Abstract
Glucocorticoids are widely used immunosuppressive drugs in treatment of autoimmune diseases and hematological malignancies. Glucocorticoids are particularly effective immune suppressants, because they induce rapid peripheral T cell and thymocyte apoptosis resulting in impaired T cell–dependent immune responses. Although glucocorticoids can induce apoptotic cell death directly in developing thymocytes, how exogenous glucocorticoids affect the thymic epithelial network that provides the microenvironment for T cell development is still largely unknown. In the present work, we show that primary thymic epithelial cells (TECs) express glucocorticoid receptors and that high-dosage dexamethasone induces degeneration of the thymic epithelium within 24 h of treatment. Changes in organ morphology are accompanied by a decrease in the TEC transcription factor FoxN1 and its regulator Wnt-4 parallel with upregulation of lamina-associated polypeptide 2α and peroxisome proliferator activator receptor γ, two characteristic molecular markers for adipose thymic involution. Overexpression of Wnt-4, however, can prevent upregulation of adipose differentiation-related aging markers, suggesting an important role of Wnt-4 in thymic senescence.
Introduction
Autoimmune diseases and hematological malignancies are significant causes of morbidity and mortality world wide.1,2 Although research is ongoing, treatment options are still often limited to high-dosage synthetic glucocorticoid (GC) analogs despite their nonspecificity and multiple side effects. Indeed, GCs are still applied in therapy for acute and chronic autoimmune diseases and hematological malignancies,3,4 because they effectively promote apoptosis of leukemia cells5 and trigger complex antiinflammatory actions by influencing both molecular and cellular components of the immune system.6 Apart from triggering decreased expression of cytokines and major histocompatibility complex class II (MHC II), GCs also induce apoptotic death of peripheral7 and developing T cells. In mouse models, GCs cause massive thymocyte depletion, especially in the CD4+CD8+ (double positive [DP]) thymocyte population,8–12 blocking de novo T cell production.
Prior experiments have also demonstrated that high-dose GCs induce a dramatic13 and apoptosis-associated14 involution of the thymus, and not only thymocytes but also thymic epithelial cells (TECs) are seriously affected.15 Additionally, a recent report by Fletcher et al.16 has highlighted that TEC depletion appears reversible, and thymic epithelial stem cells play an important role in this process.
Because physiological steroids are implicated in the regulation of aging,17,18 we theorized that GC treatment might affect thymic epithelial senescence. Although morphological similarities between physiological and induced thymic involution are striking, to date the process has not been studied in detail at the molecular level. One possible mechanism is that during physiological aging TECs undergo epithelial-to-mesenchymal transition (EMT) and then proadipose differentiation.19,20 Our studies have recently provided evidence that this process is regulated by Wnt-4 and FoxN1 decline, leading to drastic reduction in TEC identity21,22 and simultaneous upregulation of lamina-associated polypeptide (LAP) 2α as well as preadipocyte-related markers peroxisome proliferator activator receptor (PPAR) γ and adipose differentiation-related protein (ADRP).20
On the basis of the above studies, we theorized that GCs do not simply deplete thymocytes and the majority of TECs, but they also inhibit the function of the remaining epithelium via downregulation of characteristic TEC markers, leading to preadipocyte differentiation. In the present study, we provide evidence that both primary TECs and the primary TEC-derived TEP1 cell line express glucocorticoid receptors (GRs) and respond to GC treatment. Upon a single high-dose GC injection, gene expression levels of both Wnt-4 and FoxN121 become significantly downregulated, resulting in upregulation of preadipocyte differentiation markers (LAP2α, PPARγ). Regeneration of TECs—including normal FoxN1 level—occurs within 3 months after dexamethasone (DX) injection. However, if GC-treatment is continued with repeated small doses as in clinical applications, then Wnt-4 and FoxN1 transcription cannot recover and aging markers remain locked at elevated levels. Overexpression of Wnt-4 in the FoxN1-deprived TEP1 cell line is, however, able to protect against GC-induced adipose transdifferentiation, indicating Wnt-4 as the primary protector of thymic epithelium against adipose involution.
Materials and Methods
Mice and treatment of animals
Four-week-old BALB/c mice were used for the experiments. Animals received a single-dose (20 mg/kg) DX (Oradexon, Organon) injection intraperitoneally (i.p.) in phosphate-buffered saline (PBS) and then were sacrificed 24 and 168 h after injection; control animals received PBS. Another group of mice received PBS and DX for 3 months, respectively. There was also a group of mice receiving one high dose of DX, then a continuously low dose of DX (2 mg/kg) on every second day for a month, to mimic the therapeutic regimen of autoimmune diseases.23,24 All animal experiments were carried out in accordance with the regulations set out by Pécs University's committee on animal experimentation (#BA 02/2000-2/2006).
Cell lines and in vitro DX treatment
The TEP1 cell line was maintained and used for experiments as described.20 The Wnt-4-overexpressing TEP1 cell line was generated as described previously.20 Cell lines were treated with DX (Sigma, dissolved in dimethylsulfoxide [DMSO] until use) with a final concentration of 1 μM for 1 week or solvent, respectively.
