Abstract
Decreases in cardiac connexin43 (Cx43) play a critical role in abnormal cell to cell communication and have been linked to the resistance of the female heart to arrhythmias. We therefore hypothesized that Cx43 expression would be greater in female cardiomyocytes than male cardiomyocytes under pathologic conditions. Adult ventricular myocytes were isolated from male and female rats and treated with phenylephrine, a well established pathologic stimulus. Cx43 gene and protein expression was determined. microRNA-1 expression, a microRNA known to control Cx43 protein expression in cardiomyocytes, was also determined. Cx43 mRNA and protein levels were significantly higher in the female cardiomyocytes than the male cardiomyocytes (mRNA: 1.4-fold; Protein: 5-fold, both p< 0.05) under both basal and pathologic conditions. Phenylephrine treatment increased Cx43 expression only in female cardiomyocytes. Cx43 phosphorylation, a marker of preserved Cx43 function, was also higher (P<0.05) and microRNA-1 expression was lower (P<0.05) in the female cardiomyocytes after phenylephrine treatment. microRNA-1 expression was unchanged by phenylephrine treatment in male cardiomyocytes. Thus, a sex-difference in microRNA-1 may be responsible for the sex-difference in Cx43 expression in cardiomyocytes under pathologic conditions. Taken together our results demonstrate a sex-difference in Cx43 expression and site specific phosphorylation that favors cardioprotection in female cardiomyocytes.
Keywords: Gender, microRNA-1, Adult rat ventricular myocyte, phenylephrine, phosphorylation
Introduction
Connexin proteins form intercellular pores in many tissues. Connexin43 (Cx43), Cx40 and Cx45 are expressed in the heart with Cx43 being the predominant protein in the ventricular myocardium. Connexin proteins oligomerize (form connexons), migrate to the cell membrane and contribute to gap junctions. In gap junctions connexons enable rapid and coordinated electrical excitation and facilitate the intercellular exchange of small molecules, regulatory proteins and metabolites (~1kD). In the heart, normal gap junction expression and phosphorylation is essential for organized myocellular electrical activity. Disease states, such as ischemic heart disease and heart failure, decrease gap junction expression and cause Cx43 dephosphorylation [1–5]. These changes in cardiac Cx43 produce heterogeneity in intercellular communication and increase the propensity for cardiac arrhythmias [6–9].
A sex-difference in heart failure phenotype [10] and arrhythmic sudden cardiac death [11–17] has been well established in humans. In the setting of coronary heart disease women are at significantly lower risk than men of sudden cardiac death. While no study has determined differences in Cx43 expression between the sexes in the human, a rodent model has shown that higher levels of cardiac Cx43 in the female heart [18] are associated with lower lethal arrhythmia susceptibility [19]. Abnormalities in cardiac Cx43 expression and phosphorylation are believed to be the primary lesion contributing to arrhythmogenesis in a number of syndromes, as they occur prior to other structural remodeling events [20–22].
The aim of the current study was to determine whether there are sex-specific differences in myocellular Cx43 expression in isolated adult rat ventricular myocytes following a pathologic stimulus. Since Cx43 turnover occurs on the order of hours [23] we expected the sex-differences in Cx43 expression and phosphorylation to be a dynamic process following pathologic stimuli. We specifically hypothesized that male cardiomyocytes would demonstrate less Cx43 than female cardiomyocytes. To address this hypothesis we determined Cx43 mRNA and protein expression in sex-specific cultured adult rat ventricular myocytes stimulated with the α1-adrenergic receptor agonist phenylephrine, a well established pathologic stimulus in vitro.
Methods
Sex-specific Adult Rat Ventricular Myocyte Isolation and Culture
All experimental protocols adhered to the guidelines for research and were reviewed and approved by the Institutional Animal Care and Use Committee for the University of Colorado Denver. Adult rat ventricular myocytes (ARVMs) were isolated using the methods of Wolska and Solaro [24]. Briefly, rats of either sex (20 male, 20 female,15–19 weeks, 200–300 gm) were injected with heparin (5000U/kg i.p.). After 30 minutes, the rats were deeply anesthetized with ketamine and xylazine. The hearts were quickly removed and put into an ice-cold nominally calcium-free control solution (pH 7.4) containing (in mM): NaCL (133.5), KCl (4), NaH2PO4 (1.2), HEPES (10), MgSO4 (1.2), glucose (11.1) and bovine serum albumin (1g/L). Adherent non-cardiac tissue was trimmed and the heart retrograde perfused (37°C) through aortic cannulation on a modified Langendorf apparatus, first with the control solution and subsequently with the enzyme solution (control solution plus 0.02 mM Ca2+, collagenase II (Worthington Biochemical Co.) and Protease (Sigma). The aorta and atria were removed and the myocytes dissociated. To isolate any intrinsic differences between the male and female cardiomyocytes, the cells (3.0 ×105) were plated on 60 mm dishes in serum-free and phenol red-free DMEM with L-carnitine (2mM), creatine (5mM) and taurine (5mM) to remove any potential confounding due to activation of sex hormone receptors [25] and improve longevity in culture [26, 27]. Ten mM 2, 3-butandione monoxime (BDM) was added to the culture media to improve myocyte viability [28]. Cell counting of 6 random fields to measure plating density was performed prior to treatment to verify similar plating densities between sexes. Cells were cultured for 72 hours under basal conditions with daily media changes as outlined above and then treated with the α1-adrenergic receptor agonist phenylephrine (PE, 10 µM) or vehicle control for 24 hrs prior to harvesting [29, 30]. To determine that the sex specific response to PE was mediated through the α1-adrenergic receptor (AR), a subset was pretreated with prazosin (PRZ, 100nM), an α1-adrenergic receptor antagonist [31].
