Abstract
Questions concerning the role of nature and nurture in higher cognition appear to be intractable if one restricts one's attention to development in humans. However, in other domains, such as sensory development, much information has been gained from controlled rearing studies with animals. Here, we used a similar experimental strategy to investigate intuitive reasoning about occluded objects. Newborn domestic chicks (Gallus gallus) were reared singly with a small object that became their social partner. They were then accustomed to rejoin such an imprinting object when it was made to move and disappear behind either one of two identical opaque screens. After disappearance of the imprinting object, chicks were faced with two screens of different slants, or of different height or different width, which may or may not have been compatible with the presence of the imprinting object hidden beneath/behind them. Chicks consistently chose the screen of slant/height/width compatible with the presence of the object beneath/behind it. Preventing chicks from touching and pecking at the imprinting object before testing did not affect the results, suggesting that intuitive reasoning about physical objects is largely independent of specific experience of interaction with objects and of objects' occluding events.
Keywords: naive physics, filial imprinting, object permanence, experience, biological predispositions
1. Introduction
What aspects of knowledge of the physical world are apparent in biological organisms prior to their first contacts with the objects of their knowledge and what emerge thanks to the effects of experience with these objects represents an issue that has spanned more than 2000 years of philosophical and scientific inquiry (see [1,2] for reviews), with considerable social valence concerning human nature and the role of education and science in society. According to some theorists, human infants are born with a conception of objects as spatially bounded entities that exist continuously in time and move continuously in space, maintaining their internal unity and their external boundaries [1,3,4]. According to others, by contrast, infants are born with learning mechanisms that would allow them to establish object concepts on the basis of a limited set of pertinent observations, arising from, for example, infants' examination of the displacements and interactions of objects and/or infants' action upon objects [5]. The fact that human infants exhibit evidence for object permanence and inference about the physical properties of objects from about 14 weeks of age [6–13] is of course compatible with both of these views, the former arguing for maturation and the latter for learning.
Research has shown that non-human animals, both mammals [14–17] and birds [18–20], also represent and reason about physical objects. Controlled-rearing studies of animals, so influential in charting effects and non-effects of visual experience on visual development [21], can be harnessed to chart similar effects on the development of knowledge about objects [22,23]. In particular, the use of precocial species—which exhibit well-developed behaviour similar to that of adults soon after hatching—combined with rigorous control of early experience [24–27], may help to disentangle part of the traditional controversy between nativist and empiricist theories on the origins of object knowledge.
In the studies described here, we reared domestic chicks (Gallus gallus) soon after hatching in isolation with an imprinting object: a small red plastic cylinder (figure 1a). On day 4, chicks were accustomed to rejoin the imprinting object when it was made to move and disappear behind either of two identical opaque screens (figure 2a,b). At test, chicks were first shown the object moving straight towards the screens while they were confined behind a transparent partition (figure 2c). Then the partition was made opaque (figure 2d), in order to prevent chicks from seeing the object and to guarantee the slant (or, in other experiments, the height or the width) of the screens to be invisibly changed, so as to make one of the screens compatible and the other not compatible (figure 2e) with the presence of the imprinting object hidden beneath (or behind) them. Chicks were eventually released to rejoin the imprinting object (during this test phase no imprinting object was actually located beneath/behind either screen; figure 2f).
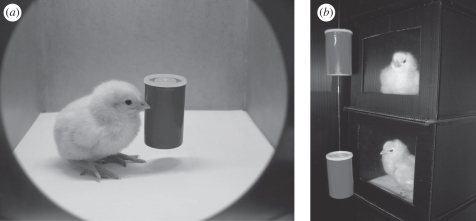
Figure 1.
(a) Standard rearing conditions for experiments (except experiment 4) showing the chicks reared singly with the imprinted object. (b) In experiment 4, chicks were again reared singly but with only visual access (and no direct contact) to the imprinting object.
