Abstract
This study sought to investigate whether normobaric hyperoxia (NH) improves brain oxygenation and brain metabolism in the early phase of severe and moderate traumatic brain injury (TBI) and whether this effect occurs uniformly in all TBI patients. Thirty patients (9 women and 21 men) with a median initial Glasgow Coma Score (GCS) of 6 (range, 3–12) were monitored using a brain microdialysis (MD) catheter with a brain tissue oxygen sensor (PtiO2) placed in the least-injured hemisphere. The inspired oxygen fraction was increased to 100% for 2 h. Patients were divided into two groups: Group 1: patients with baseline brain lactate ≤3 mmol/L and Group 2: patients with baseline brain lactate >3 mmol/L, and therefore increased anaerobic metabolism in the brain. In Group 1, no significant changes in brain metabolic parameters were found after hyperoxic challenge, whereas a significant increase in glucose and a decrease in the lactate–pyruvate ratio (LPR) were found in Group 2. In this latter group of patients, brain glucose increased on average by 17.9% (95% CI, +9.2% to +26.6%, p<0.001) and LPR decreased by 11.6% (95% CI, −16.2% to −6.9%, p<0.001). The results of our study show that moderate and severe TBI may induce metabolic alterations in the brain, even in macroscopically normal brain tissue. We observed that NH increased PaO2 and PtiO2 and significantly decreased LPR in patients in whom baseline brain lactate levels were increased, suggesting that NH improved the brain redox state. In patients with normal baseline brain lactate levels, we did not find any significant changes in the metabolic variables after NH. This suggests that the baseline metabolic state should be taken into account when applying NH to patients with TBI. This maneuver may only be effective in a specific group of patients.
Key words: anaerobic metabolism, brain tissue oxygen pressure, cerebral metabolism, hyperoxia, MD, mitochondrial dysfunction, NH, TBI
Introduction
The management of severe traumatic brain injury (TBI) is based on the assumption that for patients who arrive to the hospital alive, most of the brain damage is delayed (secondary injury) and that interventions directed toward preventing and treating secondary injuries early can make a difference both in survival and functional outcome (Park et al., 2008). However, despite of many clinical trials in the last decade targeting patients with moderate or severe TBI, at present there is no effective therapy in the treatment of these patients. It has been suggested that the heterogeneity of the TBI population is one of the most important factors in explaining the failure of all phase III neuroprotective drug trials despite these drugs having proved their efficacy in experimental studies (Saatman et al., 2008).
In the last decade, significant insight has been acquired about the underlying pathophysiology of secondary lesions and the neurochemical cascades that acute injury puts into motion. Ischemic and non-ischemic tissue hypoxia are highly prevalent after severe TBI (Sahuquillo et al., 2001). Direct assessment of brain tissue oxygen partial pressure (PtiO2) at the bedside with the use of polarographic Clark type electrodes allows the clinician to closely monitor PtiO2, a good indicator of the concentration of free oxygen (O2) available in the tissue and of the balance between O2 supply and consumption.
In TBI, the O2 flux from the capillaries to the cell and then to the mitochondria can be altered by diffusion alterations at many different stages (Menon et al., 2004; Sahuquillo et al., 2001; Siggaard-Andersen et al., 1995b). Under normal conditions, the diffusion distance of the O2 molecules from the capillaries to the cell is ∼ 60 μm, but this distance can increase after TBI as a result of the intra- and extracellular edema induced by the injury (Alves et al., 2004). Diffusion barriers to the cellular delivery of O2 can develop and persist after injury. Therefore, in some patients, it may theoretically be beneficial to increase O2 pressure and consequently the pressure drive that moves the O2 into the brain. Furthermore, evidence accumulated in the last decade suggests that mitochondrial dysfunction may play a very important role in the pathophysiology of TBI (Verweij et al., 1997, 2000). Dysfunctional mitochondria might explain the significant reduction in the cerebral metabolic rate of O2 (CMRO2) found after TBI and may be its cause. Hypothetically, if mitochondrial function is impaired in TBI, the higher tissue O2 pressure induced by hyperoxia might also improve O2 utilization.
Some authors introduced the hypothesis that the increase in O2 pressure above the levels necessary to saturate arterial hemoglobin (Hb) can produce an optimal O2 gradient that might improve mitochondrial oxygenation. Recent studies suggest that normobaric hyperoxia (FiO2 100%, 1 ATA), which is easily achieved in mechanically ventilated patients, could be used as a potential treatment to improve brain oxygenation and consequently the metabolic disorders resulting from TBI. However, significant controversy has been raised regarding this treatment because of contradictory clinical results (Magnoni et al., 2003; Menzel et al., 1997, 1999a Reinert et al., 2003; Tolias et al., 2004). This variability may be partly because of methodological differences in evaluating the metabolic response to the hyperoxic challenge or the clinical and pathophysiological heterogeneity among TBI patients. Furthermore, O2 dynamics show that an increase in PtiO2 does not necessarily mean better brain oxygenation or an improved cell's redox state and that hyperoxia does not improve metabolism when robust metabolic parameters, such as the lactate–pyruvate ratio (LPR), are evaluated after hyperoxic challenge (Menzel et al., 1999a; Tolias et al., 2004).
The aim of this study was to assess the metabolic response of the injured brain to normobaric hyperoxia (NH) using data obtained from brain microdialysis (MD) and PtiO2 monitoring. We also examined whether hyperoxia affects brain oxidative metabolism in all patients or only those in whom anaerobic metabolism is increased.
Methods
Patients
A prospective study was conducted on a cohort of patients aged 18 to 65 years who had sustained a severe or moderate TBI, had an abnormal admission CT scan, and required advanced neuromonitoring. Moderate/severe TBI was defined as an admission Glasgow Coma Scale (GCS) score ≤13 after resuscitation, which was determined when no effects from paralytic agents or sedation were present. The Traumatic Coma Data Bank (TCDB) classification (Marshall et al., 1991) was used to stratify patients. All patients in our study required intracranial pressure (ICP) monitoring and were treated following the Brain Trauma Foundation guidelines for TBI patients, which are incorporated into our routine management protocols (Bullock et al., 2000; Sahuquillo et al., 2002).
This study received institutional approval (protocol number PRTR-13798). The Institutional Review Board waived the need for informed consent, as transitory hyperoxic challenge is considered harmless and is routinely performed in most intensive care units to check the functioning of the O2 probe. Outcome was assessed by an independent evaluator at 6 months after injury using the Extended Glasgow Outcome Scale (GOSE). The scores obtained were dichotomized into two categories: bad outcome (GOSE: 1–4) and good outcome (GOSE: 5–8).