Preparation of TECs
Thymic lobes were digested with 3 mg/mL collagenase II (GIBCO) for 30 min, then washed with Dulbecco modified Eagle medium (DMEM) 10% fetal calf serum (FCS). Cell suspensions were then labeled with anti-EpCAM1-FITC (clone G8.8) and washed with magnetic cell sorting (MACS) buffer followed by incubation with anti-fluorescein isothiocyanate (FITC) microbeads (Miltenyi Biotec), the EpCAM1+-cells were used for total RNA isolation and subsequent quantitative polymerase chain reaction (PCR) analysis. The cells were purified using MACS LS separation columns (Miltenyi Biotec).
Histology using fluorescent antibodies
Frozen thymic sections (7—10 μm thick) were fixed in cold acetone, then dried and blocked using 5% bovine serum albumin (BSA) in PBS for 20 min before staining with a-Ly51-PE (clone 6C3) and a-EpCAM1 (clone G8.8) antibodies (either indirectly coupled with a-rat Northern Light 637 secondary antibody or directly labeled with FITC). GR, ER-TR7, and Wnt-4 protein levels were detected using an a-GR-FITC mouse monoclonal antibody (clone 5E4B1 developed in our laboratory25 and commercially available at Serotec), a rat monoclonal a-ER-TR7, and a polyclonal goat a-Wnt4 antibody (Abcam). Visualization was performed using an a-rat-Ig-PE secondary antibody and a-goat Ig Northern Lights 557 (RnD systems), respectively. Parallel with GR detection, corresponding sections were incubated with an irrelevant antibody. To detect GR expression in TECs, the Olympus Fluoview 300 confocal microscope with an Olympus Fluoview FV1000S-IX81 system was used. All the other sections were analysed using an Olympus BX61 microscope equipped with CCD camera and AnalySIS software.
RNA isolation, preparation of cDNA
Total RNA was isolated using RNeasy plus kit (Qiagen), following the manufacturer's instructions. Following RNA isolation, DNase digestion was performed using a DNase I digestion kit (Sigma) and cDNA was reverse transcribed using a high-capacity RNA to cDNA kit according to the manufacturer's instructions (Applied Biosystems).
Quantitative real-time and qualitative reverse transcriptase PCR analysis of purified TECs and cell lines
For real-time PCR analysis, we used an ABI 7500 Software system and ABI SYBR Green PCR master mix. The expression levels of LAP2α2, PPARγ, ADRP, and Wnt-4 were analyzed and normalized to the level of 18S rRNA. The qualitative expression of GR and CD45 in TEC was verified by PCR using ReddyMix (ABgene) according to the manufacturer's instructions, with 18S rRNA as an internal control. The PCR products were visualized on agarose gels. The sequences of primers are listed in Table 1. In the case of FoxN1 and AIRE, TaqMan chemistry was used for PCR reaction and analysis, and the levels of these genes were normalized to the TaqMan HPRT1 expression. PCR reactions were run for a maximum of 40 cycles.
Table 1.
List of Gene-Specific Primers
| Gene | Forward primer | Reverse primer |
|---|---|---|
| CD45 | 5′-CCG GAA TTC CGG ATG GGT TTG TGG CT-3′ | 5′-CCG CTC GAG CGG CTA ATC ACT GGG TG-3′ |
| GR | 5′-TGG TGT GCT CCG ATG A-3′ | 5′-AGG GTA GGG GTA AGC-3′ |
| FoxN1 | Applied Biosystems TaqMan probe PN4351272 (Mm00477457_m1) | |
| LAP2α | 5′-TGA ACT GCA GGC AGC TAA GA-3′ | 5′-TCA TAG CTA GAC TCT GAG G-3′ |
| PPARγ | 5′-CCC AAT GGT TGC TGA TTA CAA A-3′ | 5′-AAT AAT AAG GTG GAG ATG CAG GTT CT-3′ |
| ADRP | 5′-CGC CAT CGG ACA CTT CCT TA-3′ | 5′-GTG ATG GCA GGC GAC ATC T-3′ |
| Wnt4 | 5′-CTC AAA GGC CTG ATC CAG AG-3′ | 5′-TCA CAG CCA CAC TTC TCC AG-3′ |
| 18S rRNA | 5′-GGG TCG GGA GTG GGT AAT TT-3′ | 5′-AGA AAC GGC TAC CAC ATC CAA-3′ |
| HPRT1 Taqman | Applied Biosystems TaqMan probe Mm0046968_m1 | |
| AIRE | Applied Biosystems TaqMan probe Mm00477457_m1 |
GR, Glucocorticoid receptor; PPARγ, peroxisome proliferator activator receptorγ; ADRP, adipose differentiation-related protein; AIRE, autoimmune regulator.
Statistical analysis
Data are presented as mean ± standard deviation (SD), and the effects between various experimental groups were compared with the Student t-test. p < 0.05 was considered as significant.