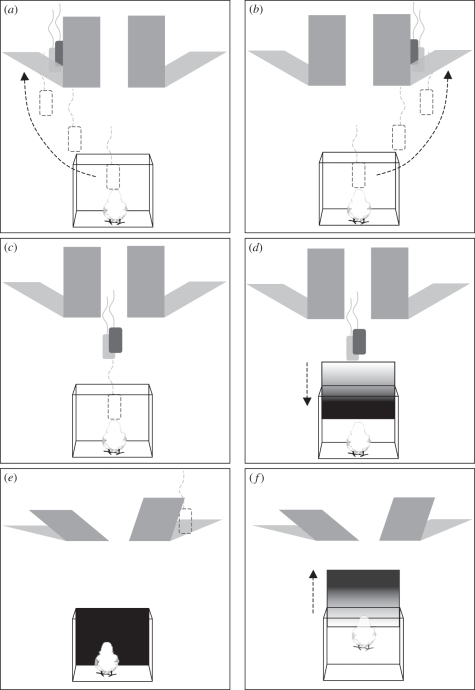
Figure 2.
An example of the general procedure of experiments. (a,b) At first, chicks were accustomed to rejoin the imprinting object that could be observed through a transparent partition moving and disappearing behind either of two identical opaque screens (training). (c) During tests, chicks were first shown the object moving along a straight trajectory midline towards the screens while they were confined behind a transparent partition. (d) Then an opaque partition was lowered and the slant (or, in other experiments, the height or the width) of the screens was changed, and (e) the imprinting object was removed without the chick being able to see it, so as to make one of the screens compatible and the other not compatible with the presence of the imprinting object hidden beneath them. (f) Eventually, the partitions were removed and the chick was allowed to choose between the two screens.
2. Material and methods
(a). Subjects and rearing conditions
We used 103 naive domestic chicks (G. gallus) of the Hybro strain (derived from the White Leghorn breed). All animals were hatched from eggs supplied weekly to our laboratory 14 days after fertilization from a local commercial hatchery (Agricola Berica s.c.r.l., Montegalda, Vicenza, Italy). Eggs were maintained until day 19 in dark conditions at a controlled temperature (37.7°C) and humidity (about 50–60%) in an automatically turning incubator FIEM snc, MG 100/150, and from days 19 to 21 (day of hatching) in a hatchery with the same temperature and complete darkness but lower humidity, to provide optimal conditions for hatching. For experiments 1, 2, 3 and 5, 6, 7, soon after hatching chicks were transferred and reared singly in standard metal home-cages (22 cm wide × 30 cm high × 40 cm deep) lit from above by fluorescent lamps with food and water available ad libitum in a room kept at a constant 30°C. An imprinting object (a red plastic cylinder; see figure 1a for an example) was suspended by a thin thread in the centre of each cage at about the chicks' head height (see [28]). The object was 5 cm high in experiments 1, 2, 4 and 5, 2.5 cm high in experiment 3, and always measured 3 cm in diameter. In experiment 6, the object was either 10 or 5 cm high and always 3 cm in diameter. In experiment 7, the object was either 6 or 3 cm in diameter and always 5 cm in height. In experiment 4, after hatching, chicks were maintained in the hatchery where they had hatched, with lower temperature (30°) for 2 days (note that for the first 3 days after hatching there are still sufficient nutrients available from the yolk sac in newborn chicks [29]). On day 3, chicks were reared in isolation for 4 h in a set of black Poliplack small boxes (14 cm wide × 14 cm high × 14 cm deep) that were completely closed apart from one side where a transparent polyester partition screen allowed the chick the sight of the imprinting object suspended 10 cm away, in front of each cage (figure 1b). At the end of experiments, chicks were collectively caged in groups of four with food and water available, and on the same day were donated to local farmers, providing the chicks with free-range conditions of rearing.