ICP monitoring
Continuous ICP monitoring was performed in all patients using an intraparenchymatous ICP sensor (Camino Model 110-4B, Integra Neurosciences, Plainsboro, NJ). Our complete ICP monitoring protocol in neurocritical patients has been previously published (Poca et al., 2002). Because of significant interhemispheric ICP gradients in focal lesions found in a previous study from our group (Sahuquillo et al., 1994), the ICP sensor was always implanted in the “worst” hemisphere —defined as the hemisphere with the most evident lesion— at the pre-coronal region, 11 cm from the nasion and 3 cm from the midline. End-hour ICP readings were recorded manually by nurses in charge of the patient.
Brain MD monitoring
A CMA-70 brain MD catheter with a 20 kDa cutoff membrane (CMA MD Stockholm, Sweden) was used in 15 patients and a CMA-71 catheter with a 100 kDa cutoff membrane (CMA Microdialysis Stockholm, Sweden) in 16 patients. Brain MD catheters were inserted in non-injured brain tissue following a methodology previously described (Poca et al., 2006). The position of the catheter was confirmed by a control CT scan in which the gold tip of the catheter was always visible. In addition, all patients were monitored with a MD catheter (CMA-60, CMA Microdialysis Stockholm, Sweden) inserted in the subcutaneous adipose tissue of the abdominal region and the data collected hourly was used as a systemic reference. Both catheters were perfused at a flow rate of 0.30 μl/min with sterile isotonic fluid containing 147 mmol/L Na+, 1.2 mmol/L CaCl2, 2.7 mmol/L KCl2, and 0.85 mmol/L MgCl2 using a CMA-106 pump (CMA Microdialysis Stockhlom, Sweden). The first microdialysate sample was always discarded and microvials were collected hourly by the nurse in charge of the patient. Bedside analysis for glucose, lactate, and pyruvate was routinely performed using the CMA-600 MD analyzer (CMA microdialysis, Stockholm, Sweden) and glutamate, urea, or glycerol was chosen as the fourth analyte, depending upon the patient.
Brain tissue O2 monitoring and hyperoxic test
Patients were monitored with a Licox® CMP system and a CC1.SB sensor (Integra Neurocare, Plainsboro, NJ). The brain tissue O2 probe was implanted near the MD catheter in non-injured brain tissue. Data obtained during the entire monitoring period were stored in a laptop and exported to a flat file for statistical analysis. The first 2 h were always discarded because of the running time defined for the Licox technology.
Hyperoxic challenge
The hyperoxic challenge was performed as soon as possible after implanting MD and PtiO2 probes. All patients received continuous sedation and analgesia by a continuous infusion of midazolam and morphine. Patients had to be hemodynamically stable and correctly sedated at the beginning of the hyperoxia test. Care was taken to maintain stable cerebral perfusion pressure, temperature, and PaCO2 to avoid confounding factors that can influence cerebral metabolism.
As a first step, we extracted a baseline arterial blood sample to determine the oxygenation status of the patient. If PaO2 was >170 mm Hg, ventilator and/or FiO2 settings were modified to obtain a PaO2 between 100 and 150 mm Hg and a PCO2 between 35 and 40 mm Hg. After these changes, at least 1 h was allowed to pass before conducting the test. Before hyperoxic challenge, systemic variables (systolic and diastolic blood pressure, heart rate, intracranial pressure, cerebral perfusion pressure), as well as brain and subcutaneous microdialysates (lactate, pyruvate, and glucose) and brain PtiO2 values, were recorded. Baseline blood samples were taken and the total hemoglobin content (ctHb), PaO2, and PCO2 were also recorded. As a second step, FiO2 was increased to 100% at 1 ATA and maintained at this level for 2 h, after which arterial blood samples were extracted again and the same physiological variables and brain and systemic microdialysates re-evaluated for lactate, pyruvate, and glucose levels. Immediately after completing the hyperoxic test, FiO2 was modified again to maintain a PaO2 of at least 100 mm Hg.
A single hyperoxic challenge was performed in all but one patient, who underwent it twice with an interval period of 1 day. The only patient in this cohort who did not present a minimum PtiO2 increase of 10 mm Hg after hyperoxia was considered a non-responder and excluded from the metabolic analysis.
Statistical analysis
Descriptive statistics were obtained for each variable. For continuous variables, summary statistics were the mean, median, range, and the standard deviation. Percentages and sample sizes were used to summarize categorical variables. To test whether or not data followed a normal distribution, the Shapiro-Wilks test and inverse probability plot was used. A paired t-test was used to compare variables before and after hyperoxic challenge when they followed a normal distribution. The Wilcoxon test was used to determine significant differences between pairs of variables that did not follow a normal distribution (i.e., baseline and post-hyperoxia samples).
The Spearman correlation coefficient was used to correlate two scale variables when they did not follow a normal distribution, whereas the Pearson linear correlation test was used for samples that followed a normal distribution.
To verify or refute our hypothesis, we checked whether or not the percentage change in any measurement was statistically significant. We also analyzed whether the changes in measurements were different in the group of patients with an increased lactate level and therefore with a potential increase in brain anaerobic metabolism. The anaerobic threshold has been defined in the literature to be within the 2.0–3.0 mmol/L range (Cesarini et al., 2002). We selected a 3.0 mmol/L threshold that assured that all selected patients had a significant increase in brain lactate. To perform this analysis, patients were divided into two groups according to baseline brain lactate levels. Group 1: patients with baseline brain lactate ≤3 mmol/L and Group 2: patients with brain lactate >3 mmol/L and therefore increased brain anaerobic metabolism.
A percentage change cannot be deduced where there is a constant term. Therefore, regression models were performed without the constant term. All variables followed a normal distribution and the assumption of the regression models was satisfied. In such regression model it is difficult to adjust for measurement error. However, the change in coefficient from a 4% measurement error as described by the CMA 600 manual (Carlsson, 2008) leads to a change in the CI by±0.35% of the coefficient or a increasing of the 95% CI width by 0.003 either way. This was the case for brain lactate, which had the highest measurement error of 4% (Carlsson, 2008). Therefore, little is lost by not adjusting for the measurement error and also none of the p values changed in significance from such an adjustment.
For regression models without a constant, the null hypothesis of no percentage change was that the coefficient equalled 1. Therefore, for any unit increase in baseline there was a unit increase in final value for any variable. A coefficient of 1.1 would mean that there is a 10% increase in baseline, whereas a coefficient of 0.9 means that there is a 10% decrease in baseline. A p≤0.05 was the threshold to reject the null hypothesis that there was no change in baseline. Statistical analyses were performed in Stata 11.0 (StataCorp LP College Station, TX).
