Abstract
Background
Endothelial dysfunction and monocyte migration are key events in the pathogenesis of atherosclerosis. Non-muscle myosin light-chain kinase (nmMLCK), the predominant MLCK isoform in endothelial cells, has been shown to contribute to vascular inflammation by altering endothelial barrier function. However, its impact on atherogenesis remains unknown.
Methods and Results
We investigated the role of nmMLCK in the development of atherosclerotic lesions in apolipoprotein E-deficient (apoE−/−) mice fed an atherogenic diet for 12 weeks. Histopathological examination demonstrated that nmMLCK deficiency (apoE−/− nmmlck−/−) reduced the size of aortic lesions by 53%, lipid contents by 44% and macrophage deposition by 40%. Western blotting and reverse-transcription polymerase chain reaction revealed the expression of nmMLCK in aortic endothelial cells and peripheral blood monocytes. Measurements of transendothelial electric resistance indicated that nmMLCK deficiency attenuated endothelial barrier dysfunction caused by thrombin, oxLDL and TNF-α. In monocytes, nmMLCK deficiency reduced their migration in response to the chemokine MCP-1. Further mechanistic studies showed that nmMLCK acted through both myosin light chain (MLC) phosphorylation-coupled and -uncoupled pathways; the latter involved Src signaling. Moreover, depletion of Src via gene silencing, site-specific mutagenesis or pharmacological inhibition of Src greatly attenuated nmMLCK-dependent endothelial barrier dysfunction and monocyte migration.
Conclusions
nmMLCK contributes to atherosclerosis by regulating endothelial barrier function and monocyte migration via mechanisms involving not only kinase-mediated MLC phosphorylation but also Src activation.
Keywords: atherogenesis, endothelial dysfunction, monocyte chemoattractant protein, atherosclerosis, vascular disease
Introduction
Atherosclerosis is an inflammatory disease characterized by lipid and macrophage deposition in the arterial wall. Although the molecular details underlying atherosclerotic lesion development have not been elucidated, endothelial dysfunction and monocyte transendothelial migration have been recognized as integral components in the initiation and progression of atherosclerosis.1
Vascular endothelium provides a selective barrier controlling the traffic of plasma proteins and circulating cells across the blood vessel wall. The integrity of this barrier is maintained based on dynamic interactions between endothelial cytoskeleton, cell-cell junctions and cell-matrix focal adhesions. Endothelial barrier dysfunction has been implicated as an important mechanism underlying many diseases or injurious conditions associated with inflammation, 2 where inflammatory mediators activate diverse signaling pathways in the endothelium leading to widened intercellular space allowing the passage of macromolecules and cells.
Activation of cytoskeletal contractile machinery is critical for cell motility (leukocyte migration) and cell-cell adhesion-based barrier properties (endothelial permeability). In endothelial monolayers, cytoskeleton contraction produces a centripetal force that counteracts cell-cell adhesions rendering widened intercellular junction. The contractile force is generated based on actin-myosin binding triggered by phosphorylation of myosin light chain (MLC), the major substrate of myosin light chain kinase (MLCK). MLCK phosphorylates MLC at Ser19/Thr18, leading to a conformational change in the myosin tertiary structure that triggers actomyosin contraction. 3
The non-muscle MLCK (nmMLCK, ~220 kD) is the predominant isoform in the endothelium of human macro/microvessels. 4 This isoform and the short smooth muscle isoform (smMLCK, ~108 kD) are encoded by the same mylk1 gene but different promoters. 5-7 A role of nmMLCK in regulating various cellular responses has recently been documented. 8-14 Consistent with its function in cell contraction, nmMLCK contributes to endothelial cell-cell junction opening and paracellular hyperpermeability in response to pro-inflammatory agents, such as histamine, 15 thrombin,16 oxygen radicals 17 and activated neutrophils. 18 Moreover, nmMLCK participates in inflammation by promoting neutrophil transendothelial migration, 19 and by activating NF-κB 20 and cytokine production. 21 nmMLCK also plays a role in nitrosative stress during endotoxin shock. 11 The pathophysiological importance of nmMLCK in inflammation is further supported by studies with genetically modified animals. In particular, nmMLCK isoform-specific knockout (nmmlck−/−) produces a phenotype indistinguishable from the wild type without major cardiovascular defects; 13, 22 however, nmMLCK deletion confers decreased susceptibility to acute lung injury 13 and improved survival in endotoxic shock 11 and severe burns. 12
During atherosclerosis development, endothelial barriers undergo conformational or structural changes promoting lipoprotein and leukocyte transendothelial flux and deposition in the vascular wall. Several pro-inflammatory mediators, including thrombin, oxidized LDL and TNF-α, have been identified as contributors to atherosclerotic lesions; however, the molecular mechanisms underlying their injurious effects have not been characterized. Based on our recent finding that nmMLCK mediates vascular hyperpermeability in systemic inflammation, 12, 23, 24 we hypothesize that nmMLCK contributes to atherosclerosis by altering endothelial barrier function and promoting monocyte infiltration. We tested this hypothesis using an apoE-deficient murine model of atherosclerosis.
Materials and Methods
Additional details are available online as Supplemental Materials.
