Abstract
Chronic elevations of endogenous cortisol levels have been shown to alter medial temporal cortical structures and to be accompanied by declarative memory impairments and depressive symptoms in human adults. These effects of elevated endogenous levels of cortisol have not been directly studied in adolescents. Because adolescents with Cushing syndrome show endogenous elevations in cortisol, they represent a unique natural model to study the effects of prolonged hypercortisolemia on brain function, and memory and affective processes during this developmental stage. Using functional magnetic resonance imaging (fMRI), we compared 12 adolescents with Cushing syndrome with 22 healthy control adolescents on amygdala and anterior hippocampus activation during an emotional faces encoding task. None of these adolescents manifested depressive symptoms. Encoding success was assessed using a memory recognition test performed after the scan. The fMRI analyses followed an event-related design and were conducted using the SPM99 platform. Compared to healthy adolescents, patients with Cushing syndrome showed greater left amygdala and right anterior hippocampus activation during successful face encoding. Memory performance for faces recognition did not differ between groups. This first study of cerebral function in adolescents with chronic endogeneous hypercortisolemia due to Cushing syndrome demonstrates the presence of functional alterations in amygdala and hippocampus, which are not associated with affective or memory impairments. Such findings need to be followed by work examining the role of age and related brain maturational stage on these effects, as well as the identification of possible protective factors conferring resilience to affective and cognitive consequences in this disease and/or during this stage of cerebral development.
Glucocorticoids are beneficial when secreted after acute stress, but prolonged glucorticoid elevations, due to chronic stress or illness, produce adverse affective, cognitive, and physical consequences, including mood symptoms and memory impairments in adults (Charney, 2004; de Kloet, Joels, & Holsboer, 2005; McEwen, 1998). These deleterious effects are thought to result from abnormal modulation of glucorticoid receptors in the medial temporal lobe (MTL), encompassing the amygdala and hippocampus (Charney, 2004; de Kloet et al., 2005; Lupien & Lepage, 2001; Nemeroff, 2004; Roozendaal, 2003).
We recently demonstrated that adolescents with major depressive disorder (MDD) exhibit abnormally high MTL responses when encoding affective stimuli into memory (Roberson-Nay et al., 2006). Such findings may relate to prior research noting associations among glucocorticoid hypersecretion, MDD, and perturbed MTL function. However, elevated cortisol levels in pediatric MDD is reported less consistently than in adult MDD (see reviews by de Kloet et al., 2005; Goodyer, Park, Netherton, & Herbert, 2001). Such data have led to the suggestion that glucocorticoids show minimal relationships with MTL function in children and adolescents.
This relationship, however, has not been thoroughly examined in youths because of the difficulty in conducting such studies. Manipulating glucocorticoid levels in pediatric populations, pharmacologically or through stress challenges, poses ethical problems. Here, we investigate the association between glucocorticoids and MTL function in adolescents who suffer from chronic endogenous hypersecretion of cortisol due to Cushing syndrome. This condition provides a unique naturalistic model for studying the cerebral consequences of excess cortisol. Similar to lesion studies, the examination of pathological development can shed light on normal development (Cicchetti, 2003; O’Connor, 2003). Adults with Cushing syndrome present reduced hippocampal volume, declarative memory impairments, and depressive symptoms (Bourdeau et al., 2005; Forget, Lacroix, Somma, & Cohen, 2000). Surprisingly, adolescents with Cushing syndrome seem to fail to manifest depressive symptoms (Magiakou & Chrousos, 2002; Merke et al., 2005). This would suggest that the combination of elevated cortisol and MTL alteration is not sufficient to trigger the emergence of mood symptoms in these adolescents, implying the presence of protective factors that are lacking in adults with depression.
Although no animal or human studies have yet examined the effects of chronically elevated corticosteroid levels on emotional memory, acute elevations in cortisol levels have been shown to enhance memory for emotional stimuli (Abercrombie, Kalin, Thurow, Rosenkranz, & Davidson, 2003; Buchanan & Lovallo, 2001; Cahill, Gorski, & Le, 2003; Roozendaal, 2003). Recent animal studies suggest that acute, as well as chronic, stress stimulates the amygdala (Mitra, Jadhav, McEwen, Vyas, & Chattarji, 2005; Poeggel et al., 2003; Roozendaal, 2003; Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002), which is implicated in emotional memory (Phelps & LeDoux, 2005).
In line with these results, we expect chronically elevated cortisol levels to predict enhanced MTL activation during the encoding of emotional information. To this aim, we compared adolescents with Cushing syndrome and healthy adolescents on MTL reactivity using the exact same paradigm and analysis employed in our previous work on adolescent MDD (Roberson-Nay et al., 2006).
Methods
Participants
Twelve adolescents with endogenous Cushing syndrome were compared to 22 healthy adolescents. Endogenous Cushing syndrome is rare, and almost always due to one of two conditions, pituitary adenoma or adrenal gland tumors (Magiakou & Chrousos, 2002). Patients with Cushing syndrome were being followed at the NIH Clinical Center and enrolled in the study on a voluntary basis. Healthy subjects were recruited in the community. The study was approved by the institutional review boards of the National Institute of Mental Health and National Institute of Child Health and Human Development. Prior to participation in the study, parents and adolescents gave written consent and assent, respectively. All subjects were compensated for participation in the study following the guidelines provided by the National Institute of Mental Health.