Results
Thirty patients (9 women and 21 men) with a median initial GCS of 6 (range, 3–12) were included in the study. Median age of the group was 27 (range, 17–59 years). We included 18 patients with a diffuse type II injury, 5 patients with a diffuse type III injury, 4 patients with a diffuse type IV injury, and 3 patients with an evacuated mass lesion. The median time from TBI until testing was 46 h (range, 15–126). Thirty-one hyperoxia tests were performed. In one test (1.9%) a significant increase in PaO2 (ΔPaO2=339.7 mm Hg) did not induce any increase in PtiO2, and therefore the patient was excluded for metabolic analysis.
Of the 29 patients included, mortality rate at 6 months was 20% (6 patients). Two patients were lost for long-term follow-up and therefore it was not possible to assess the 6-month GOSE. Of the remaining 27 patients, 16 presented a good outcome and 11 presented a bad outcome.
Systemic hemodynamic and intracranial parameters
Summary measures are shown in Table 1. Mean baseline ICP was 9.9±5.3 mm Hg and after hyperoxia treatment a non-significant increase to 10.4±6.3 mm Hg was observed. As expected most O2 parameters changed after hyperoxia (PtiO2, PaO2). A statistically significant but modest increase in the arterial content of O2 (CaO2) was obtained with hyperoxia (Table 1). Mean arterial blood pressure (MABP) at baseline was 85.2±10.5 mm Hg and did not change after hyperoxia treatment (Table 1). Baseline cerebral perfusion pressure (CPP) calculated as the difference between MABP and ICP was 75.4±12.2 mm Hg and did not change after hyperoxia challenge. Heart rate was 73.3±18.7 b.p.m. at baseline and decreased to 69.0±16.0 after hyperoxia. This moderate change did not reach statistical significance.
Table 1.
Intracranial and Extracranial Parameters Before and After Hyperoxic Challenge
| Variable | Baseline | Hyperoxia | P value |
|---|---|---|---|
| HR (mm Hg) | 73.3±18.7 | 69.0±16.0 | NS |
| SBP (mm Hg) | 131.7±16.6 | 128.8±17.0 | NS |
| DBP (mm Hg) | 62.0±9.3 | 63.0±11.6 | NS |
| MABP (mm Hg) | 85.2±10.5 | 84.9±12.5 | NS |
| Hb (g/dl) | 11.7±1.6 | 11.6±1.5 | NS |
| PaCO2 (mm Hg) | 35.6±4.4 | 36.0±4.5 | NS |
| PaO2 (mm Hg) | 121.0±29.4 | 446.2±124.9 | p<0.001* |
| SaO2 (%) | 98.5±0.7 | 99.5±0.2 | P<0.001 |
| CaO2 (ml/100 ml) | 15.7±2.3 | 17.5±2.2 | p<0.001* |
| PtiO2 (mm Hg) | 26.9±9.9 | 97.7±42.0 | p<0.001* |
Asterisks show statistically significant changes.
HR, heart rate; NS, not statistically significant; SBP, systolic blood pressure; DBP, diastolic blood pressure; MABP, mean arterial blood pressure.
Changes in systemic and brain oxygenation
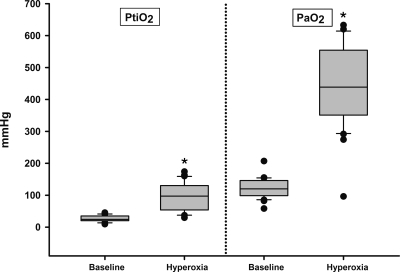
PaO2 increased significantly from 121.0±29.4 mm Hg at baseline to 446.2±124.9 mm Hg at the end of the hyperoxic challenge (paired t-test, p<0.001). In only 4 of the 30 tests (13.3%), basal PtiO2 was below the threshold of tissue hypoxia (<15 mm Hg). PtiO2 at baseline was 26.9±9.9 mm Hg and increased after hyperoxic challenge to 97.7±42.0 mm Hg (paired t-test, p<0.001). The mean increase in PaO2 (ΔPaO2) was 322.8±112.0 mm Hg (Fig. 1). This ΔPaO2 induced a ΔPtiO2 of 70.7±38.0 mm Hg (Fig. 1).
FIG. 1.
PtiO2 and PaO2 values (expressed as mmHg) at baseline and at the end of the hyperoxic challenge. Clinically statistical differences are represented with an asterisk.
MD data
Baseline and hyperoxia values for each metabolite before and after the hyperoxic challenge, as well as the statistical significance of the changes, are shown in Table 2. When the whole group was analyzed, some statistically significant but modest increase in brain glucose (6.85% increase, 95% CI, 0.4% to 13.3%) and a 9.24% reduction in brain LPR (95% CI, −15.4% to −3.4%) was observed.
Table 2.
Brain and Subcutaneous Microdialysis Data in the Whole Group
| Metabolites | Baseline | Hyperoxia | P value |
|---|---|---|---|
| Brain tissue | |||
| Glucose (mmol/L) | 1.2 (0.02–9.16) | 1.7 (0.03–10.74) | 0.037* |
| Lactate (mmol/L) | 2.8 (1.23–10.80) | 3.2 (1.25–10.5) | 0.59 |
| Pyruvate (mmol/L) | 0.12 (0.05–0.36) | 0.12 (0.06–0.38) | 0.42 |
| Lactate/Pyruvate ratio | 23.2 (4.8–116) | 23.7 (11.3–100) | 0.005* |
| Subcutaneous | |||
| Glucose (mmol/L) | 5.8 (1.71–11.5) | 6.0 (1.77–10.8) | 0.69 |
| Lactate (mmol/L) | 1.6 (0.4–6.2) | 1.4 (0.3–3.6) | <0.001* |
| Pyruvate (mmol/L) | 0.12 (0.00–0.29) | 0.09 (0.00–0.23) | 0.002* |
| Lactate/Pyruvate ratio | 13.9 (9.0–63.9) | 13.8 (5.8–91.0) | 0.80 |
Asterisks show statistically significant changes.
Significant changes both in lactate and pyruvate in the subcutaneous tissue were observed in the whole group. However, because both variables decreased (Table 2) the LPR was not affected.
Subgroup analysis
Intracranial parameters at baseline and after hyperoxia of the two groups (1 and 2) are shown in Table 3. Sixteen patients were included in Group 1 (53%) and 13 were included in Group 2 (47%). Patients in the anaerobic group did not have any detectable alteration in either brain or systemic variables that might have explained the increased baseline lactate levels. There were no significant differences between the groups in terms of the severity of injury (GCS and CT Scan classification) and baseline PtiO2, ICP, CPP, MABP, and PCO2 (Table 4). In four cases in Group 1, baseline PtiO2 was <15 mm Hg (25%) whereas no patient in the anaerobic group had a PtiO2 below the 15 mm Hg threshold.
Table 3.
