Abstract
Increased ultraviolet-B (UV-B) radiation as a consequence of ozone depletion is one of the many potential drivers of ongoing global amphibian declines. Both alone and in combination with other environmental stressors, UV-B is known to have detrimental effects on the early life stages of amphibians, but our understanding of the fitness consequences of these effects remains superficial. We examined the independent and interactive effects of UV-B and predatory chemical cues (PCC) on a suite of traits of Limnodynastes peronii embryos and tadpoles, and assessed tadpole survival time in a predator environment to evaluate the potential fitness consequences. Exposure to a 3 to 6 per cent increase in UV-B, which is comparable to changes in terrestrial UV-B associated with ozone depletion, had no effect on any of the traits measured, except survival time in a predator environment, which was reduced by 22 to 28 per cent. Exposure to PCC caused tadpoles to hatch earlier, have reduced hatching success, have improved locomotor performance and survive for longer in a predator environment, but had no effect on tadpole survival, behaviour or morphology. Simultaneous exposure to UV-B and PCC resulted in no interactive effects. These findings demonstrate that increased UV-B has the potential to reduce tadpole fitness, while exposure to PCCs improves their fitness.
Keywords: amphibian declines, ultraviolet-B radiation, predation, fitness consequences, sublethal effects, interactive effects
1. Introduction
Amphibians around the world are in decline, with nearly 2000 of over 6400 described species listed as threatened with extinction [1], and a range of potential causes for these declines have been proposed [2]. The parallel timing of rapid falls in ozone levels and the beginning of rapid amphibian declines during the late 1970s and early 1980s [3,4] generated a surge of scientific interest in the idea that increased ultraviolet-B (UV-B) radiation associated with stratospheric ozone depletion [5] may be contributing to some amphibian declines through its lethal and sublethal effects on embryonic and larval life stages [6–8]. Research over the past two decades has shown that, in some species, UV-B exposure can cause increased mortality, reduced growth, reduced rate of development, delayed metamorphosis, reduced locomotor performance, developmental abnormalities, behavioural changes and increased susceptibility to disease (e.g. [9–16]). UV-B has also been shown to interact synergistically with the pathogenic water mould Saprolegnia ferax [15], low pH [17], low temperature [16], a variety of contaminants [18] and non-lethal predatory chemical cues (PCC) [10] to enhance amphibian mortality above the additive effects of the independent stressors.
The effects of UV-B, however, are not consistent between species, nor between populations and life-history stages of the same species [7,8,19], and this has led to dispute regarding the role UV-B may play in amphibian declines. In particular, studies have reported that exposure to UV-B does not lead to a reduction in embryonic and larval survival (e.g. [19]). Nevertheless, a recent quantitative meta-analysis of 89 studies of the effects of ambient UV-B on embryonic and larval survival demonstrated that, on average, UV-B exposure reduced survival by nearly half [20]. Such a finding indicates that UV-B is indeed an important stressor in amphibian habitats, but, as Croteau et al. [7] point out in their review, ‘we must move beyond solely examining overt death of amphibians and begin to understand [the] subtle effects of [UV-B] alone and in combination with other stressors’ (p. 757). Indeed, while numerous studies on embryonic and larval amphibians have investigated the sublethal effects of UV-B alone [7,8,18] and in combination with other environmental stressors [10,16–18], few have examined whether these sublethal effects affect the ability of amphibians to garner resources and avoid predation, and thus survive through to sexual maturity and reproduce (i.e. their fitness). One study that has done so demonstrated that UV-B-induced spinal curvature in tadpoles can ultimately lead to death by causing them to swim in circles and thus preventing them from foraging [11]. Similarly, other UV-B-induced malformations and abnormalities such as oedema or bloating [21,22], eye abnormalities [23,24] and hindlimb malformations [12] may also negatively affect foraging ability, predator evasion and reproductive success, but, critically, these possibilities have not been tested directly. Other studies have measured correlates of fitness such as locomotor performance, predator-induced morphological defences, anti-predator behaviour, and age and size at metamorphosis [25–31], and have demonstrated that these are negatively impacted by exposure to UV-B [10,14,16,32], but, again, none of these studies measured the fitness variables that these parameters are meant to be correlated to (i.e. survival in a predator environment or reproductive success).
Clearly, there is a need to examine directly the fitness consequences of sublethal UV-B effects in amphibians, and to continue to do so in an ecologically relevant context with additional environmental stressors. With numerous amphibian populations disappearing rapidly from pristine habitats [4] where anthropogenic stressors (such as contaminants) are less likely to be present, it is particularly important that the effects of UV-B are examined not just in combination with anthropogenic stressors, but also natural stressors. Predation is a natural stressor that can impact greatly upon the early life stages of amphibians by causing either direct mortality through their consumption or changes in their behaviour, morphology and life-history that act as defensive mechanisms [33–36]. In a previous study on striped marsh frog Limnodynastes peronii (Duméril & Bibron 1841) tadpoles, we demonstrated that exposure to UV-B combined with non-lethal PCC, which are cues that signal risk of predation [37], causes greater tadpole mortality than the additive mortality caused by the two stressors independently, indicating a synergistic interaction [10]. We also found that exposure to UV-B suppresses the development of predator-induced morphological defences in L. peronii tadpoles, thereby potentially increasing their susceptibility to predation [10]. This study, however, did not explore the potential fitness consequences of exposure to UV-B alone and in combination with PCC by measuring tadpole survival in a predator environment.
In the present study, we examine the independent and interactive effects of UV-B and PCC on the time to hatch and the hatching success of L. peronii embryos, and the post-hatch survival, locomotor performance, morphology and behaviour of L. peronii tadpoles. Increased locomotor performance and predator-induced changes in morphology and behaviour are assumed to increase an individual's fitness because they increase its chances of survival in a predator environment [38–41]. To test this assumption, we measured tadpole survival time in a predator environment using freshwater shrimp Macrobrachium australiense (Holthius 1950) as predators, which allowed us to directly correlate changes in locomotor performance, behaviour and morphology to success in a predator environment. Since the reproductive success of an amphibian is dependent on surviving the pre-metamorphic life phase through to sexual maturity, we consider the measure of survival time in a predator environment to be indicative of a tadpole's fitness, because if the chance of a tadpole being predated upon is greater, then the tadpole is more likely to have zero fecundity and zero fitness [42].
2. Material and methods
(a). Animal collection and maintenance
Nine freshly laid L. peronii foam egg masses were collected early in the morning from an ephemeral creek near The University of Queensland, Brisbane, Australia (27°30′22.81″ S, 152°59′22.99″ E), over a two-day period. Immediately following collection on each day, egg masses were transported to The University of Queensland where an equal number of eggs were randomly selected from each mass and divided among experimental treatments (n = 48 per treatment). Individuals collected on the first day (from five egg masses) were used to gather time to hatch, hatching success, post-hatch survival, locomotor performance and morphology data, while individuals collected on the second day (from four egg masses) were used in predation trials to assess behaviour and survival time in a predator environment. All individuals were reared separately in 25 ml containers filled to a depth of 35 mm with experimental treatment water (see below). The position of containers within the UV-B experimental treatments was randomly assigned and changed daily. Upon hatching, tadpoles were fed boiled spinach ad libitum. Temperature (20 ± 1°C) and photoperiod (12 L : 12 D) were kept constant for the duration of the experimental period.