ApoE−/−nmmlck−/− mice generation and atherosclerosis characterization
Apoe−/− mice and nmmlck−/− mice were used to generate apoE−/−nmmlck+/+ (n=11) and apoE−/− nmmlck−/− (n=13) mice (Suppl. Fig. 1). Atherosclerosis was induced by feeding male mice at 6 weeks of age an atherogenic diet (Research Diet, New Brunswick, NJ) for 12 weeks. To assess lesion, heart-aorta complexes were excised. Thoracic-abdominal aortas were fixed with 10% formalin, and aortic sinuses and arches were embedded for frozen sectioning. All animal procedures were conducted in compliance with the NIH guidelines and approved by the Institutional Animal Care and Use Committee.
Primary culture of aortic endothelial cells
Primary aortic endothelial cells (AEC) were isolated from mice using positive immuno-selection with a rat anti-mouse CD31.
Monocyte isolation and transmigration
Murine peripheral monocytes were separated via histopaque (Sigma, MO) gradient centrifugation followed by negative selection (Stem Cell, BC, Canada). Transendothelial migration was examined as previously described 25 with modifications.
In vitro and in vivo permeability assays
As an indicator of barrier properties, transendothelial electrical resistance (TER) was measured following a previously described procedure. 25 In vivo vascular barrier function was examined with a modified Evans blue assay. 26
Statistical Analysis
For the vascular pathologies (atherosclerotic lesion size, intimal hyperplasia, macrophage infiltration, etc), comparisons were made between 13 nmmlck−/− and 11 nmmlck+/+ mice. For the cell studies, at least 3 independent experiments from different samples were performed under each condition, where repeated measurements from each sample was averaged and counted as one experiment. Data was presented as mean ± SE. Unpaired t test was employed for comparisons between two groups. For comparisons among multiple groups, one or two-way analysis of variance (ANOVA) was used followed by Newman-Keuls test. Significance was accepted at p≤0.05.
Results
Absence of nmMLCK reduces atherosclerosis in apoE−/− mice
Compared with apoE−/−nmmlck+/+ littermates, apoE−/−nmmlck−/− mice displayed no change in food consumption, weight and fertility; however, they showed a 53% reduction in the area of thoracic-abdominal lesion (Fig. 1A) after an atherogenic diet for 12 weeks. A similar reduction was found in the intimal area of aortic sinus cross-sections (Fig. 1B) and aortic arch longitudinal sections (Fig. 1C) from apoE−/−nmmlck−/− mice. Lipid deposition in the lesion, as demonstrated by oil red O positive region, was decreased by 44% in the aortic arch and 31% in the aortic sinus from apoE−/−nmmlck−/− mice (Fig. 1B and 1C). The absence of nmMLCK did not significantly change the serum concentration of lipids, including triglyceride, total, LDL, HDL and VLDL cholesterol (Suppl. Table 1).
Figure 1. nmMLCK deficiency reduces atherosclerosis in apoE−/− mice.
After consuming the atherogenic diet for 12 weeks, aortas were dissected from apoE−/−nmmlck−/− mice (n=13) and their apoE−/−nmmlck+/+ littermates (n=11). A, En face staining of lesion areas with oil red O in thoracic-abdominal aorta (TA). *p<0.05 vs. apoE−/−nmmlck+/+ mice. B, C, cross-sections of aortic sinuses (B) and longitudinal sections of aortic arches (C) were stained with oil red O for intimal area and lipid deposition. *p<0.05, **p<0.01, ***p<0.001 vs. apoE−/−nmmlck+/+ mice. Shown right are representative photographs.
Absence of nmMLCK alters cell and collagen contents in the intima
Consistent with reduced lesion size, decreased macrophage content was observed in the intima of nmMLCK-deficient aorta (40% reduction, Fig. 2A). While there was no significant change of T cells in the plaque (Suppl. Fig. 2A), there was a 95% increase in smooth muscle cells (Fig. 2B) and 65% increase in collagen deposition (Fig. 2C) in apoE−/−nmmlck−/− compared to apoE−/− nmmlck+/+ mice.
Figure 2. nmMLCK deficiency alters cell contents in atherosclerotic lesions.
Cryosections of aortic arches from apoE−/−nmmlck−/− mice (n=13) and their apoE−/−nmmlck+/+ littermates (n=11) were immunostained for macrophages (Mϕ) and smooth muscle cells (SMC) with anti-Mac-3 and anti-α-actin respectively. Collagen was stained with picrosirius red. A, less macrophages infiltrate into the lesion in apoE−/−nmmlck−/− mice. *p<0.05 vs. apoE−/−nmmlck+/+ mice. B, more SMCs migrate into the intima in apoE−/−nmmlck−/− mice. *p<0.05 vs. apoE−/−nmmlck+/+ mice. C, higher collagen content in apoE−/−nmmlck−/− mice. *p<0.05 vs. apoE−/−nmmlck+/+ mice. Shown right are representative photographs.
nmMLCK deficiency attenuates inflammation-induced endothelial barrier dysfunction
We compared the effects of oxLDL and thrombin on endothelial permeability with or without nmMLCK. Aortic ECs were isolated from nmmlck−/− and nmmlck+/+ mice, and nmMLCK expression in these cells was confirmed by RT-PCR and Western blotting (Fig. 3B and 3C). An electric cell-substrate impedance sensor (ECIS) was used to measure the dynamic changes in transendothelial electrical resistance (TER), an indicator of endothelial barrier function. Deficiency of nmMLCK attenuated both thrombin- and oxLDL-caused TER reduction (Fig. 3D and 3E), suggesting that nmMLCK contributes to the endothelial barrier response to these inflammatory mediators.
Figure 3. nmMLCK deficiency attenuates endothelial barrier dysfunction.