Brain Microdialysis Data in Both Groups
| Lactate baseline | Lactate hyperoxia | Pyruvate baseline | Pyruvate hyperoxia | L/P baseline | L/P hyperoxia | Glucose baseline | Glucose hyperoxia | |
|---|---|---|---|---|---|---|---|---|
| Group 1 n=16 |
1.7 (1.2–2.9) | 1.8 (1.2–5.9) | 0.1 (0.1–0.2) | 0.1 (0.1–0.3) | 16.5 (4.8–36.3) | 18.2 (11.3–37.8) | 2.5 (0.6–5.7) | 2.4 (0.5–5.5) |
| Group 2 n=13 |
5.1 (3.1–10.8) | 4.7 (2.8–10.5) | 0.2 (0.1–0.4) | 0.2 (0.1–0.4) | 30.1 (15.5–115.7) | 25.3 (13.1–99.9)* | 1 (0.0–9.2) | 1.4 (0.0–10.7)* |
Asterisks show statistically significant changes.
Table 4.
Intracranial and Extracranial Parameters in Both Groups Before and After Hyperoxic Challenge
| PtiO2 baseline | Hyperoxia | PaO2 | Hyperoxia | PCaO2 baseline | Hyperoxia | ICP baseline | Hyperoxia | MABP baseline | Hyperoxia | |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 n=16 |
20.4 (9.5–45.4) | 110.4 (29.6–174.0)* | 121.0 (82.2–207.0) | 447.8 (274.4–620.1)* | 35.9 (27.1–44.2) | 36.7 (27.3–41.4) | 8.5 (3.0–19.0) | 9.5 (−3–23) | 86.8 (65.7–105) | 86.3 (64.7–108.7) |
| Group 2 n=13 |
29.7 (20.7–41.2) | 94.3 (45–160)* | 107.8 (58.2–155.4) | 446.2 (96.3–633.0)* | 36.1 (27.3–45.5) | 35.5 (27.7–49.6) | 8.0 (4–18) | 11.0 (5.0–21.0)* | 86 (68.7–103.0) | 87 (61.3–109.3) |
Asterisks show statistically significant changes.
In Group 1, no significant changes in the brain metabolic parameters were found after hyperoxic challenge. However in the group with increased anaerobic metabolism (baseline lactate levels >3 mmol/L) a significant increase in glucose and decrease in the LPR was found. In this subgroup, brain glucose increased on average by 17.9% (95%CI, +9.2% to +26.6%, p<0.001) and there was a 11.6% decrease in LPR (95% CI, −16.2% to −6.9%, p<0.001).
We did not find any statistically significant difference in GOSE between the two groups. Of the 13 patients who presented a bad outcome (GOSE scores 1–4), 5 patients had lactate levels >3 mmol/L at baseline and 8 patients had lactate levels ≤3 mmol/L. Of the 16 patients who presented good outcome (GOSE scores 5–8), 10 patients had lactate levels >3 mmol/L at baseline and 6 patients had lactate levels ≤3 mmol/L.
Discussion
Neurocritical care in moderate and severe TBI patients is aimed at restoring and maintaining normal physiology. This enables the anatomic and functional recovery of damaged nervous cells, as well as the prevention of intracranial and extracranial secondary complications. Therefore, it is crucial to maintain correct cerebral perfusion, maintain an adequate cerebral blood flow (CBF), and supply the necessary metabolic substrates in the treatment of these patients. To reach these goals, it is essential to monitor reliable variables in order to detect alterations in brain metabolism as early as possible and to modify the therapeutic management.
The mammalian brain is a highly aerobic organ that requires a sufficient supply of O2 to the mitochondria to maintain adequate ATP production. The supply of O2 to the brain is multifactorial and depends upon CBF, the ability of the blood to transport O2, Hb O2 affinity, Hb characteristics, O2 diffusive conductance from arterial capillaries to the cells, and the arterial O2 pressure gradient between the capillaries and the intracellular compartment (Massabuau, 2001). Under normal oxygenation conditions, the two most important factors influencing cerebral O2 delivery are Hb concentration and CBF (Diringer, 2008).
When Hb is correctly saturated, NH increases the fraction of the O2 dissolved in plasma only and therefore does not significantly modify the total arterial content of O2. Under normal oxygen tension, the dissolved O2 in plasma is negligible and NH increases the amount of dissolved O2 by only 0.5–1 vol% (Diringer, 2008). NH, defined as O2 delivered at fractions of inspired O2 (FiO2) between 40 and 100% at atmospheric pressure (1 ATA), has been proposed as a simple and effective maneuver to increase brain PtiO2 and a potential alternative to hyperbaric hyperoxia (HBO2), which is very difficult to implement and is only feasible in centers with hyperbaric chambers.
The main purpose of our study was to analyze the effects of 2 h of NH in macroscopically normal brain tissue of 30 patients with TBI who were mechanically ventilated. Our data confirm the results of previous studies (Magnoni et al., 2003; Menzel et al., 1999a, b; Reinert et al., 2003; Tolias et al., 2004) showing that NH induces a significant increase in both PaO2 and brain PtiO2.
Despite the lack of available data in humans, several experimental studies have demonstrated that O2 is tightly regulated in the mammalian brain. In the last decade, evidence has accumulated to show that PtiO2 is maintained at an almost constant level in mammalian organs independently of marked changes in PaO2 (Forgue et al., 2001; Massabuau 2001, 2003). These studies have shown that increases in FiO2 and secondary increases in PaO2 promote a moderate increase in PtiO2 in any organ, which is quickly followed by a plateau. This strategy has been called the “low blood PaO2 strategy” and it is considered a phylogenetic evolutionary mechanism directed to protect organs from potential O2 toxicity (Massabuau, 2001).
The significant increases in brain PtiO2 observed both in our series and in all previously published TBI studies may be a consequence of the loss of O2 homeostatic mechanisms in the brain. However, even if this increase in PtiO2 were considered an “abnormal response”, it could be potentially beneficial if followed by an improvement in brain metabolism and an increase in brain O2 consumption. Nortje et al. have shown that NH can enhance oxidative metabolism despite the lack of significant changes in hemodynamic and metabolic variables. In their study, increased cerebral metabolic rate of oxygen (CMRO2) in brain tissue at risk (defined as a CMRO2<36 μl/100 ml/min) was observed after hyperoxia, despite the lack of changes in any brain metabolic or hemodynamic variables (Nortje et al., 2008).
Hyperoxia-induced changes in brain metabolism
All but four patients included in our study had brain tissue hypoxia and mean baseline PtiO2 levels >20 mm Hg. The remaining four patients presented baseline PtiO2 levels <15 mm Hg. Our cohort is comparable with other studies evaluating NH in TBI in which brain basal PtiO2 was within the normal range before inducing NH in most cases (Magnoni et al., 2003; Menzel et al., 1999a; Nortje et al., 2008; Reinert et al., 2003; Tolias et al., 2004).
