2390173), and Thomas H. Maren Foundation.
References
- 1.Flegal KM. Carroll MD. Ogden CL. Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner RN. Body composition in healthy aging. Ann NY Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM. Ferrucci L. Pieper CF. Leveille SG. Markides KS. Ostir GV. Studenski S. Berkman LF. Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guralnik JM. LaCroix AZ. Abbott RD. Berkman LF. Satterfield S. Evans DA. Wallace RB. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–857. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 5.Lonergan ET. Krevans JR. A national agenda for research on aging. N Engl J Med. 1991;324:1825–1828. doi: 10.1056/NEJM199106203242527. [DOI] [PubMed] [Google Scholar]
- 6.Miller SL. Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12:487–491. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- 7.Stenholm S. Rantanen T. Heliovaara M. Koskinen S. The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc. 2008;56:462–469. doi: 10.1111/j.1532-5415.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- 8.Rantanen T. Harris T. Leveille SG. Visser M. Foley D. Masaki K. Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 9.Stenholm S. Harris TB. Rantanen T. Visser M. Kritchevsky SB. Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delmonico MJ. Harris TB. Visser M. Park SW. Conroy MB. Velasquez-Mieyer P. Boudreau R. Manini TM. Nevitt M. Newman AB. Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhihetty PJ. Irrcher I. Joseph AM. Ljubicic V. Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol. 2003;88:99–107. doi: 10.1113/eph8802505. [DOI] [PubMed] [Google Scholar]
- 12.Civitarese AE. Carling S. Heilbronn LK. Hulver MH. Ukropcova B. Deutsch WA. Smith SR. Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Short KR. Vittone JL. Bigelow ML. Proctor DN. Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 14.Menshikova EV. Ritov VB. Toledo FG. Ferrell RE. Goodpaster BH. Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab. 2005;288:E818–E825. doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- 15.Jensen GL. Friedmann JM. Obesity is associated with functional decline in community-dwelling rural older persons. J Am Geriatr Soc. 2002;50:918–923. doi: 10.1046/j.1532-5415.2002.50220.x. [DOI] [PubMed] [Google Scholar]
- 16.Cesari M. Kritchevsky SB. Baumgartner RN. Atkinson HH. Penninx BW. Lenchik L. Palla SL. Ambrosius WT. Tracy RP. Pahor M. Sarcopenia, obesity, and inflammation—results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 17.Koga H. Kaushik S. Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev. 2010 doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber TA. Reichert AS. Impaired quality control of mitochondria: Aging from a new perspective. Exp Gerontol. 2010;45:503–511. doi: 10.1016/j.exger.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Ponnappan U. Ubiquitin-proteasome pathway is compromised in CD45RO + and CD45RA + T lymphocyte subsets during aging. Exp Gerontol. 2002;37:359–367. doi: 10.1016/s0531-5565(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 20.Carames B. Taniguchi N. Otsuki S. Blanco FJ. Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling D. Salvaterra PM. Brain aging and Abeta(1-42) neurotoxicity converge via deterioration in autophagy-lysosomal system: A conditional Drosophila model linking Alzheimer's neurodegeneration with aging. Acta Neuropathol. 2010;121:183–191. doi: 10.1007/s00401-010-0772-0. [DOI] [PubMed] [Google Scholar]
- 22.Wohlgemuth SE. Seo AY. Marzetti E. Lees HA. Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45:138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C. Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selsby JT. Judge AR. Yimlamai T. Leeuwenburgh C. Dodd SL. Life long calorie restriction increases heat shock proteins and proteasome activity in soleus muscles of Fisher 344 rats. Exp Gerontol. 2005;40:37–42. doi: 10.1016/j.exger.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Wohlgemuth SE. Julian D. Akin DE. Fried J. Toscano K. Leeuwenburgh C. Dunn WA., Jr. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 26.Paradies G. Petrosillo G. Paradies V. Ruggiero FM. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic Biol Med. 2010;48:1286–1295. doi: 10.1016/j.freeradbiomed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guralnik JM. Simonsick EM. Ferrucci L. Glynn RJ. Berkman LF. Blazer DG. Scherr PA. Wallace RB. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 29.Spector P GJ. Sall J. Sarle W. The GLM procedure in SAS User's guide: Statistics Version 5. Cary, NC: 1985. pp. 433–507. S. Inst (ed) [Google Scholar]