(b). Experimental treatments
Individuals collected on both days were exposed to a factorial combination of two UV-B treatments—low UV-B (LUV) and high UV-B (HUV)—and two predator treatments—PCC absent (PCC−) and PCC present (PCC+)—such that there were four experimental treatments in total (i.e. LUV PCC−, HUV PCC−, LUV PCC+ and HUV PCC+) with 96 individuals in each (48 individuals from the first collection day and 48 individuals from the second collection day).
Embryos and larvae within the low and high UV-B treatments were exposed to UV-B, UV-A and visible light emitted from two 40 W linear fluorescent light bulbs (Repti Glo 8.0, Exo Terra, Montreal, Canada) that were on for 12 h each day, and an additional two 40 W linear fluorescent light bulbs (SBL40W, NEC, Tokyo, Japan) that were on for 1 h each day at the photoperiod midpoint. The high UV-B treatment included an additional 1 h pulse of UV-B and UV-A at the photoperiod midpoint from two 40 W linear fluorescent light bulbs (T-40M, Vilber Lourmat, Marne-la-Valee, France) covered by acetate sheets to filter out wavelengths shorter than 290 nm.
The absolute irradiance of UV-B in ambient mid-day sunlight during the peak breeding season of L. peronii (i.e. summer) in Brisbane, Australia, has been measured previously as 5 W m−2 [16], which corresponds to a UV index (UVI) of 11 [43,44]. The peak absolute irradiance of UV-B generated by our low and high UV lighting regimes was 3.7 and 4.5 per cent, respectively, of 5 W m−2, and both had a UVI less than 1 (see electronic supplementary material, table S1). Over the course of a cloudless day in Brisbane during summer (December–February), the UVI is below 1 only in the early morning (before 07.00 h) and in the late afternoon (after 17.00 h), with the average daily maximum UVI between December 2004 and February 2010 being 11 [45].
For our low and high UV-B treatments, we calculated the daily dose of erythemal UV (UVery; see electronic supplementary material, table S1), which is a measure of UV weighted according to the erythemal action spectrum. The erythemal action spectrum describes the relative effectiveness of specific UV wavelengths to cause sunburn in human skin [46], but may be considered representative of other biological UV effects (e.g. DNA damage [47]) because the action spectra of these effects are similar to that of the erythemal action spectrum [48]. We also calculated the daily dose of 300 and 305 nm for our low and high UV-B treatment because these are two wavelengths that have been shown to have increased as a consequence of stratospheric ozone depletion [49,50]. The differences in daily doses of UVery, 300 and 305 nm between our low and high UV-B treatments were between 3 and 6 per cent, respectively (see electronic supplementary material, table S1), which are well within the observed increase of terrestrial UV-B of up to approximately 15 per cent per decade owing to stratospheric ozone depletion [5,51,52].
Freshwater shrimp M. australiense were used as predators and were obtained from a local aquarium supplier (Westside Pets & Aquarium, Taringa, QLD, Australia). Shrimp were maintained in two 20 l aquaria filled with dechlorinated tapwater and fed L. peronii tadpoles (Gosner stage 24–25 [53]) ad libitum. The water from these aquaria was used to rear PCC+ individuals, and thus these individuals were exposed to chemical cues from both the predators and the damaged conspecifics [37]. To maintain water quality and PCC within individual containers, 90 per cent water changes were performed daily. To control for this physical disturbance, PCC− individuals also received a 90 per cent water change with dechlorinated tap water.
(c). Time to hatch, hatching success and post-hatch survival of tadpoles
Embryos were checked daily to record time to hatch and hatching success. Hatching commenced on the fourth day of the experimental period. Once hatching commenced (0 h), embryos were checked every 2–4 h for 30 h, then again at 46 h and 70 h. Time to hatch was considered to be the midpoint between the time at which hatch was first recorded and the preceding check time. Following hatching, tadpole survival was checked daily until the termination of the experiment, which occurred on the eighth and ninth day of the experimental period.
(d). Locomotor performance and morphology
The locomotor (burst swimming) performance and morphology of each tadpole were measured on the eighth and ninth day of the experimental period when tadpoles had reached Gosner stage 25 [53] (4–5 days after hatching). Burst swimming was assessed by filming a minimum of three startle responses for each individual (n = 40, 35, 29 and 29 for LUV PCC−, LUV PCC+, HUV PCC− and HUV PCC+, respectively) with a high-speed digital camera (Redlake Motionscope, DEL Imaging Systems, Cheshire, CT, USA) recording at 250 Hz. Swims were performed without exposure to experimental treatments and were conducted in a 150 × 150 × 10 mm deep plastic container that was immersed in a temperature-controlled water bath (20 ± 1°C) and filled with dechlorinated tap water. Startle responses were induced by a small electrical stimulus (S88 Grass Stimulator), and burst swimming sequences were captured in a mirror positioned above the filming arena at a 45° angle. Recordings were played back using the accompanying Redlake software (Redlake Motionschope Media Player v. 2.21), and only burst swimming sequences that consisted of a C-start response in which the tadpole started from a stationary position and continued in a straight line parallel to the bottom were analysed. Briefly, a C-start response is a fast-escape response that has been characterized in fishes and amphibians: it initially involves a tight bend to one side, causing a C-shaped curve in the body and then a propulsive movement out of the C-shape [54,55]. The average velocity over 100 ms was used as the measure for burst swimming performance and was calculated by digitizing the snout tip at the end of C-start manoeuvre and again 25 frames (100 ms) forward, and measuring the total straight-line distance travelled. Of all the recordings taken for each individual, the fastest was taken as a measure of maximum performance and was used for statistical analysis.
Immediately after being filmed for burst swimming performance, dorsal and lateral views of each individual (n = 38, 33, 27 and 30 for LUV PCC−, LUV PCC+, HUV PCC− and HUV PCC+, respectively) were photographed using a digital camera mounted through a dissecting microscope. Nine morphological measurements (total length, body length, body height, body width, tail muscle height, tail muscle width, total tail height, tail muscle area and total tail area; see electronic supplementary material, figure S1) were taken using SigmaScan Pro 5.0 (Systat Software, CA, USA). After being photographed, tadpoles were euthanased by an overdose of MS-222.