(b). Apparatus
The training and test apparatus consisted of a completely white Poliplack arena (70 cm wide × 40 cm high × 80 cm deep) with a starting box (12 cm wide × 12 cm high × 12 cm deep) positioned in the middle of the rear wall. At the other end of the arena, 35 cm in front of the starting box, there were two identical green Poliplack opaque screens used as hiding locations and separated from each other by 5 cm. In experiments 1–5, the screens were 12 cm wide × 16 cm high; all the screens (with only exception of screens in experiment 5, which were leant against the front wall) had 3 cm sides bent back to prevent the chicks from seeing behind the screen whether the imprinting object was there. In experiment 6, the screens were 12 cm wide × 11 cm high during training, and the height was changed at test so that one measured 6 cm and the other 16 cm; in experiment 7, the screens were 7 cm wide × 16 cm high during training and the width was changed at test so that one measured 4 cm and the other 10 cm.
The arena was illuminated by a lamp of 60 W placed exactly on the centre top of the apparatus and the behaviour was video-recorded from above. The wall of the starting box facing the screens could be covered either by a removable transparent polyester substrate screen or a black Poliplack opaque screen, each used as described below.
(c). Procedure
In all experiments, with the exception of experiment 4, chicks at 2 days of age were at first gently located into the arena, accustomed to the novel environment with the imprinting cylinder freely moving in it (10–20 min), and then habituated to follow the imprinting object when it was moved and made to disappear 12 times behind either of two identical opaque screens. For the subsequent 2 days, chicks were deprived of food in the early morning (water was left available) in order to avoid sleep and/or reduced mobility. Four hours later, each chick in turn was first confined in the starting box of the training apparatus and shown, through the removable transparent partition, the imprinting object disappearing behind one or other of the two screens (figure 2a,b). After disappearance of the object, the transparent partition was removed and chicks were allowed to spontaneously rejoin the imprinting object behind the opaque screen. Chicks had to perform the choice correctly six consecutive times out of ten trials in order to access the test. The choice was scored as correct only when the chick approached the screen and made a complete turn behind it, stopping there. At test, chicks were confined in the starting box and at first could watch, through the removable transparent partition, the imprinting object moving towards the screens but this time moving in a straight line towards the point midway between the screens (figure 2c); when the imprinting object was halfway towards the screens, an opaque partition was lowered (figure 2d) and either the slant (experiments 1–5) or the height (experiment 6) or width (experiment 7) of the screens was invisibly changed and the imprinting object was removed. The partitions were then lifted up (figure 2e) and the chicks were allowed to search for the imprinting object (figure 2f).
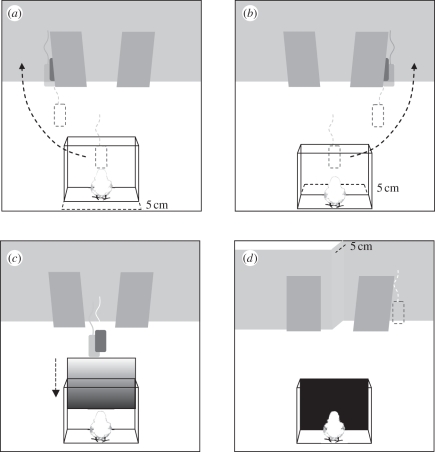
In experiment 5, the starting box was moved trial by trial 5 cm back and forth (figure 3a,b): this was done in order to prevent chicks from learning during training a specific distance between the starting position and the hiding locations. At test, after the opaque screen was lowered in front of the chick (figure 3c), the slant of the screens was changed together with the front wall, which presented this time a 5 cm protrusion: this appeared to make the two screens identical in height and with their bases aligned, as they were leant against the wall (figure 3d).
Figure 3.
The procedure used during training in experiment 5. (a,b) Screens were leant against the front wall and the starting box was displaced by 5 cm back and forth between training trials so that the chicks were accustomed to the distances used in the test, during which they were shown the imprinting object moving along a straight trajectory midline towards the screens. (c) The opaque partition was thereafter lowered to permit the slanting of the screens and the conformation of the front wall to be changed. At this point (d) the two screens had different slant but identical height and were located at identical distances from the starting cage on the floor.