All subjects underwent physical and psychiatric examinations. Exclusion criteria included chronic medical condition (except for Cushing syndrome) and pharmacological treatment unrelated to Cushing syndrome. All participants were free from any present or lifetime history of psychiatric disorders, as assessed by the Kiddie Schedule for Affective Disorders and Schizophrenia—Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997) with parents and adolescents separately. Tanner stage in all patients was determined by a pediatrician and a self-administered questionnaire in control subjects (Duke, Litt, & Gross, 1980). Patients with Cushing syndrome had hormones measured as part of their clinical evaluation. All participants scored within the average/above average on the Wechsler Abbreviated Scale of Intelligence for Children (Wechsler, 1999). Demographic and clinical characteristics are presented in Table 1.
Table 1.
Demographic and clinical characteristics of patients with Cushing syndrome and healthy adolescents.
| Cushing Syndrome Patients | Healthy Adolescents | |
|---|---|---|
| Gender | 4 boys, 8 girls | 10 boys, 12 girls |
| Age (years) | 13.5 ± 2.9 | 14.2 ± 2.4 |
| Age range (years) | ||
| Boys | 10–15 | 9–17 |
| Girls | 10–18 | 9–17 |
| Height SDS | −1.1 ± 1.2 | 0.1 ± 1.1** (n = 19) |
| Weight SDS | 1.2 ± 1.0 | 0.3 ± 0.8** (n = 19) |
| BMI SDS | 1.7 ± 1.1 | 0.4 ± 0.6* (n = 19) |
| Tanner stage | 3.3 ± 1.2 | 3.3 ± 1.3 (n = 20) |
| Wechsler IQ | 114.4 ± 13.1 (n = 10) | 108.1 ± 16.0 |
Values are reported as means  ±  SD. Reduced n indicates missing data.
p < .05.
p < .01.
Face memory task
The memory paradigm consisted of two performance phases, encoding during functional magnetic resonance imaging (fMRI) scanning and recognition outside the scanner. This memory paradigm has previously been used successfully in our laboratory for behavioral and fMRI studies of adolescents (Nelson et al., 2003; Pine et al., 2004; Roberson-Nay et al., 2006). The paradigm uses a variant of the frequently employed “subsequent memory” paradigm from cognitive neuroscience.
During the encoding phase, participants viewed 32 portraits of adult actors selected randomly from a larger pool of 56 actors. These photographs were selected from three standardized sets of gray-scale photographs depicting different facial expressions constructed by Ekman and Friesen (1976), Gur (www.uphs.upenn.edu/bbl/pubs/downloads/nptasks.shtml), and Tottenham and Nelson (www.macbrain.org/faces/index.htm). Each actor presented one of four emotional expressions (happy, fear, angry, neutral) throughout the entire encoding paradigm, and eight different actors were viewed for each expression (8 × 4 = 32). Although facial expressions for a given actor were held constant within each participant’s task, expressions varied randomly across different participants’ tasks. Thus, one participant may have viewed a given actor displaying a consistently happy expression, whereas a subsequent participant may have viewed the same actor displaying a consistently angry expression. This feature controlled for variability in nonemotional aspects of the actors (e.g., ethnicity, gender, hair texture) that influences memory formation. While in the scanner, participants viewed the series of 32 adult faces (8 happy, 8 angry, 8 fearful, 8 neutral) under four conditions. Three conditions required participants to attend to different aspects of the face stimuli: (a) How afraid did the stimulus make you feel? (b) How hostile does the face appear? (c) How large is the nose? These questions were rated on a five-button box (1 = not very to 5 = extremely). In the fourth condition, participants’ attention was unconstrained, such that the faces were viewed passively without participants making any ratings.
As described in Roberson-Nay et al. (2006), the use of these different attention manipulations facilitates encoding success by reducing boredom associated with repeated viewing of the same pictures. Moreover, as in all of our prior studies of memory encoding, fMRI analyses reported here contrast “successfully” relative to “unsuccessfully” encoded pictures by collapsing data acquired in each of the four conditions. This approach maximizes statistical power by allowing a more precise estimation of encoding-related brain activation. Moreover, as delineated in Roberson-Nay et al. (2006), the inclusion of 32 “null-event” trials, consisting of fixation crosses, occurred randomly within each condition to facilitate interpretation of brain activations.
The four conditions were presented as a 14-min single run comprising 160 trials (8 actors × 5 stimuli [4 emotions and fixation] × 4 conditions). Each facial expression was presented a total of four times, once during each of the four conditions. Trials within a given condition were blocked together, and presentation order of condition and facial expressions was randomized across participants. Rating instructions appeared for 3 s before each condition block. Facial expression and fixation trials were shown for 4 s each. Each face and fixation trial was followed by an intertrial interval showing a blank screen that varied randomly from 750 to 1250 ms.
Stimuli were displayed using Avotec Silent Vision Glasses (Stuart, FL), and responses were recorded by a five-key button box developed by MRI Devices (Waukesha, WI). Participants were trained in an MRI simulator prior to entering the scanner to become familiar with the actual MRI environment and response device. Participants were also administered a practice version of the task to ensure understanding of the task. The practice version contained pictures of only neutral facial expressions that were not shown in the MRI scanner.