Results
GR expression in primary thymic epithelium and the TEP1 cell line
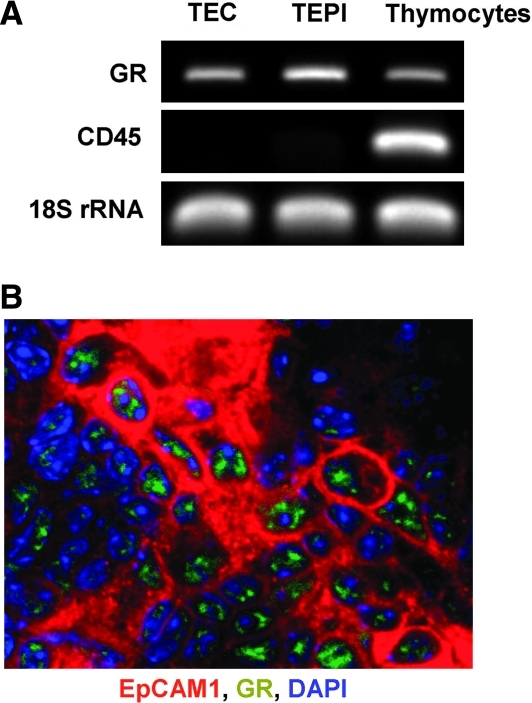
GCs regulate cellular function via GR, which belongs to the nuclear receptor superfamily26 and is required for the regulation of development and homeostasis of various epithelial-like tissues.27,28 Using reverse transcriptase (RT)-PCR analysis (Fig. 1A) and confocal laser scanning microscopy (Fig. 1B), it was demonstrated that apart from thymocytes8,9 GR is also expressed in primary TECs and the TEP1 cell line, confirming their ability to respond directly to GC stimuli.
FIG. 1.
Glucocorticoid receptor (GR) expression in the thymic epithelial cell (TEC) and TEPI cell lines. cDNA generated from mRNA of highly purified adult TECs and TEPI cells were tested in end point reverse transcriptase polymerase chain reaction (RT-PCR) analysis for GR expression. cDNA generated from thymocyte mRNA was used as positive control for GR expression, whereas TEC purity was tested for hematopoietic cell contamination using CD45 primers. 18S rRNA was used as an internal control (A). GR protein was detected by confocal microscopy using a-GR-FITC-labeled antibody (green) in frozen sections of untreated adult BALB/c thymi. TECs were detected using a-EpCAM1 antibody visualized by a-rat-Northern Light 637 secondary antibody (red). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue) (B). Corresponding sections were probed with an irrelevant antibody as a staining control. Data shown above are representative of three separate experiments.
Effects of single-dose GC administration
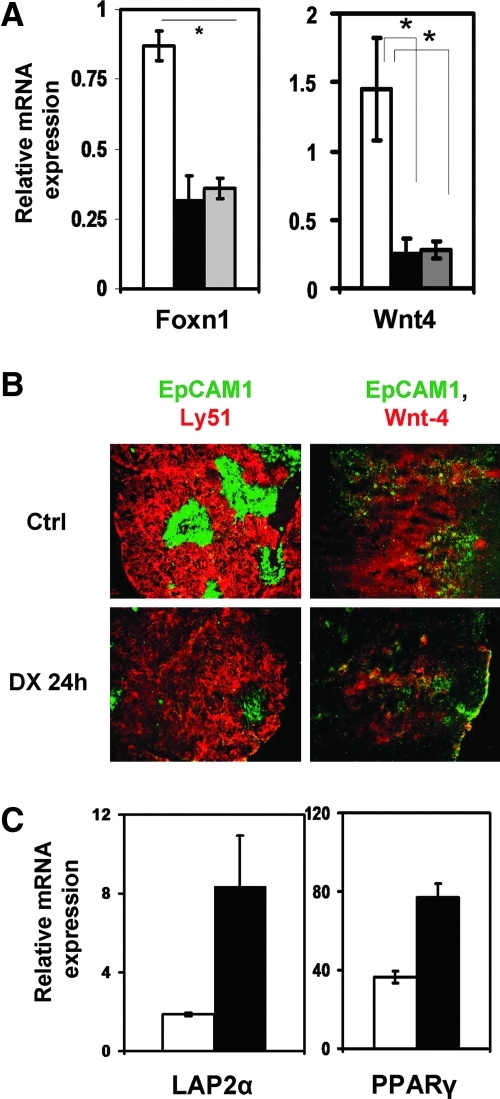
To study how the thymic epithelial network is affected by GC, sections of DX-treated and control thymi were stained for the general epithelial cell marker, EpCAM1 and Ly5129 to differentiate between medulla and cortex. Within 24 h, DX treatment induced marked reduction in epithelial cell-surface markers (Fig. 2B), particularly affecting the medullary (EpCAM1++Ly51−) thymic compartments. The remaining TECs were purified from GC-treated thymi based on EpCAM1 expression. In the purified TEC population, gene transcription was analyzed using quantitative real-time RT-PCR. During embryonic development, TEC maturation is regulated by Wnt-421; therefore Wnt-4 levels were assayed. Quantitative real-time RT-PCR analysis and immunohistochemistry confirmed that both Wnt-4 mRNA (Fig. 2A) and protein levels (Fig. 2B) decreased significantly after 24 h of DX exposure. FoxN1, a transcription factor essential for thymic organogenesis and maintenance of TEC identity directly regulated by Wnt-4 expression,30was also found to be significantly decreased (Fig. 2A). FoxN130 and Wnt-4 mRNA levels were also measured a week later and appeared to remain significantly low (Fig. 2A).
FIG. 2.