Chicks were given ten consecutive test trials in experiments 1–5 and twelve test trials in experiments 6 and 7. In experiment 4, after the imprinting period, each chick was given only two trials to become accustomed to the disappearance of the object (rather then the ten trials used in the previous experiments) and soon after given the test. The left–right positions of the screens at test were changed according to a semi-random sequence [30]. In each trial, the choice for one or other of the two screens was scored from video recordings by an independent ‘blind’ observer.
Slant (experiments 1–5). Chicks were trained in the presence of two identical screens (90° in experiment 1, 75° in experiment 5 and 45° in experiments 2–4) and allowed at test to choose between two differently slanted screens (45° versus 10° in experiment 1; 85° versus 65° in experiment 5; 80° versus 10° in experiments 2–4).
Height (experiment 6). Chicks were trained in the presence of two identical screens (11 cm high) and allowed at test to choose between two screens of different height (16 versus 6 cm).
Width (experiment 7). Chicks were trained in the presence of two identical screens (7 cm wide) and allowed at test to choose between two screens of different width (10 versus 4 cm).
3. Results
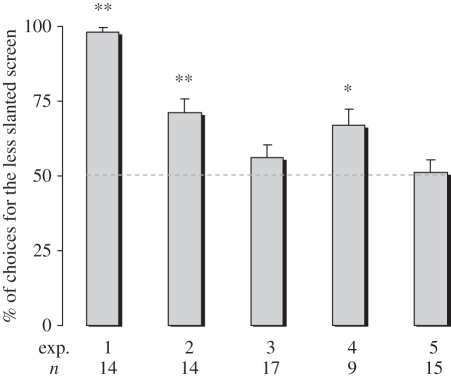
In experiment 1, chicks first observed the imprinting object disappear behind vertical screens (90°); during confinement behind the opaque partition, one of the screens was slanted at 45° and the other at 10° (the latter therefore being too flat to allow the positioning of the imprinting object beneath it). Chicks preferred the 45° slanted screen (one-sample two-tailed t-test: n = 14, t = 30.930, p < 0.001; figure 4, experiment 1). In order to account for any effect of the degree of change in slant between training and test, in experiment 2 chicks at first observed the imprinting object disappearing behind one of two screens slanted at 45° and then allowed to choose between a 80° slanted screen and a 10° slanted screen. In this way, the degree of change in slant from training to testing was exactly the same (35°) for both screens. Chicks chose the 80° slanted screen (one-sample two-tailed t-test: n = 14, t = 4.368, p = 0.001; figure 4, experiment 2). The preference was less pronounced than that observed in the previous experiment (experiment 1 versus experiment 2: p < 0.001, least significant difference test post hoc comparison), suggesting that in experiment 1 chicks were in fact also responding to the different degree of novelty associated with the change in slant between training and test. As a further control, experiment 2 was repeated with another group of chicks, this time imprinted on a smaller object, the size of which was compatible with occlusion even beneath the 10° slanted screen. Chicks did not show any preference (one-sample two-tailed t-test: n = 17, t = 1.482, p = 0.158; figure 4, experiment 3).
Figure 4.
Percentage of choice for the highest (less slanted) screen in experiments 1–5 (group means with s.e.m. are shown). *p < 0.05; **p < 0.01.
We wondered whether the possibility of pecking at the imprinting object during rearing was instrumental to the chicks' intuition of impenetrability of the object. Experiment 2 was therefore replicated (experiment 4) with another group of chicks that could not peck the imprinting cylinder because the transparent polyester substrate screens prevented the birds from touching and pecking the object during exposure (figure 1b; see §2). No difference was observed with respect to experiment 2 (t21 = 0.545, p = 0.592): chicks showed the same preference for the screen where the slant was compatible with the presence of an object beneath it (one-sample two-tailed t-test: n = 9, t = 3.015, p = 0.017; figure 4, experiment 4).