Hypertrophic Gene Expression
The expression of several genes known to be induced by PE [32] was determined by RTqPCR using SYBR green and transcript specific primer pairs (ABI 7500) for the natriuretic peptides, ANP and BNP, and the β myosin heavy chain (MyHC) isoform as previously described [33]. Briefly, cells were harvested in 1 mL of TriZOL reagent and total RNA was isolated according to the manufacturer’s recommendations. Reverse transcription of total RNA (500 ng) was performed according to the manufacturers specifications (iScript, Bio-Rad). Five ng of cDNA was used for each 25 µL PCR reaction performed according to standard protocols using the ABI7300 (Applied Biosystems). All genes of interest (including Cx43 below) were normalized to the expression of the 18S ribosomal RNA [33] and changes in expression were evaluated using the ΔΔCt method as previously described [34].
Cx43 mRNA and Protein Expression
Expression of Cx43 mRNA was determined using RTqPCR and transcript specific primer pairs (forward: 5’-GTG AAA GAG AGG TGC CCA GAC AT-3’; reverse: 5’-CCC CAA GGC ACT CCA GTC A-3’) as outlined above. The protein fraction was resuspended in Laemmli buffer (Bio-rad; 161-0737) in the presence of phosphatase and protease inhibitors (β-glycerophosphate (1mM), sodium orthovanadate (1mM), aprotinin (1µM), leupeptin (10µM) and phenylmethylsulfonyl fluoride (10mM)). Cx43 protein expression and phosphorylation was determined by separating 25 µg of total protein using standard Western blotting techniques on a 7.5% criterion gel (Bio-Rad) and transfer to a membrane (PVDF). The membrane was then probed with commercially available antibodies specific for Cx43 (Santa Cruz Biotechnology: sc-9059) and Calnexin (as a loading control). Phosphorylation at Cx43 serine 368 (S368) was demonstrated using an antibodies specific for phosphorylation (Cell Signaling #3511S) and lack of phosphorylation (Invitrogen/Zymed #13-8300) at this site. Expression was quantified using ImageJ (NIH) from chemiluminescence exposed film.
microRNA-1 Expression
Reverse transcription of microRNA (miRNA) was performed using the TaqMan™ MicroRNA Reverse Transcription Kit (Applied Biosystems) according to manufacturer’s recommendations [35]. Briefly, 5 ng of miRNA were combined with dNTPs, MultiScribe™ reverse transcriptase, and the primer specific for the target miRNA. The resulting cDNA was diluted 15-fold and used in PCR reactions. PCR was performed according to manufacturer’s recommendations (Applied Biosystems). Briefly, cDNA was combined with the TaqMan™ assay specific for the target miRNA, and PCR reaction was done using the ABI7300. Expression of microRNA-1 (miR-1) was normalized to RNU66 expression.
Statistical Analysis
All data are presented as mean ± sem. Differences between treatment groups and sexes were determined by Student’s t test for parametric data and Mann-Whitney testing for nonparametric data. Statistical significance was set a priori at P < 0.05.
Results
Sex-specific plating densities
Because Cx43 is a gap junction protein that may be influenced by cellular confluence, random fields of each plate were counted prior to PE treatment. There were no differences in plating density between the male and female cardiocytes (data not shown).
Expression of pathologic genes
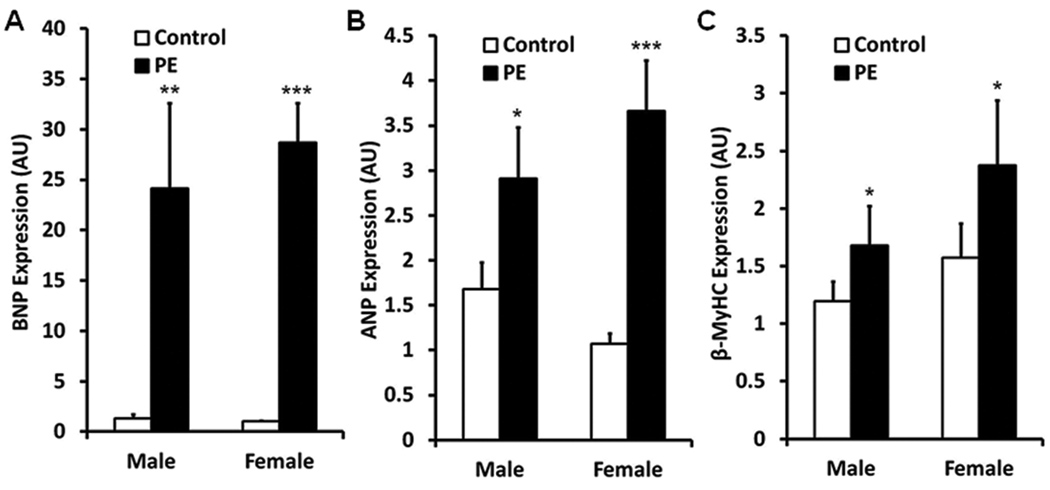
As has been documented previously in isolated neonatal [36] and male adult cardiocytes [37], PE induced the natriuretic peptides, ANP and BNP, and β-MyHC. However, there were no differences in expression between the sexes (Figure 1). These findings indicate that PE induces several of the hypertrophic genes in a similar fashion between male and female cardiomyocytes and suggest that our pathologic stimulus was similar between sexes.