We confirmed that even macroscopically normal brain tissue with normal PtiO2 and no detectable intracranial (normal ICP and CPP) or systemic insults (normal MABP and PaO2) may present severe metabolic alterations and high lactate levels. In our cohort, 13 patients (45%) had brain lactate levels >3 mmol/L despite the probe placement being in macroscopically uninjured brain tissue. In a recent MD study conducted by Nelson et al. on TBI patients, highly perturbed energy metabolism was found to be very prevalent (Nelson et al., 2011). However, the relationships between MD and ICP and/or CPP were found to be very weak and did not explain the observed energetic disturbances, suggesting that factors other than pressure and/or flow variables may be the main cause of perturbations in these patients (Nelson et al., 2011).
Marmarou's group clearly showed that patients with TBI may have profound neurochemical damage using MRI spectroscopy, even when patients' brain tissue had a normal appearance (Signoretti et al., 2008). In addition, Vespa et al. showed that TBI leads to a state of persistent metabolic impairment, not necessarily resulting from brain ischemia (Vespa et al., 2003). These authors suggested that an increase in brain lactate levels may indicate hyperglycolysis, a state of cerebral hypermetabolism directed toward restoring perturbed ion homeostasis after injury. The reason behind hypermetabolism following TBI is that oxidative phosphorylation normally runs at near maximal capacity and, consequently, an increased energy demand is supplied by increasing glycolysis (Cesarini et al., 2002). Hyperglycolysis has also been observed in spontaneous subarachnoid hemorrhage (SAH). Cesarini et al. showed an increase in brain lactate levels in some patients with spontaneous SAH, together with increased levels of pyruvate and a normal LPR. This pattern was interpreted as hyperglycolysis, not ischemia, and was found to be a predictor of good neurological outcome (Cesarini et al., 2002).
Vespa et al. found a low incidence of brain ischemia when PET scanning was performed within 24 h after TBI. The findings of this study significantly differed from previous studies in which an increased prevalence of brain ischemia was observed (Bouma, et al., 1991a, b, 1992; Graham, et al., 1978, 1989). In another study from the same group, sustained, increased LPR was found in the pericontusional tissue not related to a reduced cerebral perfusion pressure (Vespa et al., 2007). Furthermore, Bergsneider et al. showed hyperglycolysis in 56% of severe TBI patients who were studied using PET scanning within 1 week after injury, even when sufficient brain oxygen was observed (Bergsneider et al., 1997).
In our study, the brain metabolic parameter analysis of the whole group of patients showed that LPR mildly decreased from 23.7 and glucose increased from 1.2 to 1.7 after hyperoxic challenge (p<0.05). However, these small changes were clinically irrelevant and a potential consequence of multiple statistical comparisons. Interestingly, lactate and pyruvate significantly decreased in the microdialysates of the subcutaneous tissue, suggesting that the two compartments reacted to NH independently. However, subcutaneous LPR remained unmodified and therefore the changes in lactate and pyruvate did not imply that the redox state of the tissue was modified.
In order to determine whether or not patients with different metabolic profiles react in the same way to NH, we analyzed metabolic data according to baseline brain lactate levels. Patients were classified as anaerobic (lactate >3 mmol/L) or not anaerobic (≤3 mmol/L). There were no significant differences between both groups at baseline in terms of the severity of injury (GCS and CT scan classification) or baseline PtiO2, ICP, CPP, MABP, and PCO2 levels. A significant number of patients in our first group (lactate levels <3 mmol/L) had low PtiO2. In another cohort of 31 patients in whom both brain PtiO2 and lactate were measured (2938 hourly readings analyzed), we found the same pattern (low PtiO2<15 mmHg and lactate ≤3 mmol/L) in 192 readings, or 6.5% of the total samples analyzed (unpublished results). There are at least two reasons why hypoxia does not induce a lactate increase, but both are speculative and impossible to confirm in our small series. The first reason is that a significant reduction in glucose levels may coexist with hypoxia; consequently, despite a significant reduction in oxygen, lactate cannot increase because of the lack of substrate. The second possible explanation is that brain metabolism, and therefore CMRO2 might be very depressed in these patients and therefore the low levels of oxygen are enough to maintain mitochondrial activity. An in-depth analysis of a larger series of patients and experimental models in cell cultures are necessary to clarify the pathophysiology of this specific metabolic profile.
In patients with lactate levels >3 mmol/L, we found a significant decrease in LPR after 2 h of hyperoxic challenge, suggesting that NH significantly improved brain metabolism and the brain redox state. Therefore, in these patients, NH may promote the conversion of lactate to pyruvate, which would re-enter the Krebs cycle as acetyl-coenzyme-A and would be further oxidized to CO2 and H2O in the mitochondria. In addition, NH also induced a significant increase in glucose levels in our subgroup of anaerobic patients, suggesting a shift from glycolytic behavior to a predominantly aerobic pattern.
Our data suggest that the response to hyperoxia could be influenced by the metabolic state at baseline. These preliminary findings could explain why different results were obtained in previous NH studies in which patients with different metabolic profiles had been grouped together. In the last decade, there is increasing experimental and indirect clinical evidence suggesting that severe TBI may be associated with mitochondrial dysfunction. In the presence of adequate oxygenation, the only available biomarkers suggesting mitochondrial dysfunction to the clinician are lactate and the LPR. In this theoretical scenario of mitochondrial impairment, improving PtiO2 and therefore the force that moves oxygen into its natural sink, may be beneficial. A recent single-center randomized clinical trial comparing hyperbaric and normobaric O2 in patients with severe TBI was conducted by Rockswold et al. (2010). The main goal of this study was to test the effects of hyperoxia and its duration on surrogate variables that closely correlate with clinical outcome (Rockswold et al., 2010).The trial was designed as a three-treatment comparison of hyperbaric O2 (HBO2), NH, and the existing standard of care. The NH treatment consisted of 100% FiO2 at 1.0 ATA maintained over 3 h. Twenty-six patients were randomized to the HBO2 group, 21 to the NH group, and 22 to the control group. Patients enrolled in any of the hyperoxia arms (NH or HBO2) were treated—1–3 h—every 24 h over 3 consecutive days (Rockswold et al., 2010). LPR was significantly decreased from baseline following HBO2 sessions, but no changes were detected in the LPR after NH (Rockswold et al., 2010).