After completing the fMRI scanning session, participants performed the surprise recognition memory test. As indicated previously (Nelson et al., 2003; Pine et al., 2004; Roberson-Nay et al., 2006), the use of this incidental memory paradigm, with a surprise postscan recognition test, was designed to address two issues. First, these procedures mirror memory-encoding processes elicited when dealing with emotionally salient stimuli of everyday life. Second, the procedures minimize potential effects on memory encoding related to underlying between-group differences in effort, strategic approaches to memory encoding, or compliance with encoding instructions.
The recognition memory test consisted of 48 photographs, including 24 previously seen and 24 new actors, presented on a computer screen. Previous work showed that the inclusion of neutral faces at encoding and recall interfered with memory formation (see Nelson et al., 2003; Pine et al., 2004). Thus, the actors portraying the neutral faces during encoding were not used at recall. In addition, the pictures included at recall comprised only actors portraying neutral faces, that is, different expressions from the emotional expressions seen during encoding. Actors were randomly selected for each research participants to serve as “targets” that were presented both at encoding and recall, or new actors (“lures”) that were only presented at recall. This procedure minimized the possibility that actor-specific characteristics, apart from the portrayed emotion, would influence memory. During the recognition memory test, which occurred approximately 30–40 min after presentation of the faces in the scanner, participants were asked to rate the actors as old or new. The selective use of neutral expressions at recall significantly increased the difficulty of recognition. Subjects were required to encode features of individuals, who displayed a given emotion, and then to use these representations, when attempting to recognize individuals depicting no emotion. At recall, each face was presented for 4 s, during which time subjects indicated “old” or “new” by button press.
Memory scores were based on the signal detection theory. Specifically, a measure of signal detection threshold (d′) was calculated for each participant for each emotion by subtracting the z score for hits for each emotion from the overall z score for false alarms (Snodgrass & Corwin, 1988). This generated d′ estimates for each face type: “happy,” “fearful,” and “angry.” A d′ value for total hits (z score for false alarms – z score for sum of hits for all emotional face types) was also calculated for each participant. Conceptually, d′ represents the degree to which a subject is capable of differentiating signals or “true targets” from noise. Higher d′ values indicate increasing ability to correctly pick out a specific target type from a series of distracters, and positive d′ values indicate higher true signal reporting than false alarm reporting. The postscan memory test allowed data acquired at encoding in the scanner to be “binned” based on the postscan recognition (remembered vs. forgotten).
MRI data acquisition
Whole-brain blood oxygen level dependent (BOLD) fMRI data were acquired on a General Electric Signa 3-tesla magnet (Waukesha, WI). Following sagittal localization and a manual shim procedure, functional T2*-weighted images were gathered using an echo planar single-shot gradient echo pulse sequence with a matrix size of 64 × 64 mm, repetition time (TR) of 2000 ms, echo time (TE) of 40 ms, field of view (FOV) of 240 mm, and voxels of 3.75 × 3.75 × 5 mm, providing whole-brain coverage. Echo planar images (EPI) were acquired in 23 contiguous 5-mm axial slices per brain volume positioned parallel to the anterior commissure and posterior commissure (AC–PC) line. All functional data were obtained in a single 14-min run per subject. Following EPI data collection, a high-resolution T1-weighted anatomical image was acquired for each subject using a standardized magnetization-prepared gradient echo sequence (180 1-mm sagittal slices, FOV = 256, number of excitations [NEX] = 1, TR = 11.4 ms, TE = 4.4 ms, matrix size = 256 × 256, time to inversion [TI] = 300 ms, bandwidth = 130 Hz/pixel, 33 kHz/256 pixels) to facilitate spatial normalization.
Data analysis
Demographic and clinical characteristics
Demographic and clinical characteristics were compared between patients with Cushing syndrome and healthy adolescents using two-sample Student t tests for continuous measures or chi-square tests for nominal measures. Body mass index (BMI), height and weight standard deviation score (SDS) were determined using anthropometric reference data for US children (Center for Disease Control, Epi Info™, version 3.3.2).
Behavioral data
Memory recall d′ values were evaluated for assumptions of normality and sphericity. These variables were distributed normally, and Greenhouse–Geisser (1959) corrections were applied when the sphericity criterion was not met. A mixed repeated-measures analysis of variance (ANOVA) with diagnosis (Cushing syndrome vs. healthy) as the between-subjects factor and emotional faces (happy vs. fearful vs. angry) as the within-subjects factor was conducted to assess effects of diagnosis on performance and its potential interaction with emotional faces.
Imaging data
Preprocessing data procedures and fMRI data analyses were performed using the Statistical Parametric Mapping (SPM99, Wellcome Department of Cognitive Neurology, London) software and supplemental routines written in Matlab 6 (Mathworks, Natick, MA). Imaging data for participants moving more than 2.0 mm in any plane as assessed with MedX software (Medical Numerics, Sterling, VA) were excluded. Preprocessing procedures included corrections for slice timing and motion, coregistration to the anatomical data, and spatial normalization to a Montreal Neurologic Institute (MNI) T1-weighted template image supplied with SPM99b.