Figure 1.
Pathologic gene induction by phenylephrine (PE) in ARVMs. Male and female ARVMs were isolated as outlined in materials and method and treated for 24 hours with PE (10 mcM). Brain natriuretic peptide (BNP), atrial natriuretic peptide (ANP) and β-myosin heavy chain isoform (β-MyHC) mRNA expression were determined by quantitative RT-PCR. PE treatment produced similar induction of BNP (A), ANP (B) and β-MyHC (C) in both male and female ARVMs indicated a similar pathologic response between the sexes. 20 hearts/group, n = 30/group. Control vs PE: * P<0.05, ** P<0.01, *** P<0.0001.
Expression of Cx43
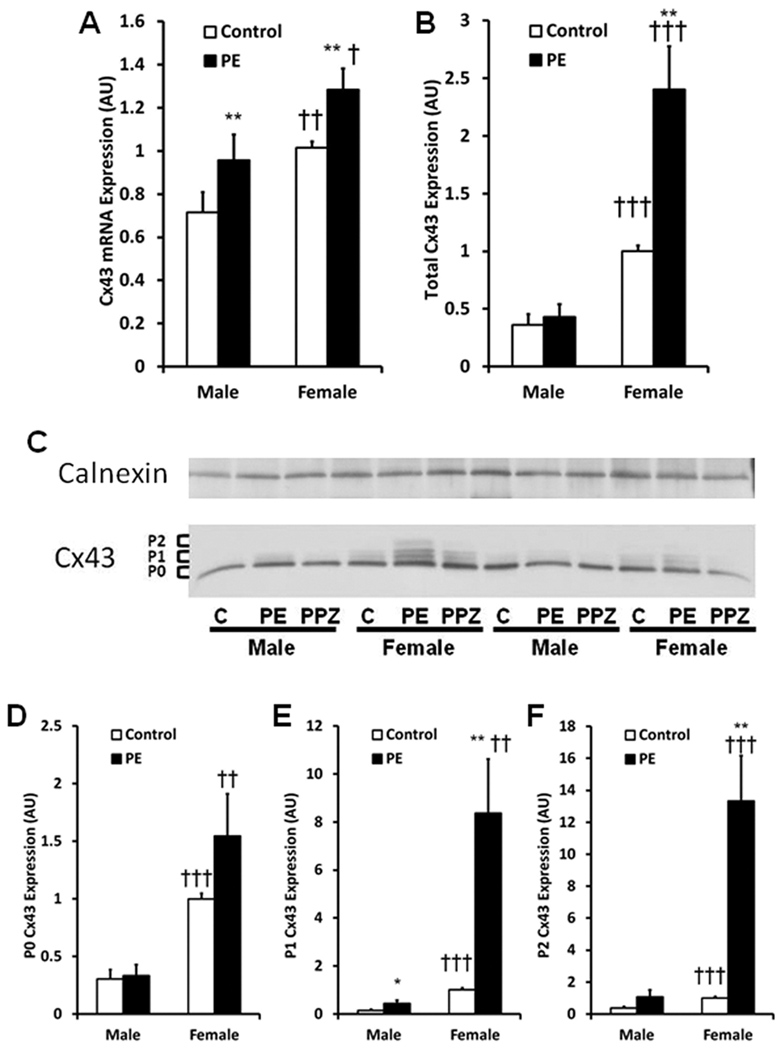
PE is known to induce Cx43 expression in neonatal rat ventricular myocytes [30, 38]. Cx43 mRNA and protein expression were higher in female than male adult cardiomyocytes under control conditions. PE treatment of sex-specific ARVMs increased Cx43 mRNA in both male and female cardiomyocytes (Figure 2A).
Figure 2.
Connexin 43 (Cx43) expression under basal and pathologic conditions. Female ARVMs demonstrate higher Cx43 mRNA (Panel A) and protein (Panels B–F) expression than male ARVMs under control and PE treated conditions. PE treatment increases Cx43 mRNA expression in both male and female cardiomyocytes (Panel A). C: Representative Western Blot of Cx43 phosphorylation isoforms separated by SDS-PAGE under control (C), PE and PE+Prazosin (PPZ) treated conditions. All protein data is normalized to Calnexin, used as a loading control. The faster migrating form includes the non-phosphorylated isoform (P0). There are also at least 2 slower migrating isoforms (P1 and P2). Although PE treatment increases the P1 isoform in male ARVMs (Panel E), PE treatment increases total Cx43 and the P1 and P2 isoforms female ARVMs (Panels C, E, F). mRNA: 20 hearts/group, n = 30/group; Protein: 11 hearts/group. Control vs PE: * P<0.05, ** P<0.01; Male vs Female: † P<0.05, †† P<0.005, ††† P<0.0005.
