Abstract
We have found modulated phase morphology in a particular region of composition within the liquid-ordered + liquid-disordered coexistence region in the four-component lipid bilayer mixture DSPC/DOPC/POPC/Chol. By controlling lipid composition, we could see distinct types of modulated liquid-liquid phase morphologies, including linear, irregular, and angular features in giant unilamellar vesicles. We used a combination of confocal, two-photon, wide-field fluorescence, and differential interference contrast microscopies, and used stringent controls to minimize light-induced artifacts. These studies establish that both the size and morphology of membrane rafts can be controlled by the concentration and the type of low-melting lipid in mixtures with cholesterol and a high-melting lipid.
The size and shape of coexisting liquid-ordered + liquid-disordered (Lo+Ld) domains in lipid mixtures might affect the behavior of cell membranes (1). We have reported previously that giant unilamellar vesicles (GUVs) of distearoylphosphatidylcholine/dioleoylphosphatidylcholine/cholesterol (DSPC/DOPC/Chol) exhibit macroscopic liquid/liquid Lo+Ld domains (2,3), whereas GUVs of (DSPC/1-palmitoyl, 2-oleoylphosphatidylcholine (POPC)/Chol) appear uniform, even though Förster resonance energy transfer (FRET) indicates liquid/liquid phase separation (3). We are interested in the transition from nano- to macroscale morphologies.
When liquid phases coexist, line tension drives the phase domains toward the minimum perimeter–a single round domain within a matrix of the other phase. This simple morphology can be modulated when a long-range repulsive interaction competes with the free-energy cost of forming excess domain interface (4). This occurs for small domains in monolayers that do not coalesce due to long-range dipole-dipole repulsion (5). In other cases, the repulsive interaction arises from contractile tension from adsorption on a substrate (6,7). Interdomain repulsion can also be generated from curvature effects in phase-separated bilayers, whereby fluid superstructures are maintained (8). Patterned phases can also arise from dynamic behavior such as critical fluctuations (9–11) or trapped domain growth (12). In all cases, competing interactions are needed for structured morphologies.
Here, we report the existence of modulated morphology at certain compositions within the Lo+Ld coexistence region in the four-component DSPC/DOPC/POPC/Chol mixture. A key aspect of this study is its simplicity, which can be attributed to the facts that 1), only lipid-lipid interactions are involved to yield both the line tension and any competing repulsive interaction; and 2), composition, the only variable, is under stringent experimental control. This control might be useful in relating model membrane studies to the existence of nanoscale rafts in cells (1).
We used fluorescence and differential interference contrast (DIC) microscopies to image domain morphologies of GUVs prepared with fixed mole fractions (χ) of both cholesterol and DSPC but varying [DOPC] and [POPC] (see Supporting Material for details). We define a replacement ratio, ρ, as
Fixing χchol = 0.25 and χDSPC = 0.45, modulated phases are observed in a narrow window of composition, with ρ = 15–25% (Fig. 1). At ρ < 15%, GUVs look uniform, whereas at ρ > 25%, they display macroscopic round Lo+Ld domains. We also varied ρ at χchol = 0.22 and χDSPC = 0.39, and found similar morphological transitions. We catalog five types of morphology (Figs. 1 B and 2, B–E; and see Table S1 for statistics), including honeycomb arrangements of Lo domains, irregular 2D bubbles, and stripes. These morphologies are intriguing: although compositions are within the liquid-liquid coexistence region, domains were not round, instead displaying linear features that come together at angles. We also observed that individual GUVs at fixed composition could show different domain morphologies (Figs. 2, B and E, and 4 C, and Fig. S3 B). These intermediate states of the nano-to-macro domain size transition might have distinct physical and chemical properties that would set them apart from fully formed macroscopic or nanoscopic domains.
Figure 1.
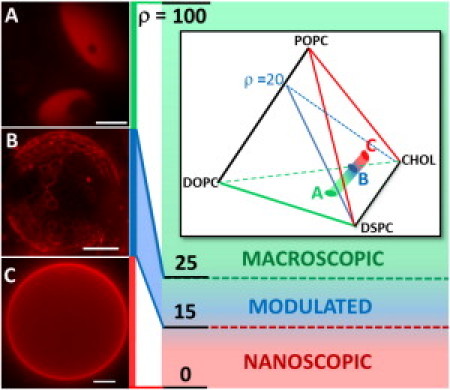
Modulated phases occur at ρ = 15–25% as POPC is replaced by DOPC along an Lo+Ld compositional trajectory (inset). GUV compositions DSPC/DOPC/POPC/Chol at 23°C: (A) 0.45/0.3/0/0.25 (ρ = 100%); (B) 0.45/0.06/0.24/0.25 (ρ = 20%); and (C) 0.45/0/0.3/0.25 (ρ = 0%). Dye C12:0-DiI (0.02 mol %, color-merged red) partitions into Ld. Scale bars 10 μm.
Figure 2.