Dexamethasone (DX)-induced effect on Wnt-4, Foxn1, adipocyte-related genes, and general morphology of the thymus. Foxn1 and Wnt-4 gene expression pattern (A) of purified control and DX-treated thymic epithelial cell (TEC) control (□), for 24 h (▪). Gray bars ( ) represent gene expression 168 h after DX treatment and asterisks (*p < 0.05) indicate significant differences (n = 3 in each group). Note the scale differences for gene expression. Thymic sections of phosphate-buffered saline (PBS)- and DX-treated mice (24 h) were stained with a-EpCAM1-FITC (green) and a-Ly51-PE (red) monoclonal antibodies to reveal medullary and cortical compartments. The staining revealed depletion of mTECs following 24 h of DX administration. Images are representative of three independent experiments (B, left). Wnt-4 expression of control and DX-treated thymi is also shown (B, right). Wnt-4-Northern Lights 557 (red) and EpCAM1-FITC (green) in Ctrl and DX-treated thymi are presented (24 h). Images are representative of three independent experiments. Induction of adipose tissue-related genes in TEC following DX-treatment. (C) Expression of lamina associated polypeptide-2α (LAP2α), peroxisome proliferator activator receptor-γ (PPARγ), and adipose differentiation-related protein (ADRP) were tested in TECs 168 h after a single DX injection and was found to be elevated in DX-treated samples (▪) (with an exception of ADRP), compared to control (□). Gene expression was normalized to 18S rRNA. All bars show means ± standard deviation (SD). Results are representative of three independent experiments (n = 3).
) represent gene expression 168 h after DX treatment and asterisks (*p < 0.05) indicate significant differences (n = 3 in each group). Note the scale differences for gene expression. Thymic sections of phosphate-buffered saline (PBS)- and DX-treated mice (24 h) were stained with a-EpCAM1-FITC (green) and a-Ly51-PE (red) monoclonal antibodies to reveal medullary and cortical compartments. The staining revealed depletion of mTECs following 24 h of DX administration. Images are representative of three independent experiments (B, left). Wnt-4 expression of control and DX-treated thymi is also shown (B, right). Wnt-4-Northern Lights 557 (red) and EpCAM1-FITC (green) in Ctrl and DX-treated thymi are presented (24 h). Images are representative of three independent experiments. Induction of adipose tissue-related genes in TEC following DX-treatment. (C) Expression of lamina associated polypeptide-2α (LAP2α), peroxisome proliferator activator receptor-γ (PPARγ), and adipose differentiation-related protein (ADRP) were tested in TECs 168 h after a single DX injection and was found to be elevated in DX-treated samples (▪) (with an exception of ADRP), compared to control (□). Gene expression was normalized to 18S rRNA. All bars show means ± standard deviation (SD). Results are representative of three independent experiments (n = 3).
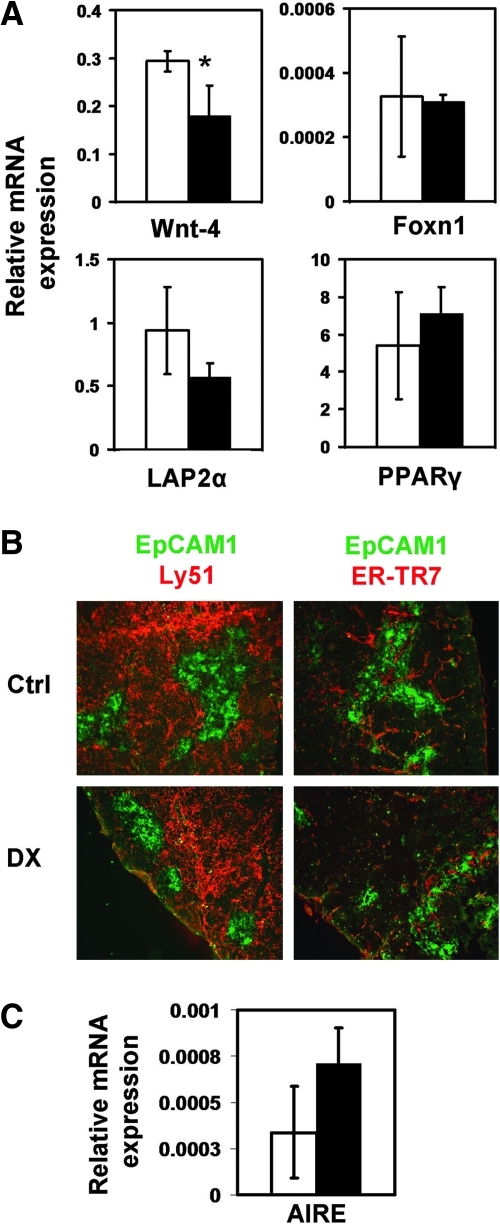
As physiological thymic involution correlates with the upregulation of preadipocyte markers LAP2α20 and PPARγ, the expression of these markers was also tested following DX treatment–induced involution. One day, 1 week, 1 month, and 3 months after DX exposure, TECs were purified and then analyzed using quantitative (q) RT-PCR. At day 1, the expression of LAP2α20 and PPARγ was low (data not shown), but it became upregulated a week later (Fig. 2C), indicating that it takes several days of Wnt-4 and FoxN1 depletion to affect preadipocyte differentiation markers. By 1 month and even by 3 months after DX treatment, Wnt-4 mRNA expression only partially increased while FoxN1, LAP2α, and PPARγ mRNA levels returned close to normal levels (within standard deviation) supporting the time frame of recovery (Fig. 3A).
FIG. 3.