Individual subject level event-related response amplitudes were estimated using a general linear model for each event type. Event-types were specified based on the emotion of the faces, and whether faces viewed at encoding were remembered or forgotten. Fixation trials served as an implicit baseline. The waveform used to model event-related responses was a rectangular pulse (4-s duration) convolved with the hemodynamic response function specified in SPM99. Contrast images were created for each subject using pairwise comparisons of the event-related BOLD response amplitudes across conditions. Before performing group-level analyses, each contrast image was divided by the subject-specific voxel time series mean, generating values proportional to percentage fMRI signal change (Zarahn, Aguire, & D’Esposito, 1997). These normalized contrast images were then smoothed with an isotropic Gaussian kernel (full width at half-maximum = 11.4) to reduce nonstationarity in the spatial autocorrelation structure produced by the previous step (Friston, Mechelli, Turner, & Price, 2000).
A priori hypotheses motivated a region of interest (ROI)-based analysis of the amygdala, and anterior hippocampus. These ROIs were ascertained from standard anatomical criteria on a single MNI template and applied to all normalized brains at the group level (Szeszko et al., 1999, 2002). Voxelwise tests were conducted in these anatomically defined volumes of interest. Consistent with the current standard (Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002; Winston, Strange, O’Doherty, & Dolan, 2002), we utilized the Gaussian random field threshold (α = .05, corrected) with small volume correction (SVC) implemented in SPM99 (Worsley, Marrett, Neelin, Vandal, Friston, & Evans, 1996). Statistical significance of activation in regions of interest was set to corrected p < .05.
Similar to our previous work in depressed adolescents (Roberson-Nay et al., 2006), we tested between-group differences in the contrast of BOLD signal changes during the viewing of the faces that were remembered on later recall versus during the viewing of the faces that were forgotten on later recall (remembered faces vs. forgotten faces). Again, consistent with our prior study, and because we had no specific a priori hypotheses regarding effects of emotion type, we pooled emotions together in this analysis. This strategy permitted to optimize statistical power.
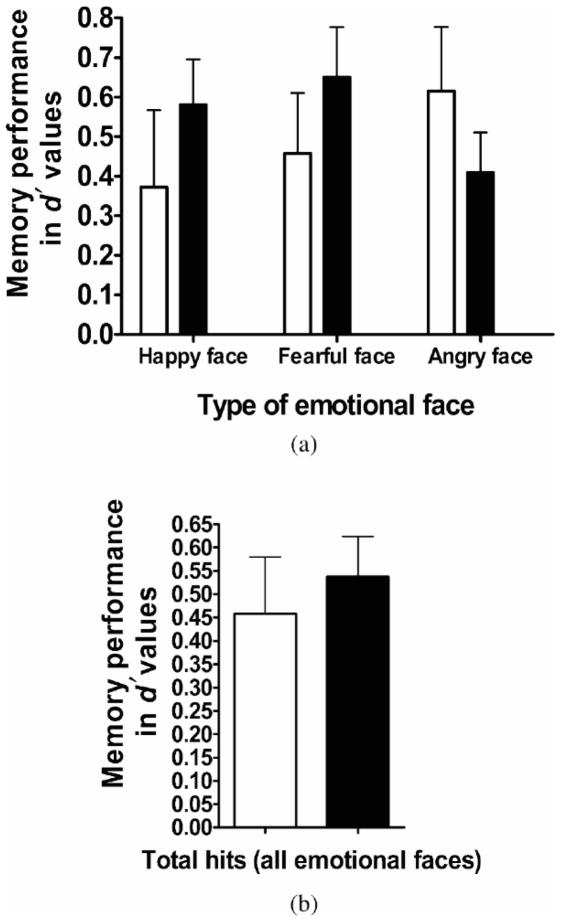
Finally, to better understand the nature of significant activations, we evaluated separately significant loci of activation for the contrasts remembered versus baseline and forgotten versus baseline in each group. These analyses were conducted in SPSS-14.0 on the mean extracted BOLD signal changes of each significant locus. In addition, correlations were performed among these extracted mean values, memory performance in the whole sample, and cortisol levels in the Cushing syndrome group (Figure 1).
Figure 1.
A comparison of memory performance between groups. Mean memory d â€2 values between patients with Cushing syndrome (open bars) and healthy adolescents (filled bars) for (a) each emotional face and (b) total hits (collapsed across all emotional faces). There were no group differences regarding memory performance (all ps > .10). Error bars represent standard error of the mean.
The regions of interest (ROI) analyses for the left amygdala and right anterior hippocampus. The statistical threshold is set at p < .05 after a small volume correction of the ROI.
Two control regions, the orbitofrontal cortex (OFC) and fusiform gyrus, were used to examine the regional specificity of the findings. These regions were chosen because of the absence of reported functional abnormalities in these areas in Cushing syndrome, and because of their role in emotion processing (OFC; Phillips, Drevets, Rauch, & Lane, 2003) and stimulus identification (Adolphs, 2003), rather than in memory encoding.