A, flow cytometric analyses with an anti-CD31 antibody confirm the identity and comparable purity of aortic endothelial cells isolated from nmmlck−/− mice and their nmmlck+/+ littermates. B, C, RT-PCR (B) and Western blotting (C) confirm nmMLCK expression in the primary culture of endothelial cells. nmMLCK expression in lung tissues serves as a positive control for Western Blotting. D, E, TER measurements show that nmMLCK deficiency in endothelial cells attenuates barrier dysfunction caused by thrombin (D) or oxLDL (E). *p<0.05 vs. nmmlck+/+ cells. D-left, representative TER tracings. F, in vivo vascular barrier function was examined with Evans blue assay in nmmlck−/− mice (n=3) vs. their nmmlck+/+ littermates (n=3), and apoE−/− nmmlck−/− mice (n=3) vs. their apoE−/−nmmlck+/+ littermates (n=3). The results show that nmMLCK deficiency leads to reserved endothelial barrier function in apoE−/− mice. *p<0.05 vs. apoE−/−nmmlck+/+ mice. Shown right are representative images of aorta from apoE−/− mice. NT, control without Evans blue injection. G, endothelial barrier function in apoE−/− mice (n=3) is decreased compared with wild type mice (n=3). Both are on C57/BL6 background. *p<0.05 vs. wild type mice.
We further assessed albumin transport across the vascular wall in vivo using Evans blue assay. 26 There was no difference between nmmlck−/− and nmmlck+/+ in apoE+/+ mice; however, in apoE −/− mice, absence of nmMLCK resulted in a 35% reduction in Evans blue deposition in the aorta compared to nmmlck+/+ littermates (Fig. 3F). Additional experiments demonstrated a small but significantly higher Evans blue staining in apoE−/− mice compared with the wild type (Fig. 3G). These findings, consistent with the normal phenotype of nmmlck−/− mice, suggest that nmMLCK plays an important role in the pathophysiological regulation of endothelial barriers.
Absence of nmMLCK reduces monocyte transmigration
Monocytes were isolated from the peripheral blood of nmmlck+/+ or nmmlck−/− mice and their transmigration across nmmlck+/+ or nmmlck−/− endothelial monolayers to a chemoattractant, MCP-1, was examined. Knockout of nmMLCK in endothelial cells did not significantly reduce monocyte transmigration (data not shown); however, nmmlck−/− monocytes displayed significantly impaired transmigration in the presence or absence of nmmlck+/+ endothelial monolayers (Fig. 4A). The data suggests that monocytic nmMLCK is relatively more important than endothelial nmMLCK in regulating monocyte motility. Consistently, nmMLCK mRNA and protein were detected in monocytes (Fig. 4B). To the best of our knowledge, this is the first report regarding nmMLCK expression in monocytes.
Figure 4. nmMLCK is expressed in monocytes and contributes to their transmigration.

A. monocytes derived from nmmlck−/− mice displayed a lower level of migration in response to MCP-1 gradient in the presence (trans-endothelial migration, TEM) or absence (Chemotaxis) of nmmlck+/+ endothelial monolayer. *p<0.05 vs. monocytes derived from nmmlck+/+ mice. B, RT-PCR and Western blotting reveal nmMLCK expression in monocytes.
Both MLCK kinase and non-kinase activities contribute to endothelial barrier dysfunction and monocyte transmigration
To determine the mechanisms of nmMLCK function, we tested the involvement of MLCK kinase activity-coupled MLC phosphorylation in response to LDL or thrombin. While native LDL did not cause significant MLC phosphorylation or TER reduction (Suppl. Fig. 3), oxLDL promoted MLC phosphorylation in endothelial cells over the same time course as TER reduction, and the phosphorylation was diminished in the absence of nmMLCK (Fig. 5A). ML-7, a pan-inhibitor of MLC kinases, attenuated oxLDL-induced hyperpermeability response (Fig. 5B). In parallel, we compared the effects of thrombin on TER and MLC phosphorylation in nmmlck+/+ and nmmlck−/− endothelial cells. The TER response was attenuated in nmmlck−/− whereas MLC phosphorylation was detected at the same level in the two cell types (Fig. 5C), suggesting that thrombin-induced hyperpermeability involves mechanisms that are uncoupled with MLC phosphorylation. Additional experiments demonstrated that MLC phosphorylation is not required for monocyte motility regardless of nmMLCK presence (Fig. 5D).
Figure 5. Different stimuli cause different responses in MLC phosphorylation in nmmlck+/+ and nmmlck−/− cells.
A, loss of nmMLCK decreases oxLDL-induced MLC phosphorylation in endothelial cells. nmmlck+/+ and nmmlck−/− aortic ECs were treated with or without 50μg/mL oxLDL for 2 hours. MLC phosphorylation was examined by Western blotting with phopho MLC (T18/S19)-specific antibody, and total MLC (tMLC) antibody on the same membrane after stripping. Left, a representative blot; right, quantitation of MLC phosphorylation. *p<0.05 vs. oxLDL-treated nmmlck+/+ cells. B, ML-7 attenuates oxLDL-caused endothelial barrier dysfunction. nmmlck+/+ aortic endothelial cells were pretreated with or without 5 μm ML-7 for 1 hour, followed by stimulation with 50μg/mL oxLDL and TER measurement. *p<0.05 vs. cells without ML-7 pretreatment. C, nmMLCK deficiency does not affect thrombin-induced MLC phosphorylation. nmmlck+/+ and nmmlck−/− aortic EC were treated with or without 25nM thrombin for 5 minutes, followed by Western blotting. D, MLC phosphorylation is undetectable in nmmlck+/+ or nmmlck−/− monocytes treated with or without MCP-1. Thrombin-treated endothelial cells serve as a positive control. Shown is representative blot.