Cx43 is known to be a phosphoprotein with several electrophoretic isoforms when analyzed by SDS/PAGE. The fastest migrating form includes the non-phosphorylated form (P0) and the two slower migrating forms commonly referred to as P1 and P2 contain unique post-translational modifications [39]. After 96 hours in culture the P0, P1 and P2 bands migrated to a similar extant as those bands from freshly isolated cells (data not shown). In contrast to the mRNA expression above, only female cardiomyocytes demonstrate significantly higher expression of total Cx43 protein (Figure 2B) and higher expression of each phosphorylation isoform (Figures 2D, 2E and 2F) following PE treatment. In addition, female ARVMs demonstrate significantly greater Cx43 expression (Figure 2B) and phosphorylation (Figures 2D, 2E and 2F) than male ARVMs under both basal and PE treated conditions. While no significant difference in total Cx43 protein expression was noted between control and PE treated male cardiomyocytes, PE treatment did produce a minor increase in the P1 band in male ARVMs. Co-treatment of ARVMs with PE and prazosin (PPZ) completely abrogated the PE stimulated increase in Cx43 expression and phosphorylation (Figure 2C) indicating the changes are occurring through a pathway requiring the α1-adrenergic receptor.
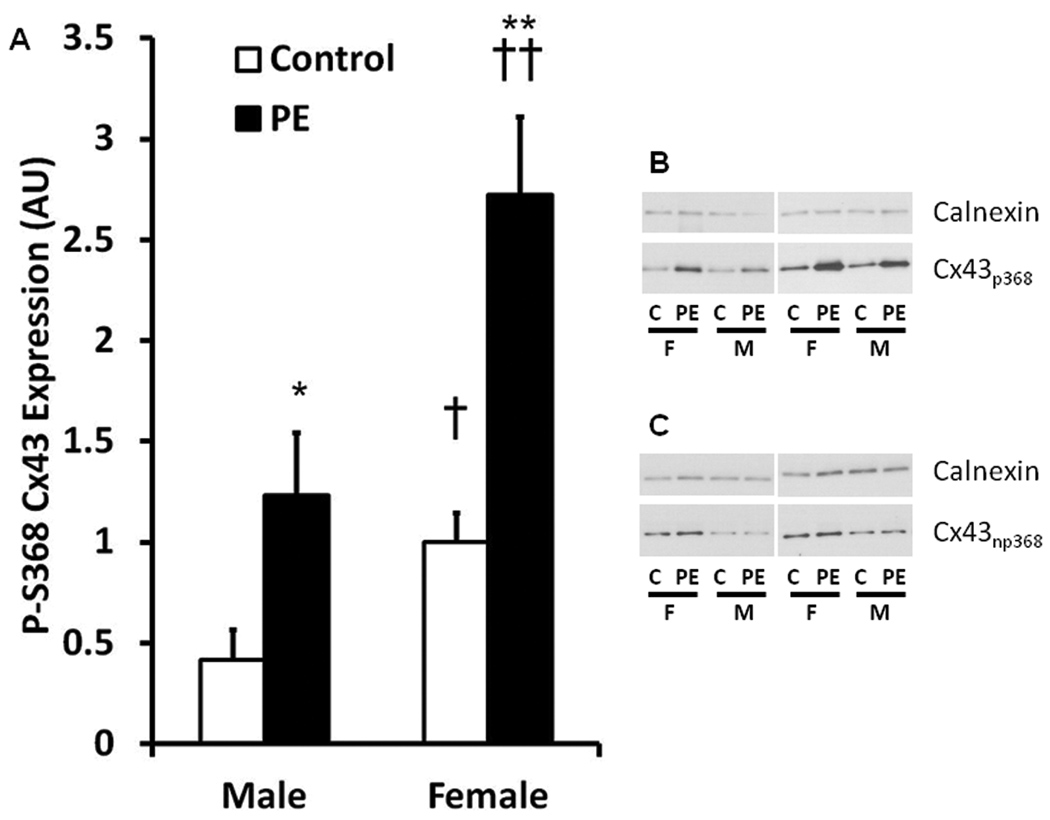
PE treatment increased phosphorylation at S368 in both male and female cells. Interestingly, phosphorylation of Cx43 at S368 was significantly higher in the female cardiomyocytes than male cardiomyocytes under both control and PE treated conditions (Figure 3A and 3B). In contrast, PE treatment did not influence the amount of Cx43 that lacked phosphorylation at S368 in either sex (Figure 3C). This finding suggests that the large increase in Cx43 expression in the female cardiomyocytes (Figure 2) was predominantly associated with phosphorylation at this site. Of note the non-phosphorylated S368 (NP-S368) Cx43 isoform was slightly higher in the female ARVMs compared to the male ARVMs under control conditions (1.0±0.1 vs 0.5 ± 0.1 AU, P < 0.001) but not following PE treatment (1.3 ± 0.3 vs 0.7 ± 0.2 AU).