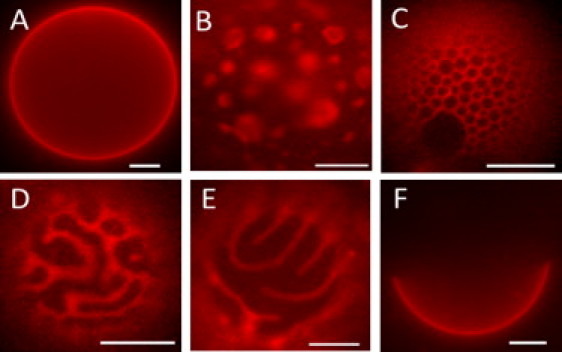
GUV patterns as POPC is replaced by DOPC are (A) uniform; (B) continuous Lo with asymmetric Ld domains; (C) honeycomb; (D) irregular patches of Lo; (E) regular striped pattern; and (F) round macro Lo+Ld domains, with compositions of DSPC/DOPC/POPC/Chol of 0.45/0.03/0.27/0.25 (A), 0.45/0.05/0.25/0.25 (B and E), 0.39/0.06/0.33/0.22 (C), 0.39/0.10/0.29/0.22 (D), and 0.45/0.30/0/0.25 (F). The dye is C12:0-DiI at 0.02 mol %. Scale bars, 10 μm; temperature, 23°C.
Figure 4.
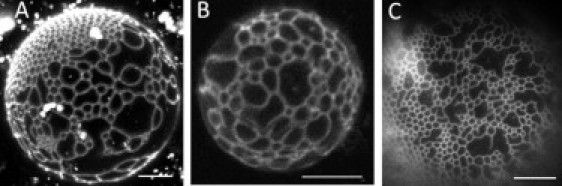
Different fluorescence microscopies show similar patterns at 23°C. (A) Confocal image, C12:0-DiI at 0.02 mol %. (B) Two-photon image Bodipy-PC at 0.02 mol %. (C) Wide-field image, C12:0-DiI at 0.02 mol %. DSPC/DOPC/POPC/Chol composition is 0.45/0.05/0.25/0.25. Scale bars, 10 μm.
Artifactual light-induced domains can occur under commonly used experimental conditions (13). Here, we have made substantial efforts to minimize light-induced artifacts. We used C12:0-DiI probe concentrations as dilute as 0.010 mol %, neutral density filters up to OD = 1.0, and shutter open time during imaging as short as 10 ms. Most important, we found modulated phase morphologies without intense illumination, obtaining images with DIC to locate GUVs prior to fluorescence imaging.
Indeed, DIC experiments were decisive in proving that 1), modulated phases exist before intense light exposure; and 2), modulated phases exist and can be observed even in samples without a probe (Fig. S1). GUVs with modulated phases have discernible DIC patterns (Fig. S2) that correlate exactly with fluorescence images of the same GUV (Fig. 3 and Fig. S3). Furthermore, the patterns are not dependent on details of the wavelength, intensity, or duration of the delivery of light energy to the samples, because two-photon, confocal, and widefield microscopies all showed modulated phases within the same composition range, ρ = 15–25% (Fig. 4). We also observed patterned domains with both DIC and fluorescence on a free-floating GUV (data not shown), confirming that the modulated domains in our system are not caused by adhesion to glass.
Figure 3.
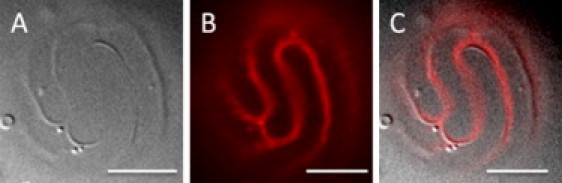
DIC and fluorescence show the same pattern. The DSPC/DOPC/POPC/Chol composition is 0.45/0.05/0.25/0.25 at 23°C. (A) DIC. (B) Fluorescence. (C) Superimposition of DIC and fluorescence. See Fig. S3 for additional images. Scale bars, 10 μm.
Theoretical modeling shows that a competition between the interdomain line energy that tends to reduce domain perimeter and a repulsive domain-domain interaction (perhaps domain curvature, dipole moment, or some other property of Lo domains) yields stable, modulated phase patterns. Calculations by Liu et al. (14) suggest that such competing interactions can control formation of nanosized rafts in cells. To model the observed phase patterns, we used a field-theory approach with competing interactions (see Supporting Material for details). We considered line tension, which favors large round domains, and a repulsive field, which tends to disperse domains. We are currently studying the nature of the repulsive field that competes with line tension, so its form is not yet known. To perform preliminary calculations, we model it here as dipole-dipole repulsion. We model the free energy phenomenologically as a Landau-Ginzburg energy functional (4) with a long-range field term as
| (1) |
where ϕ is the order parameter at position r, B sets the free-energy scale of the phase separation, K is proportional to line tension, Q is the coupling coefficient for the field term, μ is the area density of dipoles, and D[μ] is the electric field normal to the membrane. This choice of dipole-dipole interactions is an initial approach; other forms of the repulsive term, perhaps involving curvature, might prove to better model the interactions. (See the Supporting Material for a more detailed discussion.)