Recovery of the thymic epithelial cell (TEC) compartment after dexamethasone (DX) treatment takes 3 months. (A) Bars show the gene expression changes of purified TEC: Control (□) and dexamethasone (DX) treated (▪). The level of Wnt-4 remained significantly low even after 3 months (*p < 0.05), the levels of lamina associated polypeptide-2α (LAP2α) and peroxisome proliferator activator receptor-γ (PPARγ) were found to be unaltered, whereas adipose differentiation-related protein (ADRP) expression was found to be decreased. Note the scale differences for gene expression. Morphological analysis of age-matched control (Ctrl) versus DX-treated thymi after 3 months using EpCAM-Ly51 (TEC network) (B, left) and EpCAM-ER-TR7 (TEC and fibroblast) (B, right). In B (left), in the treated samples, the size of medulla appears to be smaller, but the TEC network is normal. In B (right), no remarkable differences are observed in general morphology using the ER-TR7 fibroblast marker staining on thymic sections of control and DX-treated mice. Images are representative of three independent experiments. In C, autoimmune regulator (AIRE) expression is shown in control (□) and DX- (▪) purified TEC after 3 months, with no significant difference, indicating full mTEC recovery. All bars show means ± standard deviation (SD) (n = 3).
To analyze how thymic morphology is affected, thymic sections were stained for EpCAM1/Ly51. Three months after DX treatment, no significant differences were detected between DX-treated and age-matched controls (Fig. 3B), except for the medulla, which still appeared somewhat shrunken in DX-exposed animals (Fig. 3B). To determine thymic medullar mass, qPCR was performed for the medullary marker autoimmune regulator (AIRE) gene.31 No significant difference was detected in AIRE expression after 3 months of DX treatment (Fig. 3C), indicating the full competence of the mTEC compartment. Because EMT seems to precede preadipocyte transdifferentiation during physiological aging,20 the staining pattern of fibroblast marker ER-TR7 was also examined following DX treatment. Apart from the scattered focal staining pattern of ER-TR7 in DX-treated thymi, no sign of significant EMT (Fig. 3B) was detected, supporting molecular data showing that 3 months is sufficient for TEC recovery.
Effects of sustained GC administration
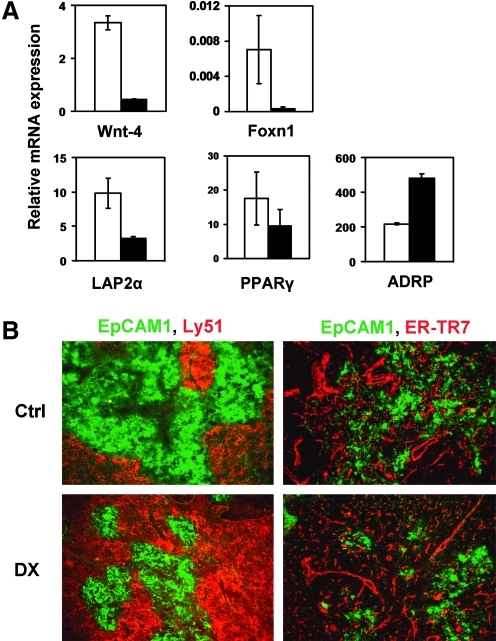
To mimic the pattern of clinical applications of GCs, mice were injected with DX repeatedly for a time course of 1 month. TECs were examined and drastic downregulation of both Wnt-4 and Foxn1 mRNA levels was detected (Fig. 4A). Although expression of early preadipocyte transdifferentiation markers LAP2α and PPARγ showed no significant alterations by this advanced time point, the downstream adipocyte differentiation factor ADRP was significantly increased in purified TECs of DX-treated animals (Fig. 4A), indicating that prolonged GC treatment pushed preadipocyte type transdifferentiation significantly further than a single-dose DX injection. In harmony with the molecular data, a strong decrease in the medullary compartment (Fig. 4B) was detected while the pattern of the fibroblast marker ER-TR7 (Fig. 4B) became punctuated in DX-treated thymi.
FIG. 4.
Effect of continuous dexamethasone (DX) treatment on thymic epithelial cell (TEC) gene expression and architecture. (A) Bars showing the mean ± standard deviation (SD) (n = 3) of Wnt-4, Foxn1, lamina associated polypeptide-2α (LAP2α), peroxisome proliferator activator receptor-γ (PPARγ), and adipose differentiation-related protein (ADRP) expression of control (□) and DX-treated (▪) TEC. The levels of Wnt-4, Foxn1, and LAP2α were found to be reduced after repeated in vivo DX injections, whereas PPARγ was unaltered. Additionally, ADRP was found to be elevated in DX-treated samples. Note the scale differences for gene expression. EpCAM1-Ly51 co-staining on thymus sections revealed that in DX-treated samples, the medullar area seems to be smaller compared to control (B, left). ER-TR7 staining showed no significant increase but became more punctuated in DX-treated samples. Images are representative of three independent experiments.