(e). Predation trials
Six 10 l aquaria, each containing five shrimp (M. australiense) and floating vegetation (Cabomba sp.) that covered approximately a third of the water surface, were used as predator tanks and were maintained at 20 ± 1°C in a temperature-controlled room and kept under laboratory fluorescent lighting without UV. Predation trials commenced on the twelfth day of the experimental period when tadpoles were at Gosner stage 25 [53] and were performed over a 6 day period, during which tadpoles continued to be exposed to experimental treatments and remained at Gosner stage 25 [53]. A single predation trial consisted of an individual tadpole, selected at random from the treatment groups, being introduced to one of the predator tanks by gently passing it through a pipette. Following entry, the tadpole was observed and time spent being active was recorded. Once the tadpole had been successfully caught and had begun to be consumed, the trial was terminated and survival time was calculated from the time of entry. Predation trials were ended if the tadpole had not been successfully caught after 1 h, which was the case for only nine out of 168 trials (one, three, three and two trials from LUV PCC−, LUV PCC+, HUV PCC− and HUV PCC+, respectively), and these were omitted from the dataset. Exploratory analysis of the frequency distribution of survival time data indicated a gap between 20 and 40 s suggesting that trials that lasted less than 20 s were a consequence of entry effects. The 11 trials (one, five, four and one trials from LUV PCC−, LUV PCC+, HUV PCC− and HUV PCC+, respectively) that fell into this category were omitted from the dataset (for the final dataset n = 41, 33, 37 and 37 for LUV PCC−, LUV PCC+, HUV PCC− and HUV PCC+, respectively).
(f). Statistical analyses
Time to hatch data were analysed using a Scheirer-Ray-Hare test [16,56] with UV-B (low or high) and PCC (present or absent) as fixed factors, and an interaction term between UV-B and PCC. Hatching success and tadpole survival data were analysed using logistic regression [57] with UV-B (low or high) and PCC (present or absent) as fixed factors, and an interaction term between UV-B and PCC. Burst swimming performance data were analysed using a two-way mixed model ANCOVA with UV-B (low or high) and PCC (present or absent) as fixed factors, an interaction term between UV-B and PCC, day and time of measurement and tadpole total length as covariates, and egg mass as a random effect.
To assess changes in body size and shape associated with treatments, we performed a principal components analysis (PCA) [25,57] on the correlation matrix of the nine morphological measurements of all treatment groups combined. This generated a new set of standardized uncorrelated morphological variables, with principal component 1 (PC 1) and principal component 2 (PC 2) accounting for 65 per cent of the variation (electronic supplementary material, table S2). The PCA matrix of component loadings (electronic supplementary material, table S2) shows the correlation between the original morphological measurements and the two principal components. PC 1 represents a measure of tadpole size because all morphological dimensions load strongly and positively (0.45–0.90) on this axis (electronic supplementary material, table S2). PC 2 represents tadpole shape and separates the variables (loadings) into two morphological aspects: strong positive loadings refer to tail muscle shape (tail muscle width, height and area), and strong negative loadings refer to body shape (body width, height and length) and total tail height (electronic supplementary material, table S2). The PC factor scores for PC 1 and PC 2 were analysed independently using a two-way mixed model ANCOVA with UV-B (low or high) and PCC (present or absent) as fixed factors, an interaction term between UV-B and PCC, day and time of measurement as covariates, and egg mass as a random effect, to identify how UV-B and PCC affected PC 1 (size) and PC 2 (shape).
For the predation trials, the proportion of the total time spent being active and survival time were arcsine square root and cube root transformed, respectively, to satisfy assumptions of normality and homogeneity of variance. These data were then analysed using a mixed-model ANCOVA with UV-B (low or high) and PCC (present or absent) as fixed factors, an interaction term between UV-B and PCC, day and time of measurement as covariates, and egg mass, recorder and predator tank as random effects.
All statistical analyses were performed using JMP 7.0 (SAS Institute Inc., Cary, NC, USA), except the PCA that was performed using StatistiXL 1.8 (www.statistixl.com).
3. Results
(a). Time to hatch, hatching success and post-hatch survival of tadpoles
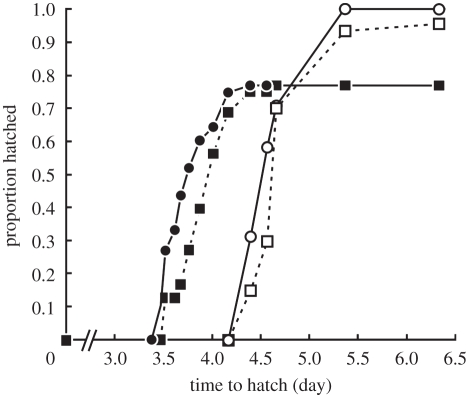
Exposure to high UV-B did not significantly affect time to hatch (H1 = 2.2, p = 0.14), but exposure to PCC caused embryos to hatch significantly earlier than those not exposed (H1 = 112.2, p < 0.001; figure 1). There was no significant interaction between UV-B and PCC (H1 = 0.1, p = 0.73).
Figure 1.
The independent and interactive effects of chronic exposure to UV-B and predatory chemical cues (PCC) on the time to hatch and hatching success of L. peronii embryos. LUV and HUV denote low and high UV-B levels, respectively, and PCC− and PCC+ denote the absence and presence of PCC, respectively. For all treatments n = 48 except for HUV PCC− where n = 47. Some data points indicating zero proportion hatched were removed for clarity. Exposure to high UV-B did not affect the time to hatch (p = 0.14) or the hatching success (p = 0.10) of embryos, but exposure to PCC caused embryos to hatch earlier (p < 0.001) and have reduced hatching success (p < 0.001). There was no interaction between UV-B and PCC for time to hatch (p = 0.73) nor hatching success (p = 0.10). Open circles with solid line, LUV PCC−; filled circles with solid line, LUV PCC+; open squares with dashed line, HUV PCC−; filled squares with dashed line, HUV PCC+.
Exposure to high UV-B did not significantly affect hatching success (χ2 = 2.6, d.f. = 1, p = 0.10), but exposure to PCC significantly reduced hatching success (χ2 = 24.1, d.f. = 1, p < 0.001), and there was no significant interaction between UV-B and PCC (χ2 = 2.6, d.f. = 1, p = 0.10; figure 1). A total of 100 and 96 per cent of embryos hatched in the LUV PCC− and HUV PCC− treatments, respectively, but only 77 per cent of embryos hatched in each of the LUV PCC+ and HUV PCC+ treatments (figure 1).
Exposure to high UV-B did not significantly affect the post-hatch survival of tadpoles (χ2 = 0, d.f. = 1, p = 1.00), nor did exposure to PCC (χ2 = 3.3, d.f. = 1, p = 0.07), and there was no significant interaction between UV-B and PCC (χ2 = 0, d.f. = 1, p = 1.00). All tadpoles survived through to the end of the experimental period in each of the LUV PCC− and HUV PCC− treatments, and 97 per cent of tadpoles survived through to the end of the experimental period in each of the LUV PCC+ and HUV PCC+ treatments (all tadpoles that did not survive died within four days of hatching).