We used Monte Carlo simulations to find equilibrium configurations that minimize Eq. 1. Fig. 5 shows the resulting patterns that could be obtained along a thermodynamic tieline, varying only the phase fractions. The fraction of each phase varies along a tieline, whereas phase properties (line tension and dipole density) are fixed, making possible a simple connection between model and experiments. For example, Fig. 5 shows a continuous transition from stripe to small isolated domains as P deviates from 0.50, where P is the area fraction of Lo. The parameters B, K, and Q were chosen to be in a region of nontrivial phase morphology.
Figure 5.
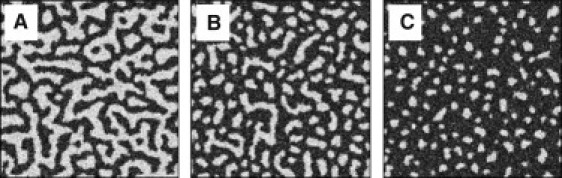
Phase patterns change with Lo area fraction P, with B = 100, K = 10, Q = 2, μ = 1 in Ld (dark) and 0 in Lo (bright). (A) P = 0.5. (B) P = 0.6. (C) P = 0.7.
Obtaining true linewidths of modulated phase patterns could aid theoretical investigations into the nature of the competing interactions. Measured linewidths for the honeycomb and 2D bubble patterns are in the range 0.4–0.7 μm. Given the small movements of the GUVs, these measurements are consistent with true linewidths being diffraction-limited. Further experiments, together with modeling, might clarify the relationship between lipid concentrations and types of patterns and linewidths of modulated phases in these four-component mixtures. We benefit greatly from studying a system with known thermodynamic tielines (2,3).
In cells, both equilibrium and nonequilibrium processes can contribute to the formation and stability of domains (9). Here, we show that lipid composition alone can control the patterns of phase-separated membrane domains. However, we have not yet indentified the long-range interaction that competes with line tension to control domain morphology (Fig. S4 and Fig. S5). Understanding the nature of this interaction might be important for understanding the nature of bilayer phase domain size and shape in cells.
Acknowledgments
We thank Warren Zipfel and Carol Bayles (Microscopy and Imaging Facility, Cornell University, Ithaca, NY) for help with two-photon and confocal microscopy, and Michael Grace and Sean Chrisitie (Micro Video Instruments, St. Louis, MO) for technical assistance.
Support was from research awards from the National Institutes of Health (R01 GM077198) and the National Science Foundation (MCB 0842839) to G.W.F. T.M.K. received support from National Institutes of Health research award 1-T32 GM 08267.
Supporting Material
References and Footnotes
- 1.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J., Wu J., Feigenson G.W. Phase studies of model biomembranes: complex behavior of DSPC/DOPC/cholesterol. Biochim. Biophys. Acta. 2007;1768:2764–2776. doi: 10.1016/j.bbamem.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heberle F.A., Wu J., Feigenson G.W. Comparison of three ternary lipid bilayer mixtures: FRET and ESR reveal nanodomains. Biophys. J. 2010;99:3309–3318. doi: 10.1016/j.bpj.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seul M., Andelman D. Domain shapes and patterns: the phenomenology of modulated phases. Science. 1995;267:476–483. doi: 10.1126/science.267.5197.476. [DOI] [PubMed] [Google Scholar]
- 5.McConnell H.M. Structures and transitions in lipid monolayers at the air-water interface. Annu. Rev. Phys. Chem. 1991;42:171–195. [Google Scholar]
- 6.Rozovsky S., Kaizuka Y., Groves J.T. Formation and spatio-temporal evolution of periodic structures in lipid bilayers. J. Am. Chem. Soc. 2005;127:36–37. doi: 10.1021/ja046300o. [DOI] [PubMed] [Google Scholar]
- 7.Plass R., Bartelt N.C., Kellogg G.L. Dynamic observations of nanoscale self-assembly on solid surfaces. J. Phys. Condens. Matter. 2002;14:4227–4240. [Google Scholar]
- 8.Kaizuka Y., Groves J.T. Bending-mediated superstructural organizations in phase-separated lipid membranes. New J. Phys. 2010;12:095001. [Google Scholar]
- 9.Fan J., Sammalkorpi M., Haataja M. Formation and regulation of lipid microdomains in cell membranes: theory, modeling, and speculation. FEBS Lett. 2010;584:1678–1684. doi: 10.1016/j.febslet.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 10.Honerkamp-Smith A.R., Veatch S.L., Keller S.L. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta. 2009;1788:53–63. doi: 10.1016/j.bbamem.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumgart T., Hess S.T., Webb W.W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 12.Semrau S., Idema T., Storm C. Membrane-mediated interactions measured using membrane domains. Biophys. J. 2009;96:4906–4915. doi: 10.1016/j.bpj.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J., Wu J., Feigenson G. Phase studies of model biomembranes: macroscopic coexistence of Lα+Lβ, with light-induced coexistence of Lα+Lo phases. Biochim. Biophys. Acta. 2007;1768:2777–2786. doi: 10.1016/j.bbamem.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Qi S., Chakraborty A.K. Phase segregation on different length scales in a model cell membrane system. J. Phys. Chem. B. 2005;109:19960–19969. doi: 10.1021/jp053562j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