Results
Demographic and clinical characteristics
Patients with Cushing syndrome and healthy adolescents did not differ on age (Cushing syndrome patients: 13.5, SD = 2.9; Healthy adolescents: 14.2, SD = 2.4), gender distribution (Cushing syndrome patients: 4 boys, 8 girls; healthy adolescents: 10 boys, 12 girls), puberty level, or Wechsler IQ (Cushing syndrome patients: 114.4, SD = 13.1; healthy adolescents: 108.1, SD = 16.0; see Table 1). As expected, patients with Cushing syndrome had significantly higher BMI, t (29) = 4.3, p < .001, and weight, t (29) = 2.5, p < .03, and shorter stature, t (29) = 2.9, p < .009, than age-matched controls. All patients with Cushing syndrome had biochemically confirmed hypercortisolism (mean ± SD, urinary free cortisol corrected for body surface area: Cushing syndrome group: 132.66 ± 55.77 μg/m2/24 hr; normal range in healthy adolescents of the same Tanner puberty stage: <68 μg/m2/24 hr) and lacked diurnal variation in plasma cortisol concentrations (mean ± SD, morning cortisol, 21.5 ± 9.7 μg/dl; midnight cortisol, 19.1 ± 8.5 μg/dl). The average duration of Cushing syndrome based on onset of decreased growth velocity was 2.6 years (range = 1–4.5 years). None of the adolescents with Cushing syndrome were receiving treatment at the time of testing, and all patients were evaluated before surgery for removal of the pituitary tumor (10 patients) or bilateral adrenalectomy (2 patients with primary pigmented neoplastic adrenal disease).
Behavioral data
The d′ values used to measure memory for happy and fearful and angry faces are displayed in Table 2. The d′ values for total hits (i.e., hits for the three types of emotional faces combined) were significantly different from zero in both the Cushing syndrome patients, t (11) = 3.8, p < .004, and healthy adolescents, t (21) = 6.2, p < .001, indicating that overall hit rates were significantly higher than the false alarm rates in both the patient and healthy groups. The mixed repeated-measures ANOVA measuring the influence of diagnosis on memory for happy, fearful and angry faces revealed no main effects of emotional faces, F (2, 64) = 0.23, p > .10, or of diagnosis, F (1, 32) = .19, p > .10, and no Emotional Faces × Diagnosis interaction, F (2, 64) = 2.02, p > .10. Therefore, both the patient and the healthy adolescents performed similarly on memory recall of each emotional face types. In addition, the absence of effect of Emotional faces on performance supported the pooling of emotions in the fMRI analysis.
Table 2.
Predictors of memory performance (dâ€2 values) in patients with Cushing syndrome and healthy adolescents
| Face Recognition Memory | Cushing Syndrome Patients (4 Boys, 8 Girls) | Healthy Adolescents (10 Boys, 12 Girls) |
|---|---|---|
| Total | .46 ± .42 | .54 ± .41 |
| Happy | .37 ± .68 | .58 ± .54 |
| Fearful | .46 ± .53 | .65 ± .59 |
| Angry | .62 ± .56 | .41 + .47 |
Values are means ± SD. significant difference between both groups on memory recall for total hits and types of emotional faces (ps > .10).
Imaging data
Amygdala ROI analysis
Amygdala response to remembered versus forgotten faces revealed greater activation in the left amygdala in Cushing syndrome patients compared to healthy adolescents, t = 2.84 (MNI x, y, z = −14, −6, −10 mm), p < .04 SVC corrected. No group differences emerged in the right amygdala (see Table 3).
Table 3.
Regional activations in the group comparison of remembered versus forgotten
| Cushing Syndrome > Healthy Adolescents Remembered Versus Forgotten | |||||||
|---|---|---|---|---|---|---|---|
| k (Cluster) | T (Peak) | x, y, z (mm) | p | k (Cluster) | T (Peak) | x, y, z (mm) | p |
| Left Amygdala | Right Amygdala | ||||||
| 117 | 2.84 | −14, −6, −10 | .04 | 39 | 2.13 | 32, −2, −20 | ns |
| Left Ant. Hippo. | Right Ant. Hippo. | ||||||
| 93 | 2.44 | −22, −18, −14 | ns | 225 | 2.98 | 36, −14, −14 | .04 |
The regions of interest (ROI) analyses for the left amygdala and right anterior hippocampus. The statistical threshold is set at p < .05 after a small volume correction of the ROI.
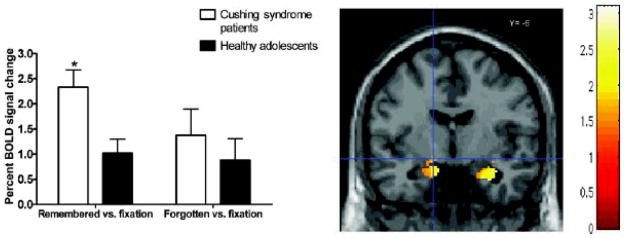
Next, a repeated-measures ANOVA was conducted in SPSS-14.0 on the extracted BOLD signal percent changes of the left amygdala ROI in the contrasts remembered versus fixation and forgotten versus fixation. This analysis revealed that the significant group difference in left amygdala for the contrast remembered versus forgotten reflected greater activation for remembered versus fixation in patients with Cushing syndrome than in healthy adolescents, F (1, 32) = 8.9, p < .007. No significant group differences emerged for forgotten versus fixation, F (1, 32) = .54, p > .10 (Figure 2).
Figure 2.
Mean BOLD signal differences in the left amygdala (MNI â??14, â??6, â??10Â mm) for contrasts remembered versus fixation and forgotten versus fixation in Cushing syndrome patients and healthy adolescents. Adolescents with Cushing syndrome showed significantly higher activation when compared to healthy adolescents (p < .05). Error bars represent standard error of the mean.
The regions of interest (ROI) analyses for the left amygdala and right anterior hippocampus. The statistical threshold is set at p < .05 after a small volume correction of the ROI.