Brain edema and endothelial swelling occur following TBI. Therefore, O2 conductance of the tissue is increased and O2 has to travel longer distances before reaching the mitochondria. This situation has been named “dysperfusion hypoxia” by Siggaard-Andersen (Siggaard-Andersen et al., 1995a, b). Dysperfusion hypoxia in the brain is mainly caused by an increased mean diffusion length, causing a decrease in the diffusion gradient for O2 from the erythrocytes to the mitochondria via a well-defined mathematical function (Siggaard-Andersen et al., 1995a, b). A decrease in this diffusion gradient causes a decrease in O2 flux to the mitochondria. In addition, the decrease in the capillary endothelium area, and therefore in the total diffusion area, is another cause of dysperfusion hypoxia.
In TBI, brain edema and the closure of capillaries caused by an increase in interstitial pressure or micro-emboli can produce both an increase in diffusion length and a decrease in the diffusion area for O2 (Siggaard-Andersen et al., 1995a, b). Diringer et al. have remarked that in this scenario “… an increase in the oxygen tension gradient may facilitate diffusion of oxygen though edematous tissue to reach the mitochondria” (Diringer, 2008).
An additional option to explain high lactate in the presence of normal PtiO2, the most frequent situation in our series, is mitochondrial dysfunction. Impairment of mitochondria has been reported both in experimental models and in human TBI (Signoretti et al., 2008; Verweij et al., 1997; Xiong et al., 1997). In an experimental model of TBI in rats, Xiong et al. showed that cortical impact injury induced mitochondrial dysfunction that was maximal at 12 to 24 h after injury and lasted 2 weeks (Xiong et al., 1997). It has also been shown that mitochondrial function is impaired in severe TBI patients (Signoretti et al., 2008; Verweij et al., 2000). Using mitochondria derived from therapeutically removed brain tissue, Verwej et al. showed that: 1) the state three respiratory rate, when mitochondria exhibit maximum activity in the presence of sufficient amounts of respiratory substrates, was slowed; 2) ATP production was impaired; and 3) intramitochondrial levels of calcium were increased (Verweij et al., 2000). Mitochondrial efficacy improved in some cases when calcium was removed from the mitochondrial membrane by adding a calcium chelator. This indicated that the mitochondrial dysfunction was not structural, but rather functional, and related to an excess of intracellular calcium (Verweij et al., 2000). Povlishock et al. suggested that shear and tensile forces that come into play during brain injury induce mechanical poration of cell membranes, which causes an initial excitatory amino acid burst, leading to an intracellular calcium influx with subsequent calcium mitochondrial overloading (Povlishock et al., 1997).
Despite increasing evidence of mitochondrial dysfunction in TBI patients, the issue of whether or not increasing brain PtiO2, and therefore O2 pressure gradients, improves mitochondrial function and brain energy metabolism is a matter of considerable debate, and needs to be verified using robust variables such as lactate, pyruvate, and the LPR. To prove that hyperoxia improves mitochondrial function, it is necessary to verify that the cerebral metabolic rate for O2 (CMRO2) increases after NH. The gold standard to measure CMRO2 is PET scanning. However, PET studies in neurocritical care patients are very difficult to conduct and only a few centers in the world have on-site PET scanning facilities.
The main limitation of our study is that we did not measure CMRO2 with PET as recent studies have done (Diringer et al., 2007; Nortje et al., 2008) and therefore we could not assess whether decreases in lactate and in the LPR were accompanied by a concomitant increase in CMRO2. Studies on the effects of hyperoxia in CMRO2 are few and controversial. Diringer and coworkers conducted PET scanning in five severe TBI patients (median GCS=7) who were mechanically ventilated and scanned between 12 and 23 h after injury (Diringer et al., 2007). In this study, patients received NH for 1 h and it was shown that hyperoxia had no impact on CMRO2 (Diringer et al., 2007). However, Nortje et al. showed that CMRO2 was increased only in brain tissue at risk, defined by a CMRO2 threshold in the absence of significant changes on other brain metabolic or hemodynamic parameters (Nortje et al., 2008). Additional studies to address this apparent contradiction should be conducted, but unfortunately very few centers in the world have the facilities to conduct such demanding studies.
O2 toxicity
A final issue to be considered when using NH is the potential toxicity of O2, both in the lungs and in the central nervous system (CNS). Pulmonary and CNS O2 toxicity has been described in both animals and humans. The side effects of O2 in the CNS are called the "Paul Bert Effect" after Paul Bert first reported hyperoxia-induced seizures in birds subjected to O2 pressure >3 ATA in 1878 (Jain 2009; Patel et al., 2003). In addition, O2 can also have side effects on the lungs, where prolonged and/or high concentrations of O2 may damage the pulmonary epithelium, inactivate surfactant and induce intra-alveolar edema and interstitial thickening (Jain 2009; Patel et al., 2003).
The development of pulmonary and CNS toxicity depends on the O2 partial pressure and the duration of exposure (Jain, 2009). However, the toxic effects of short periods of hyperoxia, either normobaric or >1 ATA, have not been proven. Recent studies on HBO2 by Rockswold et al. have shown that HBO2 at 1.5 ATA for 60 min does not appear to produce O2 toxicity and is considered safe in TBI (Rockswold et al., 2007, 2010).
In conclusion, moderate and severe TBI may induce brain metabolic alterations, even in macroscopically normal brain tissue, detected by MD that shows alterations in energy metabolism. In our study, NH significantly increased PaO2 and PtiO2, and significantly decreased LPR in patients in whom baseline brain lactate levels were increased, suggesting that NH improved the brain redox state. In patients with normal brain baseline lactate levels, we did not find any significant change in the metabolic variables after NH. This suggests that the baseline metabolic state should be taken into account when applying NH to patients with TBI. This maneuver may only be effective in a well-defined group of patients.
Aerobic metabolism depends upon the correct supply of substrates and on the existence of fully functional mitochondria. If mitochondrial dysfunction occurs, aerobic metabolism shifts toward anaerobic metabolism, even in the presence of an adequate O2 supply(Verweij et al., 2000). The mitochondrial dysfunction theory is an exciting explanation for many metabolic disorders traditionally considered to be unrelated to ischemic hypoxia. However, in order for NH or any other treatment to improve these alterations, mitochondria must be structurally intact and responsive to treatment. This theory needs more focused research before being translated to the bedside.
Further studies are needed to confirm the preliminary results described in this study. If confirmed by others, this would allow for the identification of a group of patients in whom NH improves metabolic derangements, thus improving the neurological outcome.