Wnt-mediated inhibition of steroid-induced adipose transdifferentiation
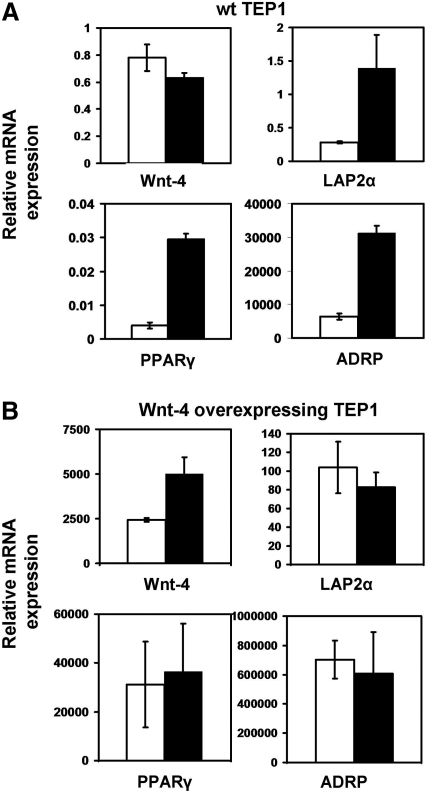
On the basis of our in vivo experiments, Wnt-4 and FoxN1 have a primary role in GC-triggered adipose involution. However, because thymocyte and TEC interactions are vitally important for the normal homeostasis of the thymus,31 it was important to test whether mass depletion of thymocyte populations was the trigger to Wnt-4 and consequently FoxN1 downregulation, or was direct consequence of DX exposure. To answer this question, the TEP1 thymic epithelial cell line was also exposed to DX for 1 week and then molecular changes were analyzed. While Wnt-4 gene transcription was moderately downregulated LAP2α PPARγ and its downstream target ADRP were significantly increased (Fig. 5A), indicating that DX treatment directly induces changes leading to preadipocyte type transdifferentiation. To test the role of Wnt-4 in this process, a Wnt-4-overexpressing TEP1 cell line was created and then treated with DX for a week. True to our expectations, Wnt-4 was able to inhibit preadipocyte-type fate commitment of the cell line, and no significant changes were detected in preadipocyte-type transdifferentiation markers (Fig. 5B). Interestingly, because the TEP1 cell line does not express FoxN1 (unpublished observation), it has become evident that the absence of Wnt-4 alone is required and sufficient to allow preadipocyte-type transdifferentiation, and apparently FoxN1 has no significant role in the process.
FIG. 5.
Wnt-4 overexpression of the TEP1 cell line overcomes dexamethasone (DX)-induced adipocyte-related gene expression in TEP1 cells. Gene expression changes in TEP1 cell lines following 168 h of DX-treatment. The mRNA levels of Wnt-4, lamina associated polypeptide-2α (LAP2α), peroxisome proliferator activator receptor-γ (PPARγ), and adipose differentiation-related protein (ADRP) was tested in solvent- and DX-treated normal (A) and Wnt-4-overexpressing TEP1 (B). White bars indicate solvent-treated control and black bars show the values of DX-treated samples. Results are representative of three independent experiments and showing means ± standard deviation (SD). Note the different scales for gene expression.
Discussion
Our studies confirm that, beside T lymphocytes,8,9 dendritic cells,32,33 and TEC lines,15 primary TECs also express GR-rendering TECs directly sensitive to GCs. Following GC exposure, and similar to physiological aging, DX treatment triggered Wnt-4 and FoxN1 downregulation, leading to increased expression of preadipocyte-type differentiation markers LAP2α and PPARγ.34,20 LAP2α and PPARγ levels increased within a week following DX exposure, highlighting the accelerated rate of GC-induced aging compared to physiological senescence. This is in harmony with literature data, because the involvement of PPARγ has also been confirmed in the induction of thymic involution and ectopic adipogenesis by recent studies using Rosiglitazone,35 an acknowledged PPARγ agonist.
The described increase of LAP2α, PPARγ, and ADRP expression is not likely to occur due to the enrichment of either mTEC or cTEC compartments. In untreated TECs, the LAP2α expression level is similar in both epithelial subsets (data not shown), indicating no difference in sensitivity to adipocyte transdifferentiation inducing factors.
Interestingly, as confirmed by histology, DX-treated thymi regained close-to-normal morphology 3 months following exposure, whereas DX-triggered effects were still detectable at the molecular level. Repeated DX administration resulted in drastic reduction of both Wnt-4 and FoxN1 expression and significant increase of ADRP expression, showing no sign of recovery at the mRNA level. Literature data are supportive, because prolonged decrease in FoxN1 and Wnt-4 expression have recently been demonstrated to lead to degeneration of the thymic epithelial network.36 The reported phenotype was strikingly similar to age-associated involution of the thymus, except for the dramatically accelerated pace similar to that observed following GC administration. Others reported that ubiquitous deletion of FoxN1 in the postnatal thymus has also caused thymic atrophy and severe deterioration of the TEC network.37 Because FoxN1 expression is Wnt-4 dependent,21 Wnt-4 appears to be a realistic candidate molecule to defend the thymus against adipose involution. Using the Wnt-4-overexpressing TEP1 cell line, the working hypothesis was confirmed, because Wnt-4 overexpression could effectively block DX-induced increase of adipocyte markers and protect thymic epithelium-derived cells against DX-induced senescence at the molecular level.
In the present work, DX was shown to trigger accelerated thymic aging accompanied by thymic involution.13,14,17 Using markers identified in our previous studies,20 we demonstrate that the processes of physiological and GC-induced accelerated thymic senescence share similar molecular mechanisms yet operate at different time scales. Because GCs are used relatively often in clinical therapies for sustained periods to suppress actual flares of autoimmune diseases, our results call attention to a currently neglected potential side effect of sustained GC treatment, namely induced accelerated thymic epithelial aging. Accelerated thymic epithelial aging impairs TEC functions, including deletion of potentially autoimmune naïve T cells, as well as allows for accumulation of T cells proliferating in the periphery. Therefore GC-induced accelerated thymic epithelial senescence could provide permissive context for the development of T cell–mediated autoimmune diseases. This would mean that GC treatment could paradoxically lead to the emergence of immune pathologies, including certain autoimmune diseases.38–40 Our findings concerning Wnt-mediated inhibition of GC-triggered accelerated thymic senescence are also particularly important, becasue Wnt-4 could potentially be a candidate molecule to inhibit adipose involution of the human thymus following GC treatment or even physiological senescence, although further in vivo experiments are required to clarify the potential application process.