Figure 3.
Densitometric analysis (Panel A) and representative Western Blots (Panel B) of expression of the Connexin 43 (Cx43) isoform phosphorylated at serine 368 (S368) under basal and pathologic conditions. Female ARVMs demonstrate higher phosphorylation at S368 (p368) under both basal and PE treated conditions. Phosphorylated S368 isoforms are higher following PE treatment in both male and female ARVMs. Panel C: Representative Western blot of nonphosphorylated Cx43 at S368 (np368). 4 hearts/group, n = 8/group. Control vs PE: * P<0.05, ** P<0.01; Male vs Female: † P<0.05, †† P<0.01.
microRNA-1 expression
micro RNA-1 (miR-1) expression modifies Cx43 expression and arrhythmia potential in cardiomyocytes [40]. We therefore determined sex-specific changes in miR-1 in response to PE as a potential mechanism underlying the sex-differences we observed in Cx43 protein expression. miR-1 expression was significantly lower with PE treatment in the female ARVMs compared with the female control treatment (Figure 4). In contrast, no difference in miR-1 expression was demonstrated in the male cardiomyocytes. As downregulation of miR-1 increases Cx43 protein expression, these data suggest that miR-1 may play a role in the sex-differences observed in Cx43 expression in ARVMs under pathologic conditions.
Figure 4.
microRNA-1 (miR-1) expression is lower in female but not male ARVMs following PE treatment. A decrease in miR-1 expression removes repression of Cx43 protein expression in the female ARVMs. 9 hearts/group, n = 13/group. Control vs PE: * P<0.05.
Discussion
To our knowledge this is the first study to evaluate sex-differences in the response of ARVMs to PE treatment. There are several major findings in the current study. First, the up-regulation of hypertrophic gene expression is similar between male and female ventricular cardiocytes in response to PE treatment. Second, female cardiomyocytes demonstrate greater Cx43 expression and phosphorylation than male ARVMs under basal conditions and PE treatment increases Cx43 expression in female ARVMs but not male cells. Finally, PE treatment decreases miR-1 expression only in female ARVMs. These results suggest both a sex-difference in basal Cx43 expression in ventricular myocytes as well as a sex-difference in Cx43 expression in response to a pathologic stimulus. These findings may be explained by the sex-differences in Cx43 mRNA and miR-1 expression, respectively.
Sex differences in cardiac disease have been noted for several decades. Although frequently associated with the vasculature [41], sex differences in the myocardium have been demonstrated [42]. For example, under basal conditions, electrocardiographic QT intervals are longer in women than men [43]. Following a similar mechanical load, the female heart has greater hypertrophic reserve and remains in a more compensated state while the male heart is more likely to decompensate to a dilated phenotype [44]. Furthermore, although this disparity decreases with advancing age, the death rate for sudden cardiac deaths is 50% higher in men than women, and the annual incidence is 3–4 times higher in men than women [16, 17]. The mechanisms underlying these sex-differences remain poorly understood.
Given the importance of normal cardiac intercellular communication through gap junctions it has been suggested that sex differences in Cx43, the major cardiac gap junction protein, may be present in basal and pathologic conditions [18, 19, 42]. Several groups have demonstrated that under basal conditions Cx43 mRNA [42] and protein [18] expression is higher in the post-pubertal female heart compared with age-match males. Knezl et. al. found that the basal sex-difference in Cx43 persisted in aging spontaneously hypertensive rats and correlated with the predisposition to develop ventricular fibrillation [19]. Unfortunately, these studies were all performed in the setting of heterogeneous endogenous hormone levels which limit the ability to determine intrinsic sex-differences in cardiomyocytes.
The alpha-adrenergic agonist phenylephrine (PE) has been widely used in neonatal rat ventricular myocytes to determine the mechanisms underlying changes in Cx43 expression in the diseased heart. However, the neonatal heart is not as dependent on Cx43 as the adult heart due to higher Cx40 expression, which may limit the utility of this model system [45]. Moreover, none of these studies use sex-specific cells in vitro and therefore provide no insight into potential mechanisms underlying clinical sex-differences in heart failure and sudden cardiac death. Consistent with prior data in vivo [46], PE treatment in ARVMs resulted in a similar increase in ANP between the sexes. However, the current study also found no sex-dependent differences in the expression of BNP and β-MyHC, two other markers of cardiac pathology. This lack of difference confirms that the degree of pathology induced by the stimulus is similar between the sexes. In contrast, this acute pathologic stimulus produces a sex-difference in Cx43 expression and phosphorylation in cardiomyocytes, thereby providing direct evidence for intrinsic differences in gap junction regulation between males and females. To our knowledge no prior study has demonstrated significant sex-differences in the phosphorylated Cx43 isoforms under pathologic conditions or determined changes in Cx43 expression in ARVMs following PE treatment, a model for pathologic hypertrophy and heart failure.