nmMLCK contributes to Src activation independently of kinase activity
We then examined the MAPK, NF-κb and Src pathways as potential signals downstream from nmMLCK. In aortic ECs, neither thrombin nor oxLDL induced detectable phosphorylation of NF-κb p65 at Ser536 (indicator of activation) (Suppl. Fig. 4A). While thrombin increased phosphorylation of p38 MAPK, Erk1/2 and SAPK/JNK (Suppl. Fig. 4B), the responses were not different in nmmlck+/+ and nmmlck−/− cells. In contrast, Src phosphorylation was different in that increased Src phosphorylation at tyrosine 416 in thrombin-treated endothelial cells (Fig. 6A) and in MCP-1-treated monocytes (Fig. 6C) was attenuated by nmMLCK deficiency. Neither thrombin nor MCP-1 induced Src phosphorylation at tyrosine 527, indicating residue-specific activation. ML-7, an inhibitor of MLC phosphorylation (Suppl. Fig. 5), failed to alter Src phosphorylation (Fig. 6B and 6D), further supporting that nmMLCK-mediated Src activation is not dependent on its kinase activity or MLC phosphorylation.
Figure 6. nmMLCK contributes to Src activation independently of kinase activity.
A, nmMLCK deficiency inhibits thrombin-induced Src phosphorylation at tyrosine 416. nmmlck+/+ and nmmlck−/− aortic endothelial cells were treated with or without 25nM thrombin for 5 minutes. Src phosphorylation was examined by Western blotting with a phopho-Src (Y416 or Y527)-specific antibody, and an antibody against non-phospho-Src at tyrosine 416 (npSrc Y416) or 527 (npSrc Y527) on the same membrane after stripping. Left, a representative blot; right, quantitation of Src phosphorylation. *p<0.05 vs. non-treated cells; #<0.05 vs. thrombin-treated nmmlck+/+ cells. B, ML-7 does not inhibit thrombin-induced Src phosphorylation. nmmlck+/+ aortic endothelial cells were treated with or without 5 μM ML-7 for 1 hour, followed by stimulation with thrombin and immunoblotting as above. *p<0.05 vs. non-treated cells. C, nmMLCK deficiency inhibits MCP-1-induced Src phosphorylation at tyrosine 416 in monocytes. nmmlck+/+ and nmmlck−/− monocytes were treated with or without 20ng/mL MCP-1 for 1 hour, followed by immunoblotting as above. *p<0.05 vs. non-treated cells; #<0.05 vs. MCP-1-treated nmmlck+/+ monocytes. D, ML-7 does not inhibit MCP-1-induced Src phosphorylation in monocytes. nmmlck+/+ monocytes were pretreated with or without 5 μM ML-7 for 1 hour, followed by stimulation with MCP-1 and immunoblotting as above. *p<0.05 vs. non-treated cells.
nmMLCK-Src pathway mediates endothelial barrier dysfunction and monocyte transmigration
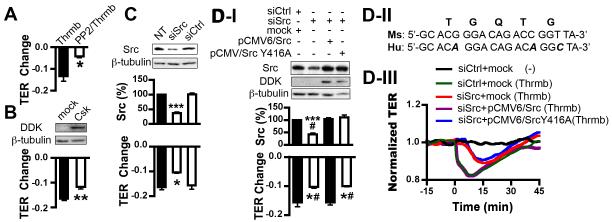
The signaling role of Src in regulating endothelial barrier function and monocyte transmigration is further tested in the following Src manipulation experiments. Pharmacological inhibition of Src activation with PP2 (Fig. 7A), over-expressing the Src endogenous inhibitor C-terminal Src kinase (Csk) (Fig. 7B), or Src gene silencing via siRNA knockdown (Fig. 7C) attenuated thrombin-induced TER reduction in endothelial cells. However, the same treatment did not significantly alter MLC phosphorylation to thrombin (Suppl. Fig. 6A). To specify the cause-effect relationship between Src phosphorylation and barrier dysfunction, murine endothelial cells were co-transfected with siRNA specific for murine Src and pCMV6/human Src. Mismatches between mouse and human Src gene sequences (Fig. 7D-II) led to the rescue expression of Src (Fig. 7D-I). Consistently, wild type Src, but not the Y416A mutant Src, restored the TER response (Fig. 7D-I and 7D-III), indicating the specific role of Src activation via Y416 phosphorylation in mediating endothelial barrier dysfunction.
Figure 7. Src activation is involved in endothelial barrier dysfunction.