Acknowledgments
This work was supported by an European Union (EU) project grant to P.J. and B.T. (“Science Please” Research Team on Innovation grant no. SROP-4.2.2/08/1/2008-0011), a Wellcome Trust (no. 079415) project grant to P.J., E.J., G.A.; a Pecs University support grant to B.T. (PTE ÁOKKA-34039-7/2009) and by the Hungarian Scientific Research Fund (PD OTKA No: 78310) to K.K.
We thank Peter Balogh, M.D., Ph.D. (Inst. for Immunology & Biotechnology, University of Pécs) for the ER-TR7 monoclonal antibody and Gergely Berta, M.D., and György Sétáló, M.D., Ph.D., for confocal microscopy (Dept. of Medical Biology, University of Pécs). The microscope was purchased from a GVOP-3.2.1-2004-04-0172/3.0 equipment grant to Pécs University.
Author Disclosure Statement
There are no competing interests.
References
- 1.Cooper GS. Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Abreu D. Bordoni A. Zucca E. Epidemiology of hematological malignancies. Ann Oncol. 2007;18(Suppl 1):i3–i8. doi: 10.1093/annonc/mdl443. [DOI] [PubMed] [Google Scholar]
- 3.Rhen T. Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 4.Greenstein S. Ghias K. Krett NL. Rosen ST. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res. 2002;8:1681–1694. [PubMed] [Google Scholar]
- 5.Schmidt S. Rainer J. Ploner C. Presul E. Rimil S. Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11(Suppl 1):S45–S55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- 6.Stahn C. Lowenberg M. Hommes DW. Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275:71–78. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Wust S. van den Brandt J. Tischner D. Kleiman A. Tuckermann JP. Gold R. Luhder F. Reichardt HM. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol. 2008;180:8434–8443. doi: 10.4049/jimmunol.180.12.8434. [DOI] [PubMed] [Google Scholar]
- 8.Wiegers GJ. Knoflach M. Bock G. Niederegger H. Dietrich H. Falus A. Boyd R. Wick G. CD4(+)CD8(+)TCR(low) thymocytes express low levels of glucocorticoid receptors while being sensitive to glucocorticoid-induced apoptosis. Eur J Immunol. 2001;31:2293–2301. doi: 10.1002/1521-4141(200108)31:8<2293::aid-immu2293>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Berki T. Palinkas L. Boldizsar F. Nemeth P. Glucocorticoid (GC) sensitivity and GC receptor expression differ in thymocyte subpopulations. Int Immunol. 2002;14:463–469. doi: 10.1093/intimm/14.5.463. [DOI] [PubMed] [Google Scholar]
- 10.Palinkas L. Talaber G. Boldizsar F. Bartis D. Nemeth P. Berki T. Developmental shift in TcR-mediated rescue of thymocytes from glucocorticoid-induced apoptosis. Immunobiology. 2008;213:39–50. doi: 10.1016/j.imbio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Boldizsar F. Palinkas L. Czompoly T. Bartis D. Nemeth P. Berki T. Low glucocorticoid receptor (GR), high Dig2 and low Bcl-2 expression in double positive thymocytes of BALB/c mice indicates their endogenous glucocorticoid hormone exposure. Immunobiology. 2006;211:785–796. doi: 10.1016/j.imbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Jondal M. Pazirandeh A. Okret S. Different roles for glucocorticoids in thymocyte homeostasis? Trends Immunol. 2004;25:595–600. doi: 10.1016/j.it.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Blomgren H. Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970;1:545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- 14.Boersma W. Betel I. van der Westen G. Thymic regeneration after dexamethasone treatment as a model for subpopulation development. Eur J Immunol. 1979;9:45–52. doi: 10.1002/eji.1830090111. [DOI] [PubMed] [Google Scholar]
- 15.Dardenne M. Itoh T. Homo-Delarche F. Presence of glucocorticoid receptors in cultured thymic epithelial cells. Cell Immunol. 1986;100:112–118. doi: 10.1016/0008-8749(86)90011-0. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher AL. Lowen TE. Sakkal S Reiseger JJ. Hammett MV. Seach N. Scott HS. Boyd RL. Chidgey AP. Ablation and regeneration of tolerance-inducing medullary thymic epithelial cells after cyclosporine, cyclophosphamide, and dexamethasone treatment. J Immunol. 2009;183:823–831. doi: 10.4049/jimmunol.0900225. [DOI] [PubMed] [Google Scholar]
- 17.Taub DD. Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 18.Qiao S. Chen L. Okret S. Jondal M. Age-related synthesis of glucocorticoids in thymocytes. Exp Cell Res. 2008;314:3027–3035. doi: 10.1016/j.yexcr.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Dixit VD. Thymic fatness and approaches to enhance thymopoietic fitness in aging. Curr Opin Immunol. 2010;22:521–528. doi: 10.1016/j.coi.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kvell K. Varecza Z. Bartis D. Hesse S. Parnell S. Anderson G. Jenkinson EJ. Pongracz JE. Wnt4 and LAP2alpha as pacemakers of thymic epithelial senescence. PLoS One. 2010;5:e10701. doi: 10.1371/journal.pone.0010701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balciunaite G. Keller MP. Balciunaite E. Piali L. Zuklys S. Mathieu YD. Gill J. Boyd R. Sussman DJ. Hollander GA. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol. 2002;3:1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- 22.Pongracz J. Hare K. Harman B. Anderson G. Jenkinson EJ. Thymic epithelial cells provide WNT signals to developing thymocytes. Eur J Immunol. 2003;33:1949–1956. doi: 10.1002/eji.200323564. [DOI] [PubMed] [Google Scholar]