- 30.Krauss RM. Eckel RH. Howard B. Appel LJ. Daniels SR. Deckelbaum RJ. Erdman JW., Jr. Kris-Etherton P. Goldberg IJ. Kotchen TA. Lichtenstein AH. Mitch WE. Mullis R. Robinson K. Wylie-Rosett J. St Jeor S. Suttie J. Tribble DL. Bazzarre TL. AHA Dietary Guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 31.Borg G. Physical performance, Perceived Exertion. Geerup; Lund, Sweden: 1962. [Google Scholar]
- 32.Marzetti E. Wohlgemuth SE. Lees HA. Chung HY. Giovannini S. Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129:542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzetti E. Carter CS. Wohlgemuth SE. Lees HA. Giovannini S. Anderson B. Quinn LS. Leeuwenburgh C. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Ageing Dev. 2009;130:272–280. doi: 10.1016/j.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Zerbetto E. Vergani L. Dabbeni-Sala F. Quantification of muscle mitochondrial oxidative phosphorylation enzymes via histochemical staining of blue native polyacrylamide gels. Electrophoresis. 1997;18:2059–2064. doi: 10.1002/elps.1150181131. [DOI] [PubMed] [Google Scholar]
- 36.Abeliovich H. Dunn WA., Jr. Kim J. Klionsky DJ. Dissection of autophagosome biogenesis into distinct nucleation and expansion steps. J Cell Biol. 2000;151:1025–1034. doi: 10.1083/jcb.151.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mammucari C. Milan G. Romanello V. Masiero E. Rudolf R. Del Piccolo P. Burden SJ. Di Lisi R. Sandri C. Zhao J. Goldberg AL. Schiaffino S. Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu M. Waguri S. Ueno T. Iwata J. Murata S. Tanida I. Ezaki J. Mizushima N. Ohsumi Y. Uchiyama Y. Kominami E. Tanaka K. Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizushima N. Noda T. Yoshimori T. Tanaka Y. Ishii T. George MD. Klionsky DJ. Ohsumi M. Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Polo RA. Boya P. Pauleau AL. Jalil A. Larochette N. Souquere S. Eskelinen EL. Pierron G. Saftig P. Kroemer G. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118(Pt 14):3091–3102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- 41.Hara T. Nakamura K. Matsui M. Yamamoto A. Nakahara Y. Suzuki-Migishima R. Yokoyama M. Mishima K. Saito I. Okano H. Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 42.Komatsu M. Waguri S. Chiba T. Murata S. Iwata J. Tanida I. Ueno T. Koike M. Uchiyama Y. Kominami E. Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 43.Nishino I. Fu J. Tanji K. Yamada T. Shimojo S. Koori T. Mora M. Riggs JE. Oh SJ. Koga Y. Sue CM. Yamamoto A. Murakami N. Shanske S. Byrne E. Bonilla E. Nonaka I. DiMauro S. Hirano M. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 44.Masiero E. Agatea L. Mammucari C. Blaauw B. Loro E. Komatsu M. Metzger D. Reggiani C. Schiaffino S. Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Bodine SC. Latres E. Baumhueter S. Lai VK. Nunez L. Clarke BA. Poueymirou WT. Panaro FJ. Na E. Dharmarajan K. Pan ZQ. Valenzuela DM. DeChiara TM. Stitt TN. Yancopoulos GD. Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 46.Leger B. Cartoni R. Praz M. Lamon S. Deriaz O. Crettenand A. Gobelet C. Rohmer P. Konzelmann M. Luthi F. Russell AP. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2006;576(Pt 3):923–933. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao J. Brault JJ. Schild A. Cao P. Sandri M. Schiaffino S. Lecker SH. Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Li YP. Chen Y. John J. Moylan J. Jin B. Mann DL. Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dirks AJ. Leeuwenburgh C. Tumor necrosis factor alpha signaling in skeletal muscle: effects of age and caloric restriction. J Nutr Biochem. 2006;17:501–508. doi: 10.1016/j.jnutbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Wright DC. Han DH. Garcia-Roves PM. Geiger PC. Jones TE. Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 51.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 52.Menshikova EV. Ritov VB. Ferrell RE. Azuma K. Goodpaster BH. Kelley DE. Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol. 2007;103:21–27. doi: 10.1152/japplphysiol.01228.2006. [DOI] [PubMed] [Google Scholar]