Left amygdala activations in the contrasts of remembered versus forgotten, remembered versus fixation, or forgotten versus fixation were not correlated with d′ performance scores in the Cushing syndrome and healthy adolescents groups, combined or separately. Moreover, measures of 24-hr urinary cortisol levels in the Cushing syndrome patients did not correlate with left amygdala activation (remembered vs. forgotten; p > .10).
Anterior hippocampal ROI analysis
Anterior hippocampal response to remembered versus forgotten faces revealed greater activation in the right anterior hippocampus, t = 2.98 (MNI x, y, z = 36, −14, −14 mm), p < .03, SVC corrected, in Cushing syndrome patients compared to healthy adolescents (see Table 3). No group differences emerged in the left anterior hippocampus.
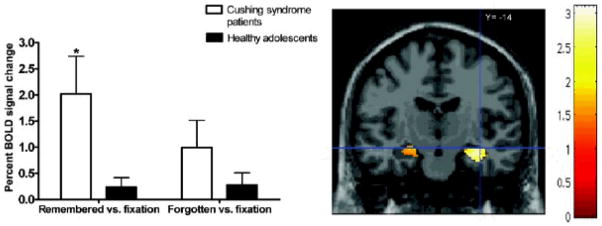
Next, a repeated-measures ANOVA was conducted in SPSS-14.0 on the extracted BOLD signal percent changes of the right anterior hippocampus ROI in the contrasts remembered versus fixation and forgotten versus fixation (Figure 3). Similarly to the findings in the left amygdala, the significant group difference in right anterior hippocampus for the contrast remembered versus forgotten reflected greater activation for remembered versus fixation in patients with Cushing syndrome than in healthy control, F (1, 32) = 9.8, p < .005. No significant group differences emerged for forgotten versus fixation, F (1, 32) = 2.21, p < .15.
Figure 3.
Mean BOLD signal differences in the right anterior hippocampus (MNI 36, â??14, 14Â mm) for contrasts remembered versus fixation and forgotten versus fixation in Cushing syndrome patients and healthy adolescents. Adolescents with Cushing syndrome showed significantly higher activation when compared to healthy adolescents (p < .05). Error bars represent standard error of the mean.
The regions of interest (ROI) analyses for the left amygdala and right anterior hippocampus. The statistical threshold is set at p < .05 after a small volume correction of the ROI.
Right anterior hippocampal activations in the contrasts of remembered versus forgotten, remembered versus fixation, or forgotten versus fixation were not correlated with d′ performance scores in the Cushing syndrome and healthy adolescents groups, combined or separately. Moreover, measures of 24-hr urinary cortisol levels in the Cushing syndrome patients did not correlate with right anterior hippocampal activation (remembered vs. forgotten contrast; p > .10).
Control ROIs: OFC and fusiform gyrus
ROI analyses of left and right OFC, and fusiform gyrus showed no significant group differences in the main contrast (remembered vs. forgotten; ps > .10), supporting a regional specificity of the significant group differences reported above.
Discussion
This study is the first to demonstrate associations between endogenous chronic hypersecretion of cortisol levels and perturbed engagement of brain substrates previously associated with depression and emotional memory. This work uses a naturalistic approach that provides a unique opportunity to address potential mechanisms of developmental psychopathology. Adolescents with Cushing syndrome suffer from chronic endogeneous hypersecretion of cortisol by pituitary adenoma or adrenal gland tumors (Magiakou & Chrousos, 2002), and represent a natural model of chronic exposure to cortisol during development. This approach is based on the tenet held in the field of developmental psychopathology, advocating for the notion that the study of atypicality is an important avenue to inform normal development and reciprocity (Cicchetti, 2003).
We show here that the study of adolescents with Cushing syndrome reproduces imaging findings previously obtained with an independent sample of depressed adolescents, using an identical paradigm and analysis (Roberson-Nay et al., 2006). Left amygdala activation in response to successful encoding of evocative faces was significantly higher in adolescents with Cushing syndrome than in healthy controls. In addition, activation of the right anterior hippocampus, a region involved in emotional processing (Bannerman et al., 2004; Dolcos, LaBar, & Cabeza, 2004), was also abnormally elevated in these adolescents.
None of the adolescents with Cushing syndrome suffered from a psychiatric disorder, including mood disorder. This is important, as it suggests that exaggerated amygdala reactivity combined with chronic elevation of glucocorticoids, a marker of depression in adults (Charney, 2004; de Kloet et al., 2005; Nemeroff, 2004), is not sufficient for mediating depression in adolescents. This observation further suggests that protective factors may be at play. A number of neurotransmitters, neurohormones, brain circuits, and genes have been linked to the protective mechanisms underlying resiliency (see Curtis & Cicchetti, 2003; Charney, 2004). Adolescents with Cushing syndrome may activate such protective neural mechanisms, which may account for their resiliency to depression. These protective mechanisms may be specific to the endocrine disease or to the potentially more plastic, still maturing neural networks in adolescents. The high prevalence of depression in adults with Cushing syndrome (see Bourdeau et al., 2005; Forget et al., 2000) relative to children and adolescents with Cushing syndrome (Magiakou & Chrousos, 2002; Merke et al., 2005) suggests that the latter proposition (plasticity) or a combination of both (plasticity interacting with disease specificity) could explain this age difference in the psychiatric symptoms associated with Cushing syndrome. Clearly, further research investigating possible protective neurobiological factors conferring resiliency to psychopathology is critical.