Tribulova et. al. [18] demonstrated higher Cx43 expression in the intact aging female rat heart when compared to age matched males. In a similar fashion, we demonstrate significantly higher total Cx43 expression under basal conditions in cardiomyocytes in vitro. However, we have expanded their findings by demonstrating greater phosphorylation under both basal and pathologic conditions. It is possible that the prior study was underpowered to see differences in basal phosphorylation, since they demonstrated marked trends toward significance with an n of 6 animals/group. Post-translational modifications of connexins are important for normal function [23, 39]. Phosphorylation appears to regulate channel function and rates of channel assembly and turnover. Protein kinase C (PKC), a kinase downstream from PE, is known to phosphorylate Cx43 at several sites including S368 [47]. On SDS-PAGE, the phosphorylated isoforms can either co-localize with the non-phosphorylated isoform or demonstrate slower migration. The migration characteristics are not completely understood but appear to be dependent on the site(s) of phosphorylation and the cell type under investigation [39].
One of the primary new findings of the current study is that under both basal and pathologic conditions female cardiomyocytes maintain higher levels of Cx43. This sex-difference is primarily found in the more highly phosphorylated isoforms. It may be that this sex difference in Cx43 expression and phosphorylation causes important differences in cell to cell connections, molecule trafficking and communication that will influence cardiac disease development. Indeed, there are several lines of evidence for this in vivo. Lower Cx43 expression and phosphorylation increase arrhythmia formation in cultured cardiac myocytes [48] and in vivo in animal models [19, 49, 50]. For example, hearts from normotensive or hypertensive female rats are less susceptible to ventricular fibrillation than male counterparts. The fibrillation risk in this study correlated with lower ventricular Cx43 levels in the males [19]. Differences in Cx43 expression is noted between compensated and decompensated cardiac disease in humans and animal models. Cx43 levels are higher in compensated hypertrophy and attenuated levels are found in decompensated heart failure and may contribute to the abnormalities in myocellular conduction and contractility and cardiac arrhythmias in these patients [51, 52].
Our results show sex-differences in phosphorylation at the S368 site. Phosphorylation at this site has several functional consequences [53]. The S368 site depends on Protein Kinase C (PKC) for phosphorylation and produces changes in the selective permeability of the gap junction complex in fibroblasts. In other model systems phosphorylation of S368 leads to unchanged Cx43 permeation by current carrying ions but enhanced permeation to some larger molecules [53, 54]. These changes are observed following ischemia ex vivo and are believed to be protective [53]. Thus our results demonstrate a sex difference in the site specific phosphorylation of Cx43 that favors a cardioprotective phenotype in the female cardiomyocytes. Higher PKC activity has been demonstrated in the female rat liver than the male liver [55]. Furthermore, sex differences in PKC activity has been attributed to greater resistance to ischemia-reperfusion injury in the female heart [56]. PE treatment is known to activate PKC through the α-adrenergic receptor. Thus it is possible that sex differences in basal and α-adrenergic activation of PKC contributed to our findings.
MicroRNA (miRNA) are small non-coding sequences that modulate gene expression by targeting mRNAs for post-translational repression through binding to the 3’-untranslated regions [40]. Although changes in mRNA levels can be responsible for the sex difference in Cx43 protein expression under basal conditions, the magnitude of change following PE treatment is likely too great to be attributed solely to the mRNA changes. We therefore considered alternative protein regulatory pathways such as miRNA. miRNA-1 controls Cx43 expression in other cell systems and has been linked to arrhythmic potential through regulation of Cx43 in vivo [40]. The current study demonstrates that following PE treatment female cardiomyocytes have lower miR-1 expression and higher Cx43 expression than under basal conditions while there are no differences in miR-1 expression in the male cardiomyocytes. These data suggest a regulatory role for miR-1 in the sex-difference in Cx43 expression under pathologic conditions. Although, higher miR-1 expression has been found in human ischemic hearts [40], no sex difference in miR-1 expression has been previously demonstrated. It is interesting to note that miR-1 expression is similar in male and female cardiocytes under unstimulated conditions. Therefore, miR-1 is unlikely to be contributing to the sex-differences in Cx43 expression in the unstimulated cells. The differences in Cx43 protein expression in the basal state may be transcriptionally regulated as it parallels the sex-difference in Cx43 mRNA expression.
There are several important limitations of the current study. First, the current evaluation was performed in the rat, thus potentially limiting the application to clinical diseases. However, Vozzi, et. al. performed an extensive characterization of Cx43 in the human heart and concluded that the ventricular Cx43 expression pattern is similar to that of other mammalian species and therefore non-human species should provide reliable models [57]. Second, an in vitro model was used. Multiple studies using nearly identical in vitro techniques have determined important molecular pathways controlling Cx43 expression at both the mRNA and protein level, as well as, functional implications of post-translational protein modifications. Furthermore, culture in a serum free media without phenol-red provides strong evidence of intrinsic sex-difference independent of the influence of sex steroids. Finally, using the described techniques we are unable to determine the influences of these sex-differences in Cx43 expression on gap junction properties. Investigations into the functional implications of our findings will require additional research beyond the scope of the current study.
In conclusion, the current study demonstrates a sex-difference in ARVM Cx43 expression and phosphorylation at baseline and under similar pathologic stimulation between the sexes. Our results suggest a sex difference in Cx43 isoform expression that favors a cardioprotective phenotype in the female cardiomyocytes. These sex-differences in Cx43 may play a role in the sex differences in phenotype found in vivo. Finally, sex-differences in miR-1 may be a mechanism underlying the differences in Cx43 protein expression between male and female ARVMs and provide novel evidence for microRNA regulation of sex-dependent phenotypes.