A, PP2 attenuates thrombin-induced endothelial barrier dysfunction. nmmlck+/+ aortic endothelial cells were pretreated with or without 5 μM PP2 for 1 hour, followed by stimulation with 25nM thrombin and TER measurement. *p<0.05 vs. cells without pretreatment of PP2. B, overexpression of Csk diminishes thrombin-induced endothelial hyperpermeability response. nmmlck+/+ aortic endothelial cells were transfected with empty vector (mock) or pCMV6/DDK-tagged Csk (Csk). Top, immunoblotting analysis for Csk overexpression using an anti-DDK antibody; bottom, quantitation of TER change. **p<0.01 vs. mock. C, knockdown of Src reverses endothelial barrier dysfunction caused by thrombin. nmmlck+/+ aortic endothelial cells were non-transfected (NT) or transfected with siRNA specific for mouse Src (siSrc) or scrambled siRNA (siCtrl). Top, immunoblotting analysis for Src expression. ***p<0.001 vs. NT. Bottom, quantitation of TER change. *p<0.05 vs. NT. D, rescue expression of wild type Src, but not Y416A mutant Src, restores thrombin-induced barrier dysfunction in Src-depleted endothelial cells. D-I, nmmlck+/+ aorta endothelial cells were co-transfected with siRNA targeting mouse Src (siSrc) and pCMV6/DDK-tagged human WT (pCMV6/Src) or Y416A mutant Src (pCMV6/Src Y416A). Control cells were co-transfected with scrambled siRNA and empty vector. Top, immunoblotting analysis for Src expression. ***p<0.001 vs. control. #p<0.05 vs. cells co-transfected with siSrc and pCMV6/WT Src. Bottom, quantitation of TER change induced by thrombin. *p<0.05 vs. control cells; #p<0.05 vs. cells co-transfected with siSrc and pCMV6/WT Src. D-II, alignment of mouse (Ms) Src siRNA target sequence and corresponding human (Hu) Src gene sequence. Mismatches are indicated in bold/italic. D-III, Representative TER tracings of three independent experiments.
In monocytes, pretreatment with PP2 reduced their transendothelial migration (Fig. 8A). Further studies using THP-1 cells, a human monocytic cell line, confirmed the constitutive expression of human nmMLCK and successful knockdown by siRNA in these cells (Fig. 8B). Consistent with the findings from murine monocytes, depletion of nmMLCK in THP-1 attenuated Src phosphorylation (Fig. 8C) and transendothelial migration in response to MCP-1 (Fig. 8E). Further, THP-1 cells were transfected with siRNA specific to human Src (Fig. 8D) and pCMV6/Csk (Fig. 8F). Both interventions significantly reduced THP-1 transendothelial migration (Fig. 8E and 8F).
Figure 8. nmMLCK/Src pathway is involved in monocyte transmigration.
A, PP2 decreases monocyte transmigration. nmmlck+/+ monocytes were treated with or without 5 μM PP2 for 1 hour, followed by migration across nmmlck+/+ endothelial monolayer in response to MCP-1 gradient. ***p<0.001 vs. non-treated cells. B, RT-PCR confirms nmMLCK expression in THP-1 cells. The expression was reduced in nmMLCK siRNA (sinmMLCK)-tranfected THP-1 cells compared with scrambled siRNA- (siCtrl) or non-transfected (NT) cells. Shown is representative of three independent experiments. C, knockdown of nmMLCK inhibits MCP-1-induced Src phosphorylation in THP-1 cells. Transfected THP-1 cells were treated with 20 ng/mL human MCP-1 for 1 hour, followed by immunoblotting analysis for Src phosphorylation as described in Fig.6. *p<0.05, **p<0.01 vs. non-treated NT; #p<0.05 vs. MCP-1 treated NT. D, knockdown of Src with siRNA in THP-1 cells. **p<0.01 vs. NT. E, knockdown of nmMLCK or Src leads to a reduction in THP-1 transmigration across endothelial cell monolayers in response to MCP-1 gradient. ***p<0.001 vs. NT. F, overexpression of Csk in THP-1 cells decreases trans-endothelial migration of THP-1 cells. Top, representative blot; bottom, quantitation of THP-1 cell transmigration. ***p<0.001 vs. mock.
Discussion
Although nmMLCK is implicated in endothelial dysfunction caused by various inflammatory mediators, 11, 12, 13 its specific role in atherosclerosis has not been reported. This study provides direct evidence for the involvement of nmMLCK in atherosclerotic lesion development. The beneficial effect of nmMLCK on atherosclerosis may involve three major cell types identified in atheroma: endothelial cells, monocytes and smooth muscle cells. In endothelial cells, nmMLCK is the predominant MLCK isoform that triggers actomyosin contraction, and its causal effect on the paracellular permeability response to pro-inflammatory or pro-thrombotic agonists has been well documented. 15, 16, 17 Consistently, inhibition of MLCK kinase activity or genetic deletion of nmMLCK leads to beneficial effects against microvascular leakage, 23, 24 acute lung injury, 13 endotoxic shock 11 and severe burns. 12 In line with these findings, our results demonstrate that nmMLCK knockout or inhibition attenuates endothelial hyperpermeability to thrombin, oxLDL and TNF-α (Suppl. Fig. 7), pro-inflammatory mediators know to associate with atherosclerosis.