The height of the screen is likely to have been the crucial physical dimension considered by chicks for making a choice. We wondered, however, whether they could have also used the slant of the surface as a cue, estimated from only the difference in the shapes of the (trapezoidal) projections of the two screens. To check for that, in experiment 5 we let chicks observe the imprinting object disappearing behind one of two screens slanted at 75° and leant against the front wall; chicks were then allowed to choose between an 85° slanted screen and a 65° slanted screen. In this way, the degree of change in slant from training to testing was exactly the same (10°) for both screens. Furthermore, in order to present the two screens at the same height and aligned to each other, the screens were leant on a stepped wall that jutted out 5 cm behind the less slanted screen (see figure 3 and §2c for further details). Chicks chose equally between the two screens (one-sample two-tailed t-test: n = 15, t = 0.147, p = 0.885; figure 4, experiment 5).
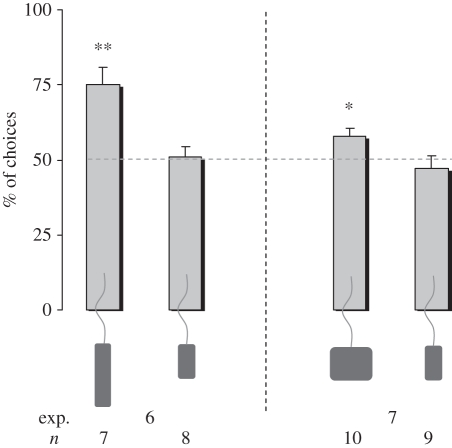
In experiment 6, we checked directly for the use of height as a relevant property of the hiding screen, by imprinting chicks on either a tall or a short imprinting object. Chicks chose preferentially the highest screen when the (tall) imprinting object could not have been hidden behind the lowest screen because of its height (one-sample two-tailed t-test: n = 7, t = 4.347, p = 0.005; figure 5, experiment 6), but not when the (short) imprinting object could have been hidden by either of the two screens (n = 8, t = 0.314, p = 0.763).
Figure 5.
Percentage of choice for the highest (left bars) and the widest (right bars) screen in experiments 6 and 7 as possible hiding locations depending on the size of the imprinting object (group means with s.e.m. are shown). *p < 0.05; **p < 0.01.
Similarly, in experiment 7, we checked for the use of width as a relevant property of the hiding screen by imprinting chicks with either a large- or a small-width object. Chicks chose preferentially the largest screen when the (large-width) imprinting object could not have been hidden behind the smallest screen because of its width (one-sample two-tailed t-test: n = 10, t = 3, p = 0.015), but not when the (small-width) imprinting object could have been hidden by either the two screens (n = 9, t = −0.632, p = 0.545; figure 5, experiment 7).
4. Discussion
Human babies exhibit object permanence and rudimentary reasoning about the properties of physical objects, such as height, size and solidity, from about 14 weeks of age [6,31]. This may be due to the fact that maturation of the sensory and motor machinery for the effective use of biologically predisposed object concept would require some time to develop, irrespective of any specific experience (e.g. [32,33]); for example, relative to recognition of partly occluded objects in chicks and babies. Alternatively, one could argue that 14 weeks provide babies with enough time to experience some physical properties of objects. In our experiments, chicks show object permanence conception and inferences about object physical properties of imprinting objects and occluding screens at a very precocial age, when their chances to have learnt object properties are, if any, extremely reduced. Of course it is impossible to prevent an organism from having sensory and motor experience. As a minimum, one could argue that chicks could have had the possibility of pecking (which occurs also in ovo in order to hatch). However, any empiricist interpretation of the data should be specific about the type of experience needed in order to account for inferences about object properties [34]. For example, generic experience of pecking in itself cannot explain the results, for the simple reason that an animal like the chick could encounter different types of physical materials—some soft, some liquid and some offering resistance to pecking. Thus, one should argue that it is the specific experience of touching and pecking the imprinting object that is crucial. However, the results of our experiment in which chicks were completely prevented from any direct physical contact with the imprinting object showed that this is not the case. Chicks chose the screen with the proper slant even after purely visual experience of the imprinting object. Note that such a visual experience did not include perceiving occlusive events associated with screens of different width or height. Moreover, the imprinting object that was visually available to the chicks was never observed to contact other objects; thus our experiments also ruled out the possibility that chicks learned something about object properties by observing the ways in which moving objects interact with each other. Apparently, chicks used their memories of the visual properties of the imprinting object to judge whether the width and height (but apparently not the projective shape as a cue to slant) of the opaque screens were compatible with the hiding of the imprinting object behind them.