Acknowledgments
Funding: NIH K08 HL080212
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1223–H1230. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 2.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 3.Luke RA, Saffitz JE. Remodeling of ventricular conduction pathways in healed canine infarct border zones. J Clin Invest. 1991;87:1594–1602. doi: 10.1172/JCI115173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol. 1991;139:801–821. [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–279. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci U S A. 2007;104:20512–20516. doi: 10.1073/pnas.0705472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 8.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–H1770. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 9.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 10.Hoppe BL, Hermann DD. Sex differences in the causes and natural history of heart failure. Curr Cardiol Rep. 2003;5:193–199. doi: 10.1007/s11886-003-0048-6. [DOI] [PubMed] [Google Scholar]
- 11.Abildstrom SZ, Rask-Madsen C, Ottesen MM, Andersen PK, Rosthoj S, Torp-Pedersen C, Kober L. Impact of age and sex on sudden cardiovascular death following myocardial infarction. Heart. 2002;88:573–578. doi: 10.1136/heart.88.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adabag AS, Therneau TM, Gersh BJ, Weston SA, Roger VL. Sudden death after myocardial infarction. JAMA. 2008;300:2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannel WB, Wilson PW, D'Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 14.Orencia A, Bailey K, Yawn BP, Kottke TE. Effect of gender on long-term outcome of angina pectoris and myocardial infarction/sudden unexpected death. JAMA. 1993;269:2392–2397. [PubMed] [Google Scholar]
- 15.Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, Vliegen HW, van Dijk AP, Bouma BJ, Grobbee DE, Mulder BJ. Gender and outcome in adult congenital heart disease. Circulation. 2008;118:26–32. doi: 10.1161/CIRCULATIONAHA.107.758086. [DOI] [PubMed] [Google Scholar]
- 16.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 17.Groh WJ, von der Lohe E, Zipes DP. What's bad for the gander…women and sudden cardiac death. Heart. 2002;88:553–554. doi: 10.1136/heart.88.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tribulova N, Dupont E, Soukup T, Okruhlicova L, Severs NJ. Sex differences in connexin-43 expression in left ventricles of aging rats. Physiol Res. 2005;54:705–708. [PubMed] [Google Scholar]
- 19.Knezl V, Bacova B, Kolenova L, Mitasikova M, Weismann P, Drimal J, Tribulova N. Distinct lethal arrhythmias susceptibility is associated with sex-related difference in myocardial connexin-43 expression. Neuro Endocrinol Lett. 2008;29:798–801. [PubMed] [Google Scholar]
- 20.McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 21.Saffitz JE. Dependence of electrical coupling on mechanical coupling in cardiac myocytes: insights gained from cardiomyopathies caused by defects in cell-cell connections. Ann N Y Acad Sci. 2005;1047:336–344. doi: 10.1196/annals.1341.030. [DOI] [PubMed] [Google Scholar]
- 22.Saffitz JE. Arrhythmogenic cardiomyopathy and abnormalities of cell-to-cell coupling. Heart Rhythm. 2009;6:S62–S65. doi: 10.1016/j.hrthm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 23.King TJ, Lampe PD. Temporal regulation of connexin phosphorylation in embryonic and adult tissues. Biochim Biophys Acta. 2005;1719:24–35. doi: 10.1016/j.bbamem.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am J Physiol. 1996;271:H1250–H1255. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 25.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellingsen O, Davidoff AJ, Prasad SK, Berger HJ, Springhorn JP, Marsh JD, Kelly RA, Smith TW. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am J Physiol. 1993;265:H747–H754. doi: 10.1152/ajpheart.1993.265.2.H747. [DOI] [PubMed] [Google Scholar]
- 27.Volz A, Piper HM, Siegmund B, Schwartz P. Longevity of adult ventricular rat heart muscle cells in serum-free primary culture. J Mol Cell Cardiol. 1991;23:161–173. doi: 10.1016/0022-2828(91)90103-s. [DOI] [PubMed] [Google Scholar]
- 28.Thum T, Borlak J. Butanedione monoxime increases the viability and yield of adult cardiomyocytes in primary cultures. Cardiovasc Toxicol. 2001;1:61–72. doi: 10.1385/ct:1:1:61. [DOI] [PubMed] [Google Scholar]
- 29.Dodge SM, Beardslee MA, Darrow BJ, Green KG, Beyer EC, Saffitz JE. Effects of angiotensin II on expression of the gap junction channel protein connexin43 in neonatal rat ventricular myocytes. J Am Coll Cardiol. 1998;32:800–807. doi: 10.1016/s0735-1097(98)00317-9. [DOI] [PubMed] [Google Scholar]
- 30.Stanbouly S, Kirshenbaum LA, Jones DL, Karmazyn M. Sodium hydrogen exchange 1 (NHE-1) regulates connexin 43 expression in cardiomyocytes via reverse mode sodium calcium exchange and c-Jun NH2-terminal kinase-dependent pathways. J Pharmacol Exp Ther. 2008;327:105–113. doi: 10.1124/jpet.108.140228. [DOI] [PubMed] [Google Scholar]