The barrier property of endothelial cells also affects leukocyte diapedesis. Based on the theory that disruption of endothelial cell-cell junctions facilitates leukocyte transmigration, 27 we hypothesized that nmMLCK deficiency would disable endothelial cell contractile machinery, thereby impairing junction opening for leukocytes to move through. To our surprise, however, nmMLCK knockout in endothelial cells attenuated endothelial hyperpermeability without significantly altering monocyte transmigration. On the other hand, nmmlck−/− monocytes showed impaired migration activity. Thus, the decreased monocyte infiltration into the atherosclerotic lesion observed in apoE−/−nmmlck−/− mice was mainly attributed to impaired motility of monocytes lacking nmMLCK. This finding indicates the complexity of leukocyte-endothelium interactions, processes controlled by both leukocyte activity and endothelial function. Indeed, endothelial hyperpermeability and leukocyte transmigration are not always coupled, and spatial or temporal dissociation between the two events has been frequently observed. 28
Another interesting aspect of this study is the constitutive expression of nmMLCK in both human and murine monocytes. The non-muscle MLCK isoform, once named endothelial MLCK (eMLCK), 4 is thought to be expressed predominantly in endothelial cells. The presence of nmMLCK in monocytes and its functional role in monocyte infiltration indicate the therapeutic potential of targeting this isoform for treating immunological disorders or inflammatory diseases. While detected in both endothelial cells and monocytes, nmMLCK is expressed in the vascular smooth muscle at a minimal level. 4 In this study, we observed an increased SMC content in the atherosclerotic lesion of apoE−/−nmmlck−/− mice. Although it remains unclear whether nmMLCK deficiency affects SMC function or changes local environments within the atheroma, 29 its effect to increase SMC contents in the plaque may be beneficial during the progress of atherosclerosis. Decreased macrophage content is usually, if not always, coupled with a higher level of SMC migration into the intima and thus more collagen deposition in the lesion, 30 leading to stabilized plaques that are less susceptible to rapture. 31
With respect to the molecular mechanisms by which nmMLCK mediates endothelial barrier dysfunction and monocyte migration, MLCK is traditionally thought to function exclusively through its kinase activity. MLCK-catalyzed MLC phosphorylation and resultant actomyosin contraction is considered the major mechanism of intercellular gap formation in response to hyperpermeability factors like histamine, 15 thrombin, 16 oxygen radicals 17 and activated neutrophils. 18 Inhibition of MLCK kinase activity attenuates microvascular leakage induced by phorbol esters, 23 neutrophils, 24 and septic agents. 13 Consistently, a MLC phosphorylation-coupled barrier response was seen in the current study with endothelial cells exposed to oxLDL.
Emerging evidence suggests the involvement of MLC phosphorylation-uncoupled mechanisms in nmMLCK-mediated cellular events. For example, nmMLCK can alter endothelial calcium entry independently of its kinase activity. 32 Telokin, a small MLCK isoform lacking the catalytic domain, regulates calcium sensitivity through activation of MLCP. 33 Most recently, nmMLCK is found to promote neutrophil infiltration into septic lungs, 34 an effect involving Pyk2 tyrosine kinase activation and independent of MLC phosphorylation. In line with these findings, our data showed that in endothelial cells, nmMLCK deficiency attenuated thrombin-induced hyperpermeability without altering its substrate activity, as a comparable MLC phosphorylation level was observed between nmmlck−/− and nmmlck+/+ cells (Fig. 5C). The data suggest that nmMLCK can mediate endothelial barrier responses to different agonists (oxLDL vs. thrombin) via different (kinase-dependent or independent) mechanisms. Moreover, while nmMLCK deficiency significantly reduced monocyte migration to MCP-1, MLC phosphorylation was not detectable in either nmmlck−/− or nmmlck+/+ monocytes. Thus, nmMLCK mediates multiple cellular responses where MLC phosphorylation is not a prerequisite or necessarily coupled event. It is noted that ML-7, a pan-MLCK inhibitor, was able to attenuate the barrier dysfunction and block MLC phosphorylation to thrombin (Suppl. Fig. 5). In another study, the same drug significantly reduced the basal permeability of microvessels in the absence of thrombin. 23
To characterize nmMLCK downstream signaling independent of MLC phosphorylation, we examined MAPK, NF-kB and Src pathways, which are known to regulate vascular inflammation. Although MAPK activation has been identified as a common step in endothelial and monocytic responses to a wide spectrum of physiological and pathological factors, 35 it did not seem to be a key event in thrombin-nmMLCK signaling, as the activation levels of p38, Erk1/2, and pSAPK/JNK in response to thrombin were consistent regardless of the presence of nmMLCK. Similarly, NF-κB activation has been considered critical in TNF-α-induced endothelial responses requiring nmMLCK activity. 20 Although we observed attenuated NF-κB phosphorylation at Ser536 coupled with a blunted barrier response to TNF-α in nmmlck−/− cells (Suppl. Fig. 7), we did not detect NF-κB phosphorylation to either thrombin or oxLDL regardless of nmMLCK presence. Therefore, NF-κB may be relatively less important in thrombin- and oxLDL-induced responses.
Interestingly, Src phosphorylation at Tyr416 in thrombin-treated endothelial cells and MCP-1-treated monocytes was greatly attenuated in the absence of nmMLCK. The binding and signaling interactions between nmMLCK and Src is supported by the molecular structure of nmMLCK characterized by a N-terminus containing multiple sites for protein-protein interactions, including several SH2/SH3-binding consensus sequences and three potential sites for Src phosphorylation. 36, 37 Recent studies have demonstrated the ability of nmMLCK to bind Src 36 and regulate its kinase activity through phosphorylation. 38 The possibility that Src serves as a key signal in nmMLCK-mediated monocyte activity and endothelial cell function is further supported by that Src controls integrin-dependent leukocyte mobility 39 and that Src mediates MCP-1-induced monocyte transendothelial migration. 35 Moreover, Src-induced protein tyrosine phosphorylation plays an important role in regulating endothelial junction structure 40 and function.16 The failure of ML-7 to inhibit Src phosphorylation has further confirmed the kinase-independency of the nmMLCK-Src pathway. In the current study, pharmacological inhibition of Src, molecular manipulation of Src via knockdown, or over-expressing CSK significantly attenuated the endothelial barrier response to thrombin. In monocytes, Src knockdown, nmMLCK depletion, or CSK overexpression impaired their transendothelial migration. Rescue experiments further demonstrated that wild type Src, but not SrcY416A mutant, recovered the hyperpermeability response, confirming the specific importance of tyrosine 416 in Src-mediated cellular events. Taken together, these experiments support the causal effects of Src activation on nmMLCK-mediated endothelial hyperpermeability and monocyte transmigration in response to pro-atherogenic factors. Src signaling is likely located downstream of nmMLCK because nmMLCK knockout can block Src activation whereas inhibition of Src failed to affect nmMLCK activity (Suppl. Fig. 6).