Age-related differences in the biological correlates of depression have also been suggested within the corticosteroid system. In a significant percentage of adults with MDD, cortisol levels were reported to be elevated throughout the day (de Kloet et al., 2005; Pariante, 2006; Pariante and Miller, 2001). In children and adolescents, however, most studies have failed to detect elevated circadian levels of cortisol (Birmaher et al., 1996; Dahl et al., 1989; Gipsen-de Wied, Jansen, Duyx, Thijssen, & van Engeland, 2000; Puig-Antich et al., 1989; see Kutcher & Sokolov, 2001). Subtle developmental differences in the pattern of cortisol secretion in depression have been proposed with respect to the typical diurnal variation of this hormone (Forbes et al., 2006). Hence, cortisol hypersecretion has been found around sleep onset in depressed adolescents (Dahl et al., 1991; Goodyer, Park, & Herberg, 2001). The density threshold (temporal and magnitude characteristics) of cortisol levels at which central effects occur is not known. Therefore, although circadian cortisol patterns in depressed adolescents seem to less perturbed than in depressed adults, brain substrates in adolescents might be exquisitely vulnerable to potentially deleterious effects of elevated corticosteroids around sleep onset, which could mediate amygdala hyperreactivity in depressed teenagers.
Pharmacological manipulations of corticosteroid levels in animals have been performed in the adult and perinatal periods, but not in the adolescent period (see review by Romeo & McEwen, 2006). This limits our ability to infer from animal work the cerebral consequences of chronic hypercortisolemia of late childhood onset, a time of unique developmental changes (e.g., Gogtay et al., 2004). The distinct neural plasticity of the adolescent period is clearly evidenced in the maturation of reward systems. Indeed, the juvenile animal has been shown to harbor vulnerabilities to addictive drugs that differ quantitatively and qualitatively from those reported neonatally or in adulthood (see reviews by Ernst, Pine, & Hardin, 2006; Spear, 2000).
Animal studies of the neural and behavioral consequences of chronic stress accompanied with hypercorticosteroidism have been examined when stressors were applied at different periods of development (perinatal, juvenile, or adult). Findings include amygdala cellular (Mitra et al., 2005; Poeggel et al., 2003; Vyas et al., 2002) and hippocampal structural alterations (de Kloet et al., 2005; Isgor et al., 2004; Kim & Diamond, 2002), which were associated with memory deficits and enhanced anxiety. The timing of these neural and behavioral changes, as well as their potential for normalization once the stress was removed, appear to differ as a function of when the chronic stress was applied (see review by Romeo & McEwen, 2006).
In humans, similar findings of hippocampal atrophy associated with memory and anxiety problems have been reported in adults who suffered a chronic stress (Bourdeau et al., 2005; de Kloet et al., 2005; Lupien et al., 2005). The only study that examined the behavioral and brain structural consequences of chronically elevated corticosteroids in 11 children and adolescents with Cushing syndrome reported reduced amygdala volume, no hippocampal size alterations, and, similar to the present findings, no affective symptoms or memory deficits (Merke et al., 2005). Here, we report for the first time the presence of functional neural abnormalities in these structures associated with hypercortisolemia.
A number of limitations need to be mentioned. First, the sample size of the Cushing syndrome patients is relatively small for the detection of memory deficits. However, this sample size is quite acceptable for an fMRI study, particularly considering the rarity of this condition and the clear-cut underlying neurobiology. We are planning to examine these same subjects after corrective surgery, and test whether the functional cerebral alterations noted here revert to normal patterns with normalized cortisol levels. Second, the healthy group, although similar on all demographic characteristics to the patient group, does not permit to control for the effects of a chronic illness on brain development. A comparison group with a chronic illness that does not directly affect brain function would be important to include in the next generation of studies.
This first study of cerebral function in adolescents with chronic endogeneous hypercortisolemia due to Cushing syndrome demonstrates the presence of functional alterations in amygdala and hippocampus, that are not associated with affective or memory symptoms. Such findings need to be followed by work examining the role of age and related brain maturational stage on these effects, as well as the identification of possible protective factors conferring resilience to affective and cognitive consequences in this disease and/or during this stage of cerebral development.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally-laden and neutral information. Behavioral Neuroscience. 2003;117:505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews in Neuroscience. 2003;4:165–167. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Bannerman D, Rawlins J, McHugh S, Deacon R, Yee B, Bast T, et al. Regional dissociations within the hippocampus—Memory and anxiety. Neuroscience and Biobehavioral Reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Dahl RE, Perel J, Williamson DE, Nelson B, Stull S, et al. Corticotropin-releasing hormone challenge in prepubertal major depression. Biological Psychiatry. 1996;39:267–277. doi: 10.1016/0006-3223(95)00177-8. [DOI] [PubMed] [Google Scholar]
- Bourdeau I, Bard C, Forget H, Boulanger Y, Cohen H, Lacroix A. Cognitive function and cerebral assessment in patients who have Cushing’s syndrome. Endocrinology and Metabolism Clinics of North America. 2005;34:357–369. doi: 10.1016/j.ecl.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning and Memory. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. American Journal of Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. Experiments of nature: Contributions to developmental theory [Editorial] Development and Psychopathology. 2003;15:833–835. doi: 10.1017/s0954579403000397. [DOI] [PubMed] [Google Scholar]
- Curtis WJ, Cicchetti D. Moving research on resilience into the 21st century theoretical and methodological considerations in examining the biological contributors to resilience. Development and Psychopathology. 2003;15:773–810. doi: 10.1017/s0954579403000373. [DOI] [PubMed] [Google Scholar]