Acknowledgments
This study was supported by Instituto de Salud Carlos III, grant number PI080480 co-financed by the European Regional Development Fund (ERDF) to Dr. J. Sahuquillo. Dr. A. Vilalta was the recipient of a pre-doctoral grant from the Institut Fundació de Recerca, Hospital Vall d'Hebron, and is currently the recipient of a Marie Curie Intra-European Fellowship grant. We thank Sabrina Voss for editorial assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- Alves O.L. Daugherty W.P. Rios M. Arterial hyperoxia in severe head injury: a useful or harmful option? Curr. Pharm. Des. 2004;10:2163–2176. doi: 10.2174/1381612043384187. [DOI] [PubMed] [Google Scholar]
- Bergsneider M. Hovda D.A. Shalmon E. Kelly D.F. Vespa P.M. Martin N.A. Phelps M.E. McArthur D.L. Caron M.J. Kraus J.F. Becker D.P. Cerebral hyperglycolysis following severe traumatic brain injury in humans: A positron emission tomography study. J. Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Bouma G.J. Muizelaar J.P. Choi S.C. Newlon P.G. Young H.F. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J. Neurosurgery. 1991a;75:685–693. doi: 10.3171/jns.1991.75.5.0685. [DOI] [PubMed] [Google Scholar]
- Bouma G.J. Muizelaar J.P. Stringer W.A. Choi S.C. Fatouros P. Young H.F. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J. Neurosurg. 1992;77:360–368. doi: 10.3171/jns.1992.77.3.0360. [DOI] [PubMed] [Google Scholar]
- Bouma G.J. Muizelaar J.P. Young H.F. Demonstration of early ischemia after severe head injury. J.Neurosurg. 1991b;74:364A–365A. [Google Scholar]
- Bullock R.M. Chesnut R.M. Clifton G.L. Ghajar J. Marion D.W. Narayan R.K. Newell D.W. Pitts L.H. Rosner M.J. Walters B.C. Wilberger J.E. Maas A.I.R. Servadei F. Teasdale G. Unterberg A. von Holst H. Contant C. Florin R. Jagoda A. Kelly J.P. Marmarou A. Queen P.C. Rosenberg J. Valadka A.B. Dearden M. Miller J.D. Stocchetti N. Management and prognosis of severe traumatic brain injury. Part 1: Guidelines for the management of severe traumatic brain injury. Part 2: Early indicators of prognosis in severe traumatic brain injury. J. Neurotrauma. 2000;17:451–627. [Google Scholar]
- Carlsson A. Application notes and Case reports. Clinical research: CMA 600 Microdialysis Analyser Manual 2008.
- Cesarini K.G. Enblad P. Ronne–Engstrom E. Marklund N. Salci K. Nilsson P. Hardemark H.G. Hillered L. Persson L. Early cerebral hyperglycolysis after subarachnoid haemorrhage correlates with favourable outcome. Acta Neurochirurg. (Wien) 2002;144:1121–1131. doi: 10.1007/s00701-002-1011-9. [DOI] [PubMed] [Google Scholar]
- Diringer M.N. Hyperoxia: good or bad for the injured brain? Curr. Opin. Crit. Care. 2008;14:167–171. doi: 10.1097/MCC.0b013e3282f57552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer M.N. Aiyagari V. Zazulia A.R. Videen T.O. Powers W.J. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J. Neurosurg. 2007;106:526–529. doi: 10.3171/jns.2007.106.4.526. [DOI] [PubMed] [Google Scholar]
- Forgue J. Legeay A. Massabuau J.C. Is the resting rate of oxygen consumption of locomotor muscles in crustaceans limited by the low blood oxygenation strategy?'. J. Exp. Biol. 2001;204:933–940. doi: 10.1242/jeb.204.5.933. [DOI] [PubMed] [Google Scholar]
- Graham D.I. Adams J.H. Doyle D. Ischaemic brain damage in fatal non-missile head injuries. J. Neurol Sci. 1978;39:213–234. doi: 10.1016/0022-510x(78)90124-7. [DOI] [PubMed] [Google Scholar]
- Graham D.I. Ford D.I. Adams J.H. Doyle D. Teasdale G.M. Lawrence A.E. McLellan D.R. Ischaemic brain damage is still common in fatal non-missile head injury. J. Neurol. Neurosurg. Psychiatry. 1989;52:346–350. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain K.K. Oxygen toxicity. In: Jain K.K., editor. Textbook of Hyperbaric Medicine. Göttingen: Hogrefe and Huber; 2009. pp. 47–58. [Google Scholar]
- Magnoni S. Ghisoni L. Locatelli M. Caimi M. Colombo A. Valeriani V. Stocchetti N. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: a microdialysis study. J. Neurosurg. 2003;98:952–958. doi: 10.3171/jns.2003.98.5.0952. [DOI] [PubMed] [Google Scholar]
- Marshall S.B. Klauber M.R. Van Berkum Clark M. Eisenberg H.M. Jane J. Luerssen T.G. Marmarou A. Marshall L.F. Foulkes M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. (Suppl.) 1991;75:14–20. [Google Scholar]
- Massabuau J.C. From low arterial- to low tissue-oxygenation strategy. An evolutionary theory. Respir. Physiol. 2001;128:249–261. doi: 10.1016/s0034-5687(01)00305-x. [DOI] [PubMed] [Google Scholar]
- Massabuau J.C. Primitive, and protective, our cellular oxygenation status? Mech. Ageing Dev. 2003;124:857–863. doi: 10.1016/s0047-6374(03)00147-7. [DOI] [PubMed] [Google Scholar]
- Menon D.K. Coles J.P. Gupta A.K. Fryer T.D. Smielewski P. Chatfield D.A. Aigbirhio F. Skepper J.N. Minhas P.S. Hutchinson P.J. Carpenter T.A. Clark J.C. Pickard J.D. Diffusion limited oxygen delivery following head injury. Crit. Care Med. 2004;32:1384–1390. doi: 10.1097/01.ccm.0000127777.16609.08. [DOI] [PubMed] [Google Scholar]
- Menzel M. Doppenberg E. Zauner A. Bullock R. Ward J. Young H.F. Marmarou A. Brockenborough P. Arterial oxygen partial pressure as a determinant of brain tissue oxygenation and brain tissue lactate levels early after severe head injury (Abstracts) Neurosurgery. 1997;41:753–754. [Google Scholar]
- Menzel M. Doppenberg E.M. Zauner A. Soukup J. Reinert M.M. Bullock R. Increased inspired oxygen concentration as a factor in improved brain tissue oxygenation and tissue lactate levels after severe human head injury. J. Neurosurg. 1999a;91:1–10. doi: 10.3171/jns.1999.91.1.0001. [DOI] [PubMed] [Google Scholar]
- Menzel M. Doppenberg E.M. Zauner A. Soukup J. Reinert M.M. Clausen T. Brockenbrough P.B. Bullock R. Cerebral oxygenation in patients after severe head injury: monitoring and effects of arterial hyperoxia on cerebral blood flow, metabolism and intracranial pressure. J. Neurosurg. Anesthesiol. 1999b;11:240–251. doi: 10.1097/00008506-199910000-00003. [DOI] [PubMed] [Google Scholar]