In conclusion, this study demonstrates a pathophysiological role of nmMLCK in atherogenesis. We suggest that nmMLCK participates in the atherosclerotic lesion development by augmenting endothelial barrier dysfunction and monocyte infiltration in response to pro-inflammatory factors, including thrombin, oxLDL and TNF-α. The molecular mechanisms of nmMLCK-mediated cellular responses involve not only the kinase-dependent MLC phosphorylation, but also Src signaling that is independent of MLC phosphorylation. This work has shed light on, as well as raised questions about, the diverse cellular and molecular effects of nmMLCK. Given the finding that nmMLCK is dispensable in physiological conditions 13 but required for the action of certain pro-atherogenic factors, selective blockage of this isoform may represent a new area of drug development for the treatment of atherosclerosis.
Supplementary Material
Clinical Perspective.
Endothelial dysfunction and monocyte migration have been implicated in the pathogenesis of atherosclerosis. Non-muscle myosin light chain kinase (nmMLCK) is known to contribute to inflammation-associated endothelial barrier dysfunction by activating the cytoskeletal contractile response via its kinase activity on myosin light chain phosphorylation. The specific contribution of nmMLCK to atherosclerotic injury and its mechanism of action have not been evaluated. In this study, we tested the hypothesis that nmMLCK promoted atherosclerotic lesion development by altering endothelial barrier properties. In the aorta of apoE−/− mice fed an atherogenic diet, nmMLCK deficiency significantly reduced lesion size , intimal hyperplasia, and macrophage deposition in the vascular wall, indicating a pathogenic role of nmMLCK in atherosclerosis. Consistent with the in vivo observations, nmMLCK expression was detected in both aortic endothelial cells and peripheral monocytes, and nmMLCK deficiency attenuated endothelial hyperpermeability and monocyte transendothelial migration caused by atherosclerosis-relevant inflammatory stimuli, including thrombin, oxidized LDL, TNF-α and MCP-1. Further mechanistic studies demonstrated that in addition to myosin light chain phosphorylation, Src signaling contributed to nmMLCK-induced cellular responses. Pharmacological blockade or genetic manipulation of Src inhibited nmMLCK-mediated hyperpermeability and monocyte transmigration. Taken together, the data suggests a novel function of nmMLCK in atherosclerosis that involves a non-conventional signaling pathway independent of MLC phosphorylation. Further characterization of specific cellular responses to isoform-specific MLCK kinase activity and kinase-independent mechanisms would contribute to the development of new therapeutic targets for treating atherosclerosis.
Acknowledgements
We thank Drs. Laurel Beckett and Erin Dienes from Department of Public Health Sciences for statistical consultation. We thank Chris Pivetti, Bert Frederich, Robert Pitts and Olesya Litovka for excellent technical assistance.
Funding This work was supported by NIH grants RO1 HL061507, HL084542, HL096640 and UL1 RR024146.
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Cinel I, Dellinger RP. Advances in pathogenesis and management of sepsis. Curr Opin Infect Dis. 2007;20:345–352. doi: 10.1097/QCO.0b013e32818be70a. [DOI] [PubMed] [Google Scholar]
- 3.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 4.Verin AD, Lazar V, Torry RJ, Labarrere CA, Patterson CE, Garcia JG. Expression of a novel high molecular-weight myosin light chain kinase in endothelium. Am J Respir Cell Mol Biol. 1998;19:758–766. doi: 10.1165/ajrcmb.19.5.3125. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher PJ, Herring BP. The carboxyl terminus of the smooth muscle myosin light chain kinase is expressed as an independent protein, telokin. J Biol Chem. 1991;266:23945–23952. [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- 7.Yin F, Hoggatt AM, Zhou J, Herring BP. 130-kDa smooth muscle myosin light chain kinase is transcribed from a CArG-dependent, internal promoter within the mouse mylk gene. Am J Physiol Cell Physiol. 2006;290:C1599–1609. doi: 10.1152/ajpcell.00289.2005. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher PJ, Jin Y, Killough G, Blue EK, Lindner V. Alterations in expression of myosin and myosin light chain kinases in response to vascular injury. Am J Physiol Cell Physiol. 2000;279:C1078–1087. doi: 10.1152/ajpcell.2000.279.4.C1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, Lin A, Turner JR. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 10.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 11.Ranaivo H Ralay, Carusio N, Wangensteen R, Ohlmann P, Loichot C, Tesse A, Chalupsky K, Lobysheva I, Haiech J, Watterson DM, Andriantsitohaina R. Protection against endotoxic shock as a consequence of reduced nitrosative stress in MLCK210-null mice. Am J Pathol. 2007;170:439–446. doi: 10.2353/ajpath.2007.060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynoso R, Perrin RM, Breslin JW, Daines DA, Watson KD, Watterson DM, Wu MH, Yuan S. A role for long chain myosin light chain kinase (MLCK-210) in microvascular hyperpermeability during severe burns. Shock. 2007;28:589–595. doi: 10.1097/SHK.0b013e31804d415f. [DOI] [PubMed] [Google Scholar]