- 31.Barron AJ, Finn SG, Fuller SJ. Chronic activation of extracellular-signal-regulated protein kinases by phenylephrine is required to elicit a hypertrophic response in cardiac myocytes. Biochem J. 2003;371:71–79. doi: 10.1042/BJ20021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schluter KD, Simm A, Schafer M, Taimor G, Piper HM. Early response kinase and PI 3-kinase activation in adult cardiomyocytes and their role in hypertrophy. Am J Physiol. 1999;276:H1655–H1663. doi: 10.1152/ajpheart.1999.276.5.H1655. [DOI] [PubMed] [Google Scholar]
- 33.Sucharov CC, Dockstader K, McKinsey TA. YY1 protects cardiac myocytes from pathologic hypertrophy by interacting with HDAC5. Mol Biol Cell. 2008;19:4141–4153. doi: 10.1091/mbc.E07-12-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stauffer BL, Kushner EJ, Wulfman T, Zeller T, Sobus R, Westby CM. Transcriptional regulation of β2-microgobulin demonstrated via a novel genomic and proteomic analysis of percutaneously collected peripheral atheroma. Clin Transl Sci. 2008;1:240–244. doi: 10.1111/j.1752-8062.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–212. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeong MY, Kinugawa K, Vinson C, Long CS. AFos dissociates cardiac myocyte hypertrophy and expression of the pathological gene program. Circulation. 2005;111:1645–1651. doi: 10.1161/01.CIR.0000160367.99928.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markou T, Hadzopoulou-Cladaras M, Lazou A. Phenylephrine induces activation of CREB in adult rat cardiac myocytes through MSK1 and PKA signaling pathways. J Mol Cell Cardiol. 2004;37:1001–1011. doi: 10.1016/j.yjmcc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Salameh A, Krautblatter S, Baessler S, Karl S, Rojas Gomez D, Dhein S, Pfeiffer D. Signal transduction and transcriptional control of cardiac connexin43 up-regulation after alpha 1-adrenoceptor stimulation. J Pharmacol Exp Ther. 2008;326:315–322. doi: 10.1124/jpet.108.136663. [DOI] [PubMed] [Google Scholar]
- 39.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 42.Rosenkranz-Weiss P, Tomek RJ, Mathew J, Eghbali M. Gender-specific differences in expression of mRNAs for functional and structural proteins in rat ventricular myocardium. J Mol Cell Cardiol. 1994;26:261–270. doi: 10.1006/jmcc.1994.1029. [DOI] [PubMed] [Google Scholar]
- 43.Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ. 2007;31:17–22. doi: 10.1152/advan.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carroll JD, Carroll EP, Feldman T, Ward DM, Lang RM, McGaughey D, Karp RB. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099–1107. doi: 10.1161/01.cir.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 45.Severs NJ, Dupont E, Coppen SR, Halliday D, Inett E, Baylis D, Rothery S. Remodelling of gap junctions and connexin expression in heart disease. Biochim Biophys Acta. 2004;1662:138–148. doi: 10.1016/j.bbamem.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Kilic A, Javadov S, Karmazyn M. Estrogen exerts concentration-dependent pro- and anti-hypertrophic effects on adult cultured ventricular myocytes. Role of NHE-1 in estrogen-induced hypertrophy. J Mol Cell Cardiol. 2009;46:360–369. doi: 10.1016/j.yjmcc.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 48.Nakagami T, Tanaka H, Dai P, Lin SF, Tanabe T, Mani H, Fujiwara K, Matsubara H, Takamatsu T. Generation of reentrant arrhythmias by dominant-negative inhibition of connexin43 in rat cultured myocyte monolayers. Cardiovasc Res. 2008;79:70–79. doi: 10.1093/cvr/cvn084. [DOI] [PubMed] [Google Scholar]
- 49.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 51.Teunissen BE, Jongsma HJ, Bierhuizen MF. Regulation of myocardial connexins during hypertrophic remodelling. Eur Heart J. 2004;25:1979–1989. doi: 10.1016/j.ehj.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Kostin S, Dammer S, Hein S, Klovekorn WP, Bauer EP, Schaper J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res. 2004;62:426–436. doi: 10.1016/j.cardiores.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zangar RC, Reiners JJ, Jr, Novak RF. Gender-specific and developmental differences in protein kinase C isozyme expression in rat liver. Carcinogenesis. 1995;16:2593–2597. doi: 10.1093/carcin/16.10.2593. [DOI] [PubMed] [Google Scholar]
- 56.Edwards AG, Rees ML, Gioscia RA, Zachman DK, Lynch JM, Browder JC, Chicco AJ, Moore RL. PKC-permitted elevation of sarcolemmal KATP concentration may explain female-specific resistance to myocardial infarction. J Physiol. 2009;587:5723–5737. doi: 10.1113/jphysiol.2009.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vozzi C, Dupont E, Coppen SR, Yeh HI, Severs NJ. Chamber-related differences in connexin expression in the human heart. J Mol Cell Cardiol. 1999;31:991–1003. doi: 10.1006/jmcc.1999.0937. [DOI] [PubMed] [Google Scholar]