- Nelson D.W. Thornquist B. Maccallum R.M. Nystrom H. Holst A. Rudehill A. Wanecek M. Bellander B.M. Weitzberg E. Analyses of cerebral microdialysis in patients with traumatic brain injury: relations to intracranial pressure, cerebral perfusion pressure and catheter placement. BMC Med. 2011;9:21. doi: 10.1186/1741-7015-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nortje J. Coles J.P. Timofeev I. Fryer T.D. Aigbirhio F.I. Smielewski P. Outtrim J.G. Chatfield D.A. Pickard J.D. Hutchinson P.J. Gupta A.K. Menon D.K. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: Preliminary findings. Crit. Care Med. 2008;36:273–281. doi: 10.1097/01.CCM.0000292014.60835.15. [DOI] [PubMed] [Google Scholar]
- Park E. Bell J.D. Baker A.J. Traumatic brain injury: can the consequences be stopped? CMAJ. 2008;178:1163–1170. doi: 10.1503/cmaj.080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D.N. Goel A. Agarwal S.B. Garg P. Lakhani K.K. Oxygen toxicity. J. Ind. Acad. Clin. Med. 2003;4:234–237. [Google Scholar]
- Poca M.A. Sahuquillo J. Arribas M. Baguena M. Amoros S. Rubio E. Fiberoptic intraparenchymal brain pressure monitoring with the Camino V420 monitor: reflections on our experience in 163 severely head-injured patients. J. Neurotrauma. 2002;19:439–448. doi: 10.1089/08977150252932398. [DOI] [PubMed] [Google Scholar]
- Poca M.A. Sahuquillo J. Vilalta A. de los Rios J. Robles A. Exposito L. Percutaneous implantation of cerebral microdialysis catheters by twist-drill craniostomy in neurocritical patients: description of the technique and results of a feasibility study in 97 patients. J. Neurotrauma. 2006;23:1510–1517. doi: 10.1089/neu.2006.23.1510. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Marmarou A. McIntosh T. Trojanowski J.Q. Moroi J. Impact acceleration injury in the rat: evidence for focal axolemmal change and related neurofilament sidearm alteration. J. Neuropathol. Exp. Neurol. 1997;56:347–359. [PubMed] [Google Scholar]
- Reinert M. Barth A. Rothen H.U. Schaller B. Takala J. Seiler R.W. Effects of cerebral perfusion pressure and increased fraction of inspired oxygen on brain tissue oxygen, lactate and glucose in patients with severe head injury. Acta Neurochirurg. 2003;145:341–349. doi: 10.1007/s00701-003-0027-0. [DOI] [PubMed] [Google Scholar]
- Rockswold S.B. Rockswold G.L. Defillo A. Hyperbaric oxygen in traumatic brain injury. Neurol. Res. 2007;29:162–172. doi: 10.1179/016164107X181798. [DOI] [PubMed] [Google Scholar]
- Rockswold S.B. Rockswold G.L. Zaun D.A. Zhang X. Cerra C.E. Bergman T.A. Liu J. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J. Neurosurg. 2010;112:1080–1094. doi: 10.3171/2009.7.JNS09363. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. Workshop Scientific Team and Advisory Panel. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuquillo J. Biestro A. Mena M.P. Amoros S. Lung M. Poca M.A. de Nadal M. Baguena M. Panzardo H. Mira J.M. Garnacho A. Lobato R.D. First tier measures in the treatment of intracranial hypertension in the patient with severe craniocerebral trauma. Proposal and justification of a protocol. Neurocirugía (Astur) 2002;13:78–100. doi: 10.1016/s1130-1473(02)70628-3. [DOI] [PubMed] [Google Scholar]
- Sahuquillo J. Poca M.A. Amoros S. Current aspects of pathophysiology and cell dysfunction after severe head injury. Curr. Pharm. Des. 2001;7:1475–1503. doi: 10.2174/1381612013397311. [DOI] [PubMed] [Google Scholar]
- Sahuquillo J. Poca M.A. Monforte L. Sanchez–Massa L.L. Campos L. Rubio E. Zapater P. Interhemispheric supratentorial ICP gradients in head injury patients: Are they clinically important? In: Nagai H., editor; Kamiya K., editor; Ishii S., editor. Intracranial Pressure IX. Tokyo: Springer–Verlag; 1994. pp. 48–51. [Google Scholar]
- Siggaard–Andersen O. Fogh–Andersen N. Gothgen I.H. Larsen V.H. Oxygen status of arterial and mixed venous blood. Crit. Care Med. 1995a;23:1284–1293. doi: 10.1097/00003246-199507000-00020. [DOI] [PubMed] [Google Scholar]
- Siggaard–Andersen O. Ulrich A. Gothgen I.H. Classes of tissue hypoxia. Acta Anaesthesiol. Scand. 1995b;39:137–142. doi: 10.1111/j.1399-6576.1995.tb04348.x. [DOI] [PubMed] [Google Scholar]
- Signoretti S. Marmarou A. Aygok G.A. Fatouros P.P. Portella G. Bullock R.M. Assessment of mitochondrial impairment in traumatic brain injury using high-resolution proton magnetic resonance spectroscopy. J. Neurosurg. 2008;108:42–52. doi: 10.3171/JNS/2008/108/01/0042. [DOI] [PubMed] [Google Scholar]
- Tolias C.M. Reinert M. Seiler R. Gilman C. Scharf A. Bullock M.R. Normobaric hyperoxia-induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J. Neurosurg. 2004;101:435–444. doi: 10.3171/jns.2004.101.3.0435. [DOI] [PubMed] [Google Scholar]
- Verweij B.H. Muizelaar J.P. Vinas F.C. Peterson P.L. Xiong Y. Lee C.P. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N–type calcium channel antagonist (SNX-111) Neurol. Res. 1997;19:334–339. doi: 10.1080/01616412.1997.11740821. [DOI] [PubMed] [Google Scholar]
- Verweij B.H. Muizelaar J.P. Vinas F.C. Peterson P.L. Xiong Y. Lee C.P. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 2000;93:815–820. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- Vespa P.M. McArthur D. O'Phelan K. Glenn T. Etchepare M. Kelly D. Bergsneider M. Martin N.A. Hovda D.A. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J. Cereb. Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Vespa P.M. O'Phelan K. McArthur D. Miller C. Eliseo M. Hirt D. Glenn T. Hovda D.A. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit. Care Med. 2007;35:1153–1160. doi: 10.1097/01.CCM.0000259466.66310.4F. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Gu Q. Peterson P.L. Muizelaar J.P. Lee C.P. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]