- 13.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, Van Eldik LJ, Watterson DM. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci U S A. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moy AB, Shasby SS, Scott BD, Shasby DM. The effect of histamine and cyclic adenosine monophosphate on myosin light chain phosphorylation in human umbilical vein endothelial cells. J Clin Invest. 1993;92:1198–1206. doi: 10.1172/JCI116690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VW. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res. 1998;83:1115–1123. doi: 10.1161/01.res.83.11.1115. [DOI] [PubMed] [Google Scholar]
- 17.Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- 18.Hixenbaugh EA, Goeckeler ZM, Papaiya NN, Wysolmerski RB, Silverstein SC, Huang AJ. Stimulated neutrophils induce myosin light chain phosphorylation and isometric tension in endothelial cells. Am J Physiol. 1997;273:H981–988. doi: 10.1152/ajpheart.1997.273.2.H981. [DOI] [PubMed] [Google Scholar]
- 19.Saito H, Minamiya Y, Kitamura M, Saito S, Enomoto K, Terada K, Ogawa J. Endothelial myosin light chain kinase regulates neutrophil migration across human umbilical vein endothelial cell monolayer. J Immunol. 1998;161:1533–1540. [PubMed] [Google Scholar]
- 20.Wadgaonkar R, Linz-McGillem L, Zaiman AL, Garcia JG. Endothelial cell myosin light chain kinase (MLCK) regulates TNFalpha-induced NFkappaB activity. J Cell Biochem. 2005;94:351–364. doi: 10.1002/jcb.20250. [DOI] [PubMed] [Google Scholar]
- 21.Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohlmann P, Tesse A, Loichot C, Ranaivo H Ralay, Roul G, Philippe C, Watterson DM, Haiech J, Andriantsitohaina R. Deletion of MLCK210 induces subtle changes in vascular reactivity but does not affect cardiac function. Am J Physiol Heart Circ Physiol. 2005;289:H2342–2349. doi: 10.1152/ajpheart.00511.2004. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y, Huang Q, Wu HM. Myosin light chain phosphorylation: modulation of basal and agonist-stimulated venular permeability. Am J Physiol. 1997;272:H1437–1443. doi: 10.1152/ajpheart.1997.272.3.H1437. [DOI] [PubMed] [Google Scholar]
- 24.Yuan SY, Wu MH, Ustinova EE, Guo M, Tinsley JH, De Lanerolle P, Xu W. Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ Res. 2002;90:1214–1221. doi: 10.1161/01.res.0000020402.73609.f1. [DOI] [PubMed] [Google Scholar]
- 25.Sun C, Wu MH, Guo M, Day ML, Lee ES, Yuan SY. ADAM15 regulates endothelial permeability and neutrophil migration via Src/ERK1/2 signalling. Cardiovasc Res. 2010;87:348–355. doi: 10.1093/cvr/cvq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007;176:719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He P. Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled? Cardiovasc Res. 2010;87:281–290. doi: 10.1093/cvr/cvq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niemann-Jonsson A, Ares MP, Yan ZQ, Bu DX, Fredrikson GN, Branen L, Porn-Ares I, Nilsson AH, Nilsson J. Increased rate of apoptosis in intimal arterial smooth muscle cells through endogenous activation of TNF receptors. Arterioscler Thromb Vasc Biol. 2001;21:1909–1914. doi: 10.1161/hq1201.100222. [DOI] [PubMed] [Google Scholar]
- 30.Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452–1458. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- 31.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H, Tran QK, Takeuchi K, Fukao M, Liu MY, Kanno M, Hayashi T, Iguchi A, Seto M, Ohashi K. Myosin light-chain kinase regulates endothelial calcium entry and endothelium-dependent vasodilation. FASEB J. 2001;15:282–284. doi: 10.1096/fj.00-0587fje. [DOI] [PubMed] [Google Scholar]
- 33.Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R, Owens GK, Somlyo AP, Somlyo AV. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc Natl Acad Sci U S A. 2006;103:2440–2445. doi: 10.1073/pnas.0508566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Gao XP, Ramchandran R, Zhao YY, Vogel SM, Malik AB. Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating beta2 integrins. Nat Immunol. 2008;9:880–886. doi: 10.1038/ni.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cambien B, Pomeranz M, Millet MA, Rossi B, Schmid-Alliana A. Signal transduction involved in MCP-1-mediated monocytic transendothelial migration. Blood. 2001;97:359–366. doi: 10.1182/blood.v97.2.359. [DOI] [PubMed] [Google Scholar]
- 36.Garcia JG, Verin AD, Schaphorst K, Siddiqui R, Patterson CE, Csortos C, Natarajan V. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60(src) Am J Physiol. 1999;276:L989–998. doi: 10.1152/ajplung.1999.276.6.L989. [DOI] [PubMed] [Google Scholar]
- 37.Lazar V, Garcia JG. A single human myosin light chain kinase gene (MLCK; MYLK) Genomics. 1999;57:256–267. doi: 10.1006/geno.1999.5774. [DOI] [PubMed] [Google Scholar]
- 38.Birukov KG, Csortos C, Marzilli L, Dudek S, Ma SF, Bresnick AR, Verin AD, Cotter RJ, Garcia JG. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60(Src) J Biol Chem. 2001;276:8567–8573. doi: 10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]
- 39.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 40.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.