- 23.Buttgereit F. Straub RH. Wehling M. Burmester GR. Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum. 2004;50:3408–3417. doi: 10.1002/art.20583. [DOI] [PubMed] [Google Scholar]
- 24.Buttgereit F. da Silva JA. Boers M. Burmester G-R. Cutolo M. Jacobs J. Kirwan J. Kohler L. van Riel P. Vischer T. Bijisma JWJ. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis. 2002;61:718–722. doi: 10.1136/ard.61.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berki T. Kumanovics G. Kumanovics A. Falus A. Ujhelyi E. Nemeth P. Production and flow cytometric application of a monoclonal anti-glucocorticoid receptor antibody. J Immunol Methods. 1998;214:19–27. doi: 10.1016/s0022-1759(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 26.Revollo JR. Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann NY Acad Sci. 2009;1179:167–178. doi: 10.1111/j.1749-6632.2009.04986.x. [DOI] [PubMed] [Google Scholar]
- 27.Bayo P. Sanchis A. Bravo A. Cascallana JL. Buder K. Tuckermann J. Schutz G. Perez P. Glucocorticoid receptor is required for skin barrier competence. Endocrinology. 2008;149:1377–1388. doi: 10.1210/en.2007-0814. [DOI] [PubMed] [Google Scholar]
- 28.Manwani N. Gagnon S. Post M. Joza S. Muglia L. Cornejo S. Kaplan F. Sweezey NB. Reduced viability of mice with lung epithelial-specific knockout of glucocorticoid receptor. Am J Respir Cell Mol Biol. 2010;43:503–606. doi: 10.1165/rcmb.2009-0263OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray DH. Chidgey AP. Boyd RL. Analysis of thymic stromal cell populations using flow cytometry. J Immunol Methods. 2002;260:15–28. doi: 10.1016/s0022-1759(01)00493-8. [DOI] [PubMed] [Google Scholar]
- 30.Rodewald HR. Thymus organogenesis. Annu Rev Immunol. 2008;26:355–388. doi: 10.1146/annurev.immunol.26.021607.090408. [DOI] [PubMed] [Google Scholar]
- 31.Alves NL. Huntington ND. Rodewald HR. Di Santo JP. Thymic epithelial cells: the multi-tasking framework of the T cell “cradle.”. Trends Immunol. 2009;30:468–474. doi: 10.1016/j.it.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Sacedon R. Vicente A. Varas A. Jimenez E. Munoz JJ. Zapata AG. Glucocorticoid-mediated regulation of thymic dendritic cell function. Int Immunol. 1999;11:1217–1224. doi: 10.1093/intimm/11.8.1217. [DOI] [PubMed] [Google Scholar]
- 33.Pan J. Ju D. Wang Q. Zhang M. Xia D. Zhang L. Cao X. Dexamethasone inhibits the antigen presentation of dendritic cells in MHC class II pathway. Immunol Lett. 2001;76:153–161. doi: 10.1016/s0165-2478(01)00183-3. [DOI] [PubMed] [Google Scholar]
- 34.Youm YH. Yang H. Sun Y. Smith RG. Manley NR. Vandanmagsar B. Dixit VD. Deficient ghrelin receptor-mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J Biol Chem. 2009;284:7068–7077. doi: 10.1074/jbc.M808302200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youm YH. Yang H. Amin R. Smith SR. Leff T. Dixit VD. Thiazolidinedione treatment and constitutive-PPARgamma activation induces ectopic adipogenesis and promotes age-related thymic involution. Aging Cell. 2010;9:478–489. doi: 10.1111/j.1474-9726.2010.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L. Xiao S. Manley NR. Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner. Blood. 2009;113:567–574. doi: 10.1182/blood-2008-05-156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng L. Guo J. Sun L. Fu J. Barnes PF. Metzger D. Chambon P. Oshima RG. Amagai T. Su D-M. Postnatal tissue-specific disruption of transcription factor FoxN1 triggers acute thymic atrophy. J Biol Chem. 2010;285:5836–5847. doi: 10.1074/jbc.M109.072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann NY Acad Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- 39.Bassett D. Hirata F. Gao X. Kannan R. Kerr J. Doyon-Reale N. Wilson S. Lieh-Lai M. Reversal of methylprednisolone effects in allergen-exposed female BALB/c mice. J Toxicol Environ Health A. 2010;73:711–724. doi: 10.1080/15287391003614018. [DOI] [PubMed] [Google Scholar]
- 40.Royer B. Lee K. Gruson B. Roszkiewicz F. Al Khedr A. Colson A. Camboulives A. Bierling P. Marolleau JP. Methylprednisolone-induced immune thrombocytopenia. Blood. 2010;115:5431–5432. doi: 10.1182/blood-2010-01-264986. [DOI] [PubMed] [Google Scholar]