(b). Locomotor performance
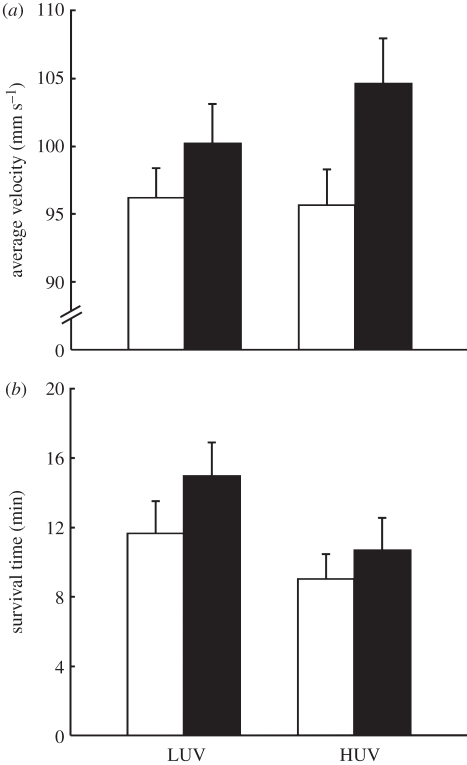
Exposure to high UV-B did not significantly affect the burst swimming performance of tadpoles (F1,126 = 0.002, p = 0.97), but exposure to PCC significantly increased it (F1,126 = 4.8, p = 0.03), and there was no significant interaction between UV-B and PCC (F1,126 = 0.6, p = 0.45; figure 2a). PCC− and PCC+ tadpoles had a pooled average velocity of 96.0 ± 1.7 and 102.2 ± 2.2 mms−1, respectively (figure 2a). There was a significant effect of tadpole total length (F1,126 = 20.1, p < 0.001) on burst swimming performance, with longer tadpoles swimming faster, but there was no significant effect of day or time of measurement (F1,126 = 0.2, p = 0.69 and F1,126 = 0.4, p = 0.55, respectively).
Figure 2.
The independent and interactive effects of chronic exposure to UV-B and predatory chemical cues (PCC) on (a) burst swimming performance (i.e. the average swimming velocity (mm s−1) over 100 ms) and (b) survival time (min) in a predator environment of L. peronii tadpoles. LUV and HUV denote low and high UV-B levels, respectively, and PCC− and PCC+ denote PCC absence and presence, respectively. In (a), n = 40, 35, 29 and 29 for LUV PCC−, LUV PCC+, HUV PCC− and HUV PCC+, respectively. In (b), n = 41, 33, 37 and 37 for LUV PCC−, LUV PCC+, HUV PCC− and HUV PCC+, respectively. Data represent means ± s.e. Exposure to high UV-B did not affect the burst swimming performance (p = 0.97) of tadpoles, but reduced their survival time in a predator environment (p = 0.02). Exposure to PCC significantly increased the burst swimming performance of tadpoles (p = 0.03), and increased their survival time in a predator environment (p = 0.03). There was no interaction between UV-B and PCC for burst swimming performance (p = 0.45), nor survival time in a predator environment (p = 0.58). Open bars, PCC−; filled bars, PCC+.
(c). Morphology
Exposure to high UV-B did not significantly affect tadpole size (PC 1: F1,121 = 0.003, p = 0.96), nor did exposure to PCC (PC 1: F1,118 = 3.2, p = 0.08), and there was no significant interaction between UV-B and PCC (PC 1: F1,118 = 1.3, p = 0.26). There was also no significant effect of day or time of measurement (PC 1: F1,121 = 1.1, p = 0.31 and F1,120 = 0.4, p = 0.55, respectively).
Exposure to high UV-B did not significantly affect tadpole shape (PC 2: F1,121 = 1.4, p = 0.23), nor did exposure to PCC (PC 2: F1,119 = 2.2, p = 0.14), and there was no significant interaction between UV-B and PCC (PC 2: F1,119 = 1.7, p = 0.19). There was also no significant effect of day or time of measurement (PC 2: F1,118 = 2.4, p = 0.13 and F1,122 = 0.1, p = 0.72, respectively).
(d). Predation trials
Exposure to high UV-B did not significantly affect the proportion of time tadpoles spent being active during predation trials (F1,133 = 0.5, p = 0.47), nor did exposure to PCC (F1,132 = 3.0, p = 0.09), and there was no significant interaction between UV-B and PCC (F1,134 = 1.8, p = 0.18). Tadpoles were active for 10 ± 6 per cent (mean ± s.d.) of the trials. There was no significant effect of day or time of measurement (F1,138 = 0.6, p = 0.45 and F1,135 = 0.1, p = 0.82, respectively).
Exposure to high UV-B significantly reduced the survival time of tadpoles in a predator environment (F1,117 = 5.3, p = 0.02), while exposure to PCC significantly increased it (F1,111 = 4.7, p = 0.03), but there was no significant interaction between UV-B and PCC (F1,132 = 0.3, p = 0.58; figure 2b). Exposure to high UV-B reduced PCC− and PCC+ tadpole survival time by 22 and 28 per cent, respectively, while exposure to PCC increased LUV and HUV tadpole survival time by 28 and 18 per cent, respectively (figure 2b). Day of measurement had a significant effect on survival time (F1,114 = 4.1, p = 0.045), but time of measurement did not (F1,73 = 73.2, p = 0.27), with tadpoles surviving for longer as the days progressed.
4. Discussion
(a). Independent effects of UV-B
In the present study, we demonstrate for the first time that an ecologically relevant increase in UV-B may have significant fitness consequences for tadpoles living with predators. Here, we show that a small (3–6%) increase in the daily dose of UV-B, which is comparable to changes in terrestrial UV-B associated with stratospheric ozone depletion [5,51,52], causes a significant (22–28%) reduction in the time it takes for L. peronii tadpoles to be captured and eaten by a predator (figure 2b). This is particularly interesting given that this small increase in UV-B did not affect any of the other traits measured, namely the time to hatch and hatching success of embryos (figure 1), and the post-hatch survival, morphology, locomotor performance (figure 2a) and behaviour of tadpoles. Romansic et al. [58] similarly found that exposure to non-lethal UV-B increases the susceptibility of Cascades frog Rana cascadae tadpoles to predation by rough-skinned newts Taricha granulosa, although in their study there was a 74-fold difference in the daily dose of UV-B between their control and UV-B treatments. Romansic et al. [58] suspected that tail deformities caused by exposure to UV-B may have caused tadpoles to have reduced swimming ability, and therefore reduced chances of escape, but failed to find a correlation between deformities and time to predation. Unlike Romansic et al. [58] and many other studies (e.g. [11,12,21,22]), we did not see an effect of UV-B on tadpole morphology, and presumably this is why there was no effect on locomotor performance. In contrast to the effects of increased UV-B, exposure to PCC caused tadpoles to have increased locomotor performance (figure 2a) and longer survival times in a predator environment (figure 2b). This suggests that an increase in locomotor performance does confer some benefit to survival in a predator environment as one would expect [27], but with regard to the effects of UV-B, there are clearly additional factors that need to be considered when investigating predator–prey interactions.