- Dahl R, Puig-Antich J, Ryan N, Nelson B, Novacenko H, Twomey J, et al. Cortisol secretion in adolescents with major depressive disorder. Acta Psychiatrica Scandinavica. 1989;80:18–26. doi: 10.1111/j.1600-0447.1989.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, et al. 24-hour cortisol measures in adolescents with major depression: A controlled study. Biological Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- de Kloet E, Joels M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Reviews in Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar K, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biological Psychiatry. 2006;59:24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget H, Lacroix A, Somma A, Cohen H. Cognitive decline in patients with Cushing’s syndrome. Journal of the International Neurospychological Society. 2000;6:20–29. doi: 10.1017/s1355617700611037. [DOI] [PubMed] [Google Scholar]
- Friston K, Mechelli A, Turner R, Price C. Nonlinear responses in fMRI: The balloon model, Volterra kernels, and other hemodynamics. NeuroImage. 2000;12:466–477. doi: 10.1006/nimg.2000.0630. [DOI] [PubMed] [Google Scholar]
- Gispen-de Wied CC, Jansen LM, Duyx JH, Thijssen JH, van Engeland H. Pituitary–adrenal function in adolescent psychiatric patients: Impact of depressive symptoms. Journal of Affective Disorders. 2000;59:71–76. doi: 10.1016/s0165-0327(99)00116-0. [DOI] [PubMed] [Google Scholar]
- Greenhouse S, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of the human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Herbert J. Psychosocial and endocrine features of chronic first-episode major depression in 8–16 year olds. Biological Psychiatry. 2001;50:351–357. doi: 10.1016/s0006-3223(01)01120-9. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. British Journal of Psychiatry. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Hariri A, Tessitore A, Mattay V, Fera F, Weinberger D. The amygdala response to emotional stimuli: A comparison of faces and scenes. NeuroImage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal–juvenile period of hippocampal morphology and on cognitive and stress axis function in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children—Present and life-time version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DD. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews in Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kutcher S, Sokolov S. Adolescent depression: Neuroendocrine aspects. In: Goodyer I, editor. The depressed child and adolescent: Developmental and clinical perspectives. Cambridge: Cambridge University Press; 2001. pp. 195–215. [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu FS, Lord C, Schramek TE, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behavioral Brain Research. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Chrousos GP. Cushing’s syndrome in children and adolescents: Current diagnostic and therapeutic strategies. Journal of Endocrinological Investigation. 2002;25:181–194. doi: 10.1007/BF03343985. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Merke DP, Giedd JN, Keil MF, Mehlinger SL, Wiggs EA, Holzer S, et al. Children experience cognitive decline despite reversal of brain atrophy one year after resolution of Cushing syndrome. Journal of Clinical Endocrinology and Metabolism. 2005;90:2531–2536. doi: 10.1210/jc.2004-2488. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modualtes the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, et al. Developmental differences in neuronal engagement during implicit encoding of emotional faces: An event-related fMRI study. Journal of Child Psychology and Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. Journal of Clinical Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- O’Connor TG. Natural experiments to study the effects of early experience: Progress and limitations. Development and Psychopathology. 2003;15:837–852. doi: 10.1017/s0954579403000403. [DOI] [PubMed] [Google Scholar]
- Pariante CM. The glucocorticoid receptor: Part of the solution or part of the problem? Journal of Psychopharmacology. 2006;20(Suppl):79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biological Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips M, Drevets W, Rauch S, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pine DS, Lissek S, Klein R, Mannuzza S, Moulton J, III, Guardino M, et al. Face-memory and emotion: Associations with major depression in children and adolescents. Journal of Child Psychology and Psychiatry. 2004;45:1199–1208. doi: 10.1111/j.1469-7610.2004.00311.x. [DOI] [PubMed] [Google Scholar]
- Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P, Braun K. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proceedings of the National Academy of Science of the United Sates of America. 2003;100:16137–16142. doi: 10.1073/pnas.2434663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Antich J, Dahl RE, Ryan N, Novacenko H, Goetz D, Goetz R, et al. Cortisol secretion in prepubertal children with major depressive disorder. Episode and recovery. Archives of General Psychiatry. 1989;46:801–809. doi: 10.1001/archpsyc.1989.01810090043008. [DOI] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Froom S, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An fMRI study. Biological Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Annals of the New York Academy of Sciences. 2006;14:1094–2002. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:1213–1223. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology, General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain development and animal models. Annals of the New York Academy of Sciences. 2000;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Szeszko P, Robinson D, Alvir J, Bilder R, Lencz T, Ashtari M, et al. Orbital frontal and amygdala volume reductions in obsessive–compulsive disorder. Archives of General Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Szeszko P, Strous R, Goldman R, Ashtari M, Knuth K, Lieberman J, et al. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. American Journal of Psychiatry. 2002;159:217–226. doi: 10.1176/appi.ajp.159.2.217. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao B, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Winston J, Strange B, O’Doherty J, Dolan R. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:8–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. Empirical analyses of BOLD fMRI statistics. I. Spatially unsmoothed data collected under null-hypothesis conditions. NeuroImage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]