Consideration of behavioural defence mechanisms and the energetic costs associated with exposure to UV-B may provide an explanation for the observed reduction in survival time of tadpoles exposed to the high UV-B treatment. In a detailed examination of the behavioural defences of red-eyed tree frog Agalychnis callidryas tadpoles in response to predatory shrimp Macrobrachium americanum, Warkentin [59] found that tadpoles that are more active have better chances of survival. Specifically, she found that fleeing in response to an approach or attack by a shrimp was an effective behavioural defence adopted by tadpoles. Although we found no treatment effects on the proportion of time tadpoles spent being active during predation trials, we did observe in the present study the defence strategy described by Warkentin [59]: tadpoles sprinted away from shrimp in bouts of activity lasting up to several seconds following encounters with shrimp that occurred once every minute on average. It is possible that this level of activity in tadpoles is quite intense and exhaustive. We therefore hypothesize that the tadpoles exposed to the high UV-B treatment took less time to be predated upon because they became fatigued more quickly than those exposed to the low UV-B treatment. We suggest this because exposure to UV affects the metabolic rate of tadpoles [60] and Daphnia [61], and reduces the metabolic scope of larval fish [62]. Therefore, tadpoles exposed to the high UV-B treatment may have succumbed to predation faster because they had less energy available for sustained swimming.
(b). Independent effects of predatory chemical cues
The effects of predators and PCC on embryonic and larval amphibians have been well documented, and our findings in the present study correspond well with previous work. Here, we show that exposure to PCC derived from predatory shrimp feeding on conspecific tadpoles induces earlier hatching of L. peronii embryos (figure 1), which is an effect that has been found for a number of amphibian species in response to exposure to both egg and tadpole predators and PCC [63–68]. Less common is our finding that exposure to PCC reduced the hatching success of L. peronii embryos, which is shared by only one other study, by Anderson & Brown [64] on green frog Rana clamitans embryos. An accumulation of nitrogenous waste in the predator tanks may have been responsible for the reduced hatching success in the present study, but the water in the predator tanks was aerated and filtered constantly, making this possibility seem unlikely. Interestingly, the present study and the study by Anderson & Brown [64] both used crustacean predators (the freshwater shrimp M. australiense and the crayfish Procambarus nigrocinctus, respectively), indicating that reduced hatching success may be a consequence of the stress imposed on embryos by this specific type of predator.
For L. peronii tadpoles, exposure to PCC resulted in improved locomotor performance (figure 2a) and longer survival times in a predator environment (figure 2b), but had no effect on post-hatch survival, morphology or behaviour during predation trials. Previous studies have shown that enhanced burst swimming performance of tadpoles is accompanied by changes in tadpole morphology [69–71], and that predator-induced change in morphology rather than increased locomotor performance is the mechanism by which tadpoles increase their chances of survival in a predator environment [39,70–73]. Here, however, we show that tadpoles can have faster escape responses without changing their morphology (which suggests that improved locomotor performance may be associated with physiological changes), and that changes in locomotor performance rather than changes in morphology are correlated with survival time in predation trials. It is important to point out, however, that while changes in tadpole morphology were not detectable at the time measurements were taken, it is possible that tadpoles may have developed morphological defences by the time they were used in predation trials because young hatchlings such as ours are known to exhibit predator-induced morphological defences [74,75]. We therefore cannot dismiss the possibility that changes in tadpole morphology may have contributed to the better survival of PCC-exposed tadpoles in predation trials. Despite this, however, the capacity to swim faster is likely to be an important trait for tadpole survival in an environment with predatory shrimp, particularly given that sprinting away from shrimp has been shown to be an effective defence adopted by tadpoles [59].
(c). Conclusions
UV-B is potentially contributing to the ongoing phenomenon of global amphibian declines. While our knowledge of the lethal and sublethal effects of UV-B alone and in combination with other environmental stressors on amphibians has grown considerably over the past two decades [6–8], our understanding of how UV-B impacts upon the individual fitness and population dynamics of amphibians remains relatively superficial (but see [76]). Here, we show that exposure to a small (3–6%) increase in the daily dose of UV-B has the potential to significantly reduce tadpole fitness by increasing its susceptibility to predation. Interestingly, however, we did not see an effect of increased UV-B on locomotor performance, a measure of performance that is a commonly used proxy for fitness owing to its presumed connection to escape from predators [27]. This suggests that there is potential to misinterpret the effects of UV-B if future research relies only on assumed correlations between performance and fitness measures. Thus, future research that endeavours to understand the role of environmental stressors in species declines should aim to test directly the functional benefits of performance measures, rather than assume their connection to fitness. Importantly, our research arrived at these conclusions using levels of UV-B that correspond well with the observed increases in terrestrial UV-B associated with stratospheric ozone depletion [5,51,52], indicating that continued interest in UV-B as an environmental stressor in amphibian habitats is warranted.
Acknowledgements
All experimental procedures were approved by The University of Queensland Native and Exotic Wildlife and Marine Animals Ethics Committee (SIB/626/08/URG).
We thank Vincent van Uitregt, Philip Matthews, Sean Fitzgibbon, Andrew Trappett, Sara Kayes, Kirstin Pratt and Natalie Schimpf for technical support, and Craig White and anonymous reviewers for their comments on earlier versions of this manuscript. This research was funded by The University of Queensland Research Grant to C.E.F.
References
- 1.IUCN 2010. IUCN red list of threatened species. Version 2010.1. See http://www.iucnredlist.org (accessed on 23 June 2010).
- 2.Alford R. A., Richards S. J. 1999. Global amphibian declines: a problem in applied ecology. Annu. Rev. Ecol. Syst. 30, 133–165 10.1146/annurev.ecolsys.30.1.133 (doi:10.1146/annurev.ecolsys.30.1.133) [DOI] [Google Scholar]
- 3.Farman J. C., Gardiner B. G., Shanklin J. D. 1985. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature 315, 207–210 10.1038/315207a0 (doi:10.1038/315207a0) [DOI] [Google Scholar]
- 4.Stuart S. N., Chanson J. S., Cox N. A., Young B. E., Rodrigues A. S. L., Fischman D. L., Waller R. W. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786 10.1126/science.1103538 (doi:10.1126/science.1103538) [DOI] [PubMed] [Google Scholar]
- 5.WMO 2007. Scientific assessment of ozone depletion: 2006, global ozone research and monitoring project—report No. 50. Geneva, Switzerland: World Meteorological Organization [Google Scholar]
- 6.Blaustein A. R., Belden L. K., Hatch A. C., Kats L. B., Hoffman P. D., Hays J. B., Marco A., Chivers D. P., Kiesecker J. M. 2001. Ultraviolet radiation and amphibians. In Ecosystems, evolution, and ultraviolet radiation (eds Cockell C. S., Blaustein A. R.), pp. 63–79 Berlin, Germany: Springer [Google Scholar]
- 7.Croteau M. C., Davidson M. A., Lean D. R. S., Trudeau V. L. 2008. Global increases in ultraviolet B radiation: potential impacts on amphibian development and metamorphosis. Physiol. Biochem. Zool. 81, 743–761 10.1086/591949 (doi:10.1086/591949) [DOI] [PubMed] [Google Scholar]
- 8.Crump D. 2001. The effects of UV-B radiation and endocrine-disrupting chemicals (EDCs) on the biology of amphibians. Environ. Rev. 9, 61–80 10.1139/er-9-2-61 (doi:10.1139/er-9-2-61) [DOI] [Google Scholar]
- 9.Belden L. K., Blaustein A. R. 2002. Exposure of red-legged frog embryos to ambient UV-B radiation in the field negatively affects larval growth and development. Oecologia 130, 551–554 10.1007/s00442-001-0843-y (doi:10.1007/s00442-001-0843-y) [DOI] [PubMed] [Google Scholar]
- 10.Alton L. A., Wilson R. S., Franklin C. E. 2010. Risk of predation enhances the lethal effects of UV-B in amphibians. Global Change Biol. 16, 538–545 10.1111/j.1365-2486.2009.02010.x (doi:10.1111/j.1365-2486.2009.02010.x) [DOI] [Google Scholar]
- 11.Croteau M. C., Martyniuk C. J., Trudeau V. L., Lean D. R. S. 2008. Chronic exposure of Rana pipiens tadpoles to UVB radiation and the estrogenic chemical 4-tert-octylphenol. J. Toxicol. Environ. Health Part A 71, 134–144 10.1080/15287390701613330 (doi:10.1080/15287390701613330) [DOI] [PubMed] [Google Scholar]
- 12.Ankley G. T., Tietge J. E., Holcombe G. W., DeFoe D. L., Diamond S. A., Jensen K. M., Degitz S. J. 2000. Effects of laboratory ultraviolet radiation and natural sunlight on survival development of Rana pipiens. Can. J. Zool. 78, 1092–1100 10.1139/cjz-78-6-1092 (doi:10.1139/cjz-78-6-1092) [DOI] [Google Scholar]
- 13.Blaustein A. R., Hoffman P. D., Hokit D. G., Kiesecker J. M., Walls S. C., Hays J. B. 1994. UV repair and resistance to solar UV-B in amphibian eggs: a link to population declines? Proc. Natl Acad. Sci. USA 91, 1791–1795 10.1073/pnas.91.5.1791 (doi:10.1073/pnas.91.5.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kats L. B., Kiesecker J. M., Chivers D. P., Blaustein A. R. 2000. Effects of UV-B on antipredator behavior in three species of amphibians. Ethology 106, 921–932 10.1046/j.1439-0310.2000.00608.x (doi:10.1046/j.1439-0310.2000.00608.x) [DOI] [Google Scholar]
- 15.Kiesecker J. M., Blaustein A. R. 1995. Synergism between UV-B radiation and a pathogen magnifies amphibian embryo mortality in nature. Proc. Natl Acad. Sci. USA 92, 11 049–11 052 10.1073/pnas.92.24.11049 (doi:10.1073/pnas.92.24.11049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Uitregt V. O., Wilson R. S., Franklin C. E. 2007. Cooler temperatures increase sensitivity to ultraviolet B radiation in embryos and larvae of the frog Limnodynastes peronii. Global Change Biol. 13, 1114–1121 10.1111/j.1365-2486.2007.01353.x (doi:10.1111/j.1365-2486.2007.01353.x) [DOI] [Google Scholar]
- 17.Pahkala M., Räsänen K., Laurila A., Johanson U., Björn L. O., Merilä J. 2002. Lethal and sublethal effects of UV-B/pH synergism on common frog embyros. Conserv. Biol. 16, 1063–1073 10.1046/j.1523-1739.2002.00527.x (doi:10.1046/j.1523-1739.2002.00527.x) [DOI] [Google Scholar]
- 18.Blaustein A. R., Romansic J. M., Kiesecker J. M., Hatch A. C. 2003. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers. Distrib. 9, 123–140 10.1046/j.1472-4642.2003.00015.x (doi:10.1046/j.1472-4642.2003.00015.x) [DOI] [Google Scholar]
- 19.Blaustein A. R., Kiesecker J. M., Chivers D. P., Hokit D. G., Marco A., Belden L. K., Hatch A. 1998. Effects of ultraviolet radiation on amphibians: field experiments. Am. Zool. 38, 799–812 10.1093/icb/38.6.799 (doi:10.1093/icb/38.6.799) [DOI] [Google Scholar]
- 20.Bancroft B. A., Baker N. J., Blaustein A. R. 2008. A meta-analysis of the effects of ultraviolet B radiation and its synergistic interactions with pH, contaminants, and disease on amphibian survival. Conserv. Biol. 22, 987–996 10.1111/j.1523-1739.2008.00966.x (doi:10.1111/j.1523-1739.2008.00966.x) [DOI] [PubMed] [Google Scholar]
- 21.Blaustein A. R., Kiesecker J. M., Chivers D. P., Anthony R. G. 1997. Ambient UV-B radiation causes deformities in amphibian embryos. Proc. Natl Acad. Sci. USA 94, 13 735–13 737 10.1073/pnas.94.25.13735 (doi:10.1073/pnas.94.25.13735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays J. B., Blaustein A. R., Kiesecker J. M., Hoffman P. D., Pandelova I., Coyle D., Richardson T. 1996. Developmental response to amphibians to solar and artificial UVB sources: a comparative study. Photochem. Photobiol. 64, 449–456 10.1111/j.1751-1097.1996.tb03090.x (doi:10.1111/j.1751-1097.1996.tb03090.x) [DOI] [PubMed] [Google Scholar]
- 23.Fite K. V., Blaustein A. R., Bengston L., Hewitt H. E. 1998. Evidence of retinal light damage in Rana cascadae: a declining amphibian species. Copeia 1998, 906–914 10.2307/1447337 (doi:10.2307/1447337) [DOI] [Google Scholar]
- 24.Flamarique I. N., Ovaska K., Davis T. M. 2000. UV-B induced damage to the skin and ocular system of amphibians. Biol. Bull. 199, 187–188 10.2307/1542889 (doi:10.2307/1542889) [DOI] [PubMed] [Google Scholar]
- 25.Kraft P. G., Franklin C. E., Blows M. W. 2006. Predator-induced phenotypic plasticity in tadpoles: extension or innovation. J. Evol. Biol. 19, 450–458 10.1111/j.1420-9101.2005.01015.x (doi:10.1111/j.1420-9101.2005.01015.x) [DOI] [PubMed] [Google Scholar]
- 26.Skelly D. K. 1994. Activity level and susceptibility of anuran larvae to predation. Anim. Behav. 47, 465–468 10.1006/anbe.1994.1063 (doi:10.1006/anbe.1994.1063) [DOI] [Google Scholar]
- 27.Walker J. A., Ghalambor C. K., Griset O. L., McKenney D., Reznick D. N. 2005. Do faster starts increase the probability of evading predators? Funct. Ecol. 19, 808–815 10.1111/j.1365-2435.2005.01033.x (doi:10.1111/j.1365-2435.2005.01033.x) [DOI] [Google Scholar]
- 28.Berven K. A. 1990. Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 71, 1599–1608 10.2307/1938295 (doi:10.2307/1938295) [DOI] [Google Scholar]
- 29.Goater C. P. 1994. Growth and survival of postmetamorphic toads: interactions among larval history, density, and parasitism. Ecology 75, 2264–2274 10.2307/1940882 (doi:10.2307/1940882) [DOI] [Google Scholar]
- 30.Scott D. E. 1994. Effect of larval density on life history traits in Ambystoma opacum. Ecology 75, 1383–1396 10.2307/1937462 (doi:10.2307/1937462) [DOI] [Google Scholar]
- 31.Smith D. C. 1987. Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68, 344–350 10.2307/1939265 (doi:10.2307/1939265) [DOI] [Google Scholar]
- 32.Pahkala M., Merilä J., Ots I., Laurila A. 2003. Effects of ultraviolet-B radiation on metamorphic traits in the common frog Rana temporaria. J. Zool. 259, 57–62 10.1017/S0952836902002984 (doi:10.1017/S0952836902002984) [DOI] [Google Scholar]
- 33.Relyea R. A. 2001. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82, 523–540 10.1890/0012-9658(2001)082[0523:MABPOL]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[0523:MABPOL]2.0.CO;2) [DOI] [Google Scholar]
- 34.Relyea R. A. 2007. Getting out alive: how predators affect the decision to metamorphose. Oecologia 152, 389–400 10.1007/s00442-007-0675-5 (doi:10.1007/s00442-007-0675-5) [DOI] [PubMed] [Google Scholar]
- 35.Sih A. 1987. Predators and prey lifestyles: an evolutionary and ecological overview. In Predation: direct and indirect impacts on aquatic communities (eds Kerfoot W. C., Sih A.). Hanover, Germany: University Press of New England [Google Scholar]
- 36.Skelly D. K., Werner E. E. 1990. Behavioral and life-historical changes responses of larval American toads to an odonate predator. Ecology 71, 2313–2322 10.2307/1938642 (doi:10.2307/1938642) [DOI] [Google Scholar]
- 37.Chivers D. P., Smith R. J. F. 1998. Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience 5, 338–352 [Google Scholar]
- 38.Lind J., Cresswell W. 2005. Determining the fitness consequences of antipredation behavior. Behav. Ecol. 16, 945–956 10.1093/beheco/ari075 (doi:10.1093/beheco/ari075) [DOI] [Google Scholar]
- 39.Johnson J. B., Burt D. B., DeWitt T. J. 2008. Form, function, and fitness: pathways to survival. Evolution 62, 1243–1251 10.1111/j.1558-5646.2008.00343.x (doi:10.1111/j.1558-5646.2008.00343.x) [DOI] [PubMed] [Google Scholar]
- 40.Bennett A. F., Huey R. B. 1990. Studying the evolution of physiological performance. In Oxford surveys in evolutionary biology, vol. 7 (eds Futuyma D. J., Antonovics J.), pp. 251–284 Oxford, UK: Oxford University Press [Google Scholar]
- 41.Garland T. J., Losos J. B. 1994. Ecological morphology of locomotor performance in squamate reptiles. In Ecological morphology: integrative organismal biology (eds Wainwright P. C., Reilly S. M.), pp. 240–302 Chicago, IL: University of Chicago Press [Google Scholar]
- 42.Artacho P., Nespolo R. G. 2009. Natural selection reduces energy metabolism in the garden snail, Helix aspersa (Cornu aspersum). Evolution 63, 1044–1050 10.1111/j.1558-5646.2008.00603.x (doi:10.1111/j.1558-5646.2008.00603.x) [DOI] [PubMed] [Google Scholar]
- 43.ARPANSA 2010. UV-index summary for Brisbane 10th December 2005. See http://www.arpansa.gov.au/uvindex/historical/images/bri10122005.gif (accessed on 4 October 2010)
- 44.WHO 2002. Global solar UV index: a practical guide. Geneva, Switzerland: World Health Organization [Google Scholar]
- 45.ARPANSA 2010. Monthly UV-index summaries for Brisbane. See http://www.arpansa.gov.au/uvindex/monthly/brimonthlysumm.htm (accessed on 4 October 2010)
- 46.McKinlay A. F., Diffey B. L. 1987. A reference action spectrum for ultraviolet-induced erythema in skin. In Human exposure to ultraviolet radiation: risks and regulations (eds Passchier W. F., Bosnjakovic B. F. M.), pp. 83–87 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 47.Setlow R. B. 1974. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc. Natl Acad. Sci. USA 71, 3363–3366 10.1073/pnas.71.9.3363 (doi:10.1073/pnas.71.9.3363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKenzie R. L., Björn L. O., Bais A., Ilyas M. 2003. Changes in biologically active ultraviolet radiation reaching the Earth's surface. In Environmental effects of ozone depletion and its interactions with climate change: 2002 assessment, pp. 1–23 Nairobi, Kenya: United Nations Environment Programme [Google Scholar]
- 49.Wardle D. I., Kerr J. B., McElroy C. T., Francis D. R. 1997. Ozone science: a Canadian perspective on the changing ozone layer. Toronto, ON: Environment Canada [Google Scholar]
- 50.Zerefos C., Meleti C., Balis D., Tourpali K., Bais A. F. 1998. Quasi-biennial and longer-term changes in clear-sky UV-B solar irradiance. Geophys. Res. Lett. 25, 4345–4348 10.1029/1998GL900160 (doi:10.1029/1998GL900160) [DOI] [Google Scholar]
- 51.Blumthaler M., Webb A. R. 2003. UVR climatology. In UV effects in aquatic organisms and ecosystems (eds Helbling E. R., Zagarese H.), pp. 21–58 Cambridge, UK: Royal Society of Chemistry [Google Scholar]
- 52.Udelhofen P. M., Gies P., Roy C., Randel W. J. 1999. Surface UV radiation over Australia, 1979–1992: effects of ozone and cloud cover changes on variations of UV radiation. J. Geophys. Res. 104, 19 135–19 159 10.1029/1999JD900306 (doi:10.1029/1999JD900306) [DOI] [Google Scholar]
- 53.Gosner K. L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 [Google Scholar]
- 54.Domenici P., Blake R. W. 1997. The kinematics and performance of fish fast-start swimming. J. Exp. Biol. 200, 1165–1178 [DOI] [PubMed] [Google Scholar]
- 55.von Seckendorf Hoff K., Wassersug R. J. 2000. Tadpole locomotion: axial movement and tail functions in a largely vertebrateless vertebrate. Am. Zool. 40, 62–76 10.1093/icb/40.1.62 (doi:10.1093/icb/40.1.62) [DOI] [Google Scholar]
- 56.Sokal R. R., Rohlf F. J. 1995. Biometry: the principles and practices of statistics in biological research. New York, NY: W. H. Freeman and Co [Google Scholar]
- 57.Quinn G. P., Keough M. J. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press [Google Scholar]
- 58.Romansic J. M., Waggener A. A., Bancroft B. A., Blaustein A. R. 2009. Influence of ultraviolet-B radiation on growth, prevalence of deformities, and susceptibility to predation in Cascades frog (Rana cascadae) larvae. Hydrobiologia 624, 219–233 10.1007/s10750-009-9703-2 (doi:10.1007/s10750-009-9703-2) [DOI] [Google Scholar]
- 59.Warkentin K. M. 1999. The development of behavioral defenses: a mechanistic analysis of vulnerability in red-eyed tree frog hatchlings. Behav. Ecol. 10, 251–262 10.1093/beheco/10.3.251 (doi:10.1093/beheco/10.3.251) [DOI] [Google Scholar]
- 60.Formicki G., Zamachowski W., Stawarz R. 2003. Effect of UV-A and UV-B on oxygen consumption in common toad (Bufo bufo) tadpoles. J. Zool. 259, 317–326 10.1017/S0952836902003345 (doi:10.1017/S0952836902003345) [DOI] [Google Scholar]
- 61.Fischer J. M., Fields P. A., Pryzbylkowski P. G., Nicolai J. L., Neale P. J. 2006. Sublethal exposure to UV radiation affects respiration rates of the freshwater cladoceran Daphnia catawba. Photochem. Photobiol. 82, 547–550 10.1562/2005-08-30-RA-664 (doi:10.1562/2005-08-30-RA-664) [DOI] [PubMed] [Google Scholar]
- 62.Ylönen O., Heikkilä J., Karjalainen J. 2004. Metabolic depression in UV-B exposed larval coregonids. Ann. Zool. Fenn. 41, 577–585 [Google Scholar]
- 63.Capellán E., Nicieza A. G. 2007. Trade-offs across life stages: does predator-induced hatching plasticity reduce anuran post-metamorphic performance? Evol. Ecol. 21, 445–458 10.1007/s10682-006-9133-9 (doi:10.1007/s10682-006-9133-9) [DOI] [Google Scholar]
- 64.Anderson A. L., Brown W. D. 2009. Plasticity of hatching in green frogs (Rana clamitans) to both egg and tadpole predators. Herpetologica 65, 207–213 10.1655/08-016R1.1 (doi:10.1655/08-016R1.1) [DOI] [Google Scholar]
- 65.Warkentin K. M. 1995. Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proc. Natl Acad. Sci. USA 92, 3507–3510 10.1073/pnas.92.8.3507 (doi:10.1073/pnas.92.8.3507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chivers D. P., Kiesecker J. M., Marco A., DeVito J., Anderson M. T., Blaustein A. R. 2001. Predator-induced life history changes in amphibians: egg predation induces hatching. Oikos 92, 135–142 10.1034/j.1600-0706.2001.920116.x (doi:10.1034/j.1600-0706.2001.920116.x) [DOI] [Google Scholar]
- 67.Saenz D., Johnson J. B., Adams C. K., Dayton G. H. 2003. Accelerated hatching of sourthern leopard frog (Rana sphenocephala) eggs in response to the presence of a crayfish (Procambarus nigrocinctus) predator. Copeia 2003, 646–649 10.1643/CE-02-172R1 (doi:10.1643/CE-02-172R1) [DOI] [Google Scholar]
- 68.Laurila A., Pakkasmaa S., Crochet P.-A., Merilä J. 2002. Predator-induced plasticity in early life history and morphology of two anuran amphibians. Oecologia 132, 524–530 10.1007/s00442-002-0984-7 (doi:10.1007/s00442-002-0984-7) [DOI] [PubMed] [Google Scholar]
- 69.McCollum S. A., Leimberger J. D. 1997. Predator-induced morphological changes in an amphibian: predation by dragonflies affects tadpole shape and color. Oecologia 109, 615–621 10.1007/s004420050124 (doi:10.1007/s004420050124) [DOI] [PubMed] [Google Scholar]
- 70.Teplitsky C., Plénet S., Léna J.-P., Mermet N., Malet E., Joly P. 2005. Escape behaviour and ultimate causes of specific induced defences in an anuran tadpole. J. Evol. Biol. 18, 180–190 10.1111/j.1420-9101.2004.00790.x (doi:10.1111/j.1420-9101.2004.00790.x) [DOI] [PubMed] [Google Scholar]
- 71.Wilson R. S., Kraft P. G., Van Damme R. 2005. Predator-specific changes in morphology and swimming performance of larval Rana lessonae. Funct. Ecol. 19, 238–244 10.1111/j.1365-2435.2005.00958.x (doi:10.1111/j.1365-2435.2005.00958.x) [DOI] [Google Scholar]
- 72.Van Buskirk J., Anderwald P., Lüpold S., Reinhardt L., Schuler H. 2003. The lure effect, tadpole tail shape, and the target of dragonfly strikes. J. Herpetol. 37, 420–424 10.1670/0022-1511(2003)037[0420:TLETTS]2.0.CO;2 (doi:10.1670/0022-1511(2003)037[0420:TLETTS]2.0.CO;2) [DOI] [Google Scholar]
- 73.Van Buskirk J., McCollum S. A. 2000. Influence of tail shape on tadpole swimming performance. J. Exp. Biol. 203, 2149–2158 [DOI] [PubMed] [Google Scholar]
- 74.Laurila A., Crochet P.-A., Merilä J. 2001. Predation-induced effects on hatchling morphology in the common frog (Rana temporaria). Can. J. Zool. 79, 926–930 10.1139/cjz-79-5-926 (doi:10.1139/cjz-79-5-926) [DOI] [Google Scholar]
- 75.Mandrillon A.-L., Saglio P. 2009. Effects of single and combined embryonic exposures of herbicide and conspecific chemical alarm cues on hatching and larval traits in the common fog (Rana temporaria). Arch. Environ. Contam. Toxicol. 56, 566–576 10.1007/s00244-008-9196-4 (doi:10.1007/s00244-008-9196-4) [DOI] [PubMed] [Google Scholar]
- 76.Kiesecker J. M., Blaustein A. R., Belden L. K. 2001. Complex causes of amphibian population declines. Nature 410, 681–684 10.1038/35070552 (doi:10.1038/35070552) [DOI] [PubMed] [Google Scholar]