Interestingly, human infants do not refer to height initially: their judgements seem rather restricted to a simple behind/not behind variable. Only later (by about 3.5 months) do they identify height as an occlusion variable (e.g. [35]). Again, differences in the pattern of development and the ecological requests faced by precocial and altricial species may be crucial: chicks, which move immediately after hatching, need to know immediately about the whole range of physical properties associated with their mobile social partners in order to survive in a natural environment, whereas babies could easily delay development of some aspects of such knowledge for some months, allowing more time to the completion of neural maturation.
Animals can certainly learn a lot about the physical properties of objects but what these results seem to suggest is that they also possess innate core knowledge mechanisms [13] that guide and constrain their intuitive inferences (and thus presumably their learning) concerning physical objects' properties, such as occlusion and size.
Acknowledgements
All experimental procedures were licensed by the Italian Ministero della Salute, Dipartimento Alimenti, Nutrizione e Sanità Pubblica Veterinaria according to EC regulations. We thank Chiara Bechini and Francesca Domini for help in training the animals. We also thank Lesley Rogers and Elizabeth Spelke for comments on a previous version of this paper. C.C. was supported by a L'Oréal–UNESCO Fellowship for Women in Science. This work has been realized also thanks to the support from the Provincia Autonoma di Trento and the Fondazione Cassa di Risparmio di Trento e Rovereto. There are no conflicts of interest to report.
References
- 1.Carey S. 2009. The origin of concepts. New York, NY: Oxford University Press [Google Scholar]
- 2.Spelke E. S. 1998. Nativism, empiricism, and the origin of knowledge. Infant Behav. Dev. 21, 181–200 10.1016/S0163-6383(98)90002-9 (doi:10.1016/S0163-6383(98)90002-9) [DOI] [Google Scholar]
- 3.Spelke E. S., Kinzler K. D. 2007. Core knowledge. Dev. Sci. 10, 89–96 10.1111/j.1467-7687.2007.00569.x (doi:10.1111/j.1467-7687.2007.00569.x) [DOI] [PubMed] [Google Scholar]
- 4.Spelke E. S. 2004. Initial knowledge: six suggestions. Cognition 50, 431–445 10.1016/0010-0277(94)90039-6 (doi:10.1016/0010-0277(94)90039-6) [DOI] [PubMed] [Google Scholar]
- 5.Mandler J. M. 1988. How to build a baby: on the development of an accessible representational system. Cogn. Dev. 3, 113–136 10.1016/0885-2014(88)90015-9 (doi:10.1016/0885-2014(88)90015-9) [DOI] [Google Scholar]
- 6.Aguiar A., Baillargeon R. 2002. Development in young infants' reasoning about occluded objects. Cogn. Psycol. 45, 267–336 10.1016/S0010-0285(02)00005-1 (doi:10.1016/S0010-0285(02)00005-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baillargeon R. 1987. Object permanence in 3 ½- and 4 ½ - month-old infants. Dev. Psychol. 23, 655–664 10.1037/0012-1649.23.5.655 (doi:10.1037/0012-1649.23.5.655) [DOI] [Google Scholar]
- 8.Baillargeon R., Spelke E. S., Wasserman S. 1985. Object permanence in five-month-old infants. Cognition 20, 191–208 10.1016/0010-0277(85)90008-3 (doi:10.1016/0010-0277(85)90008-3) [DOI] [PubMed] [Google Scholar]
- 9.Baillargeon R. 1995. Physical reasoning in infancy. In The cognitive neurosciences. (ed. Gazzaniga M. S.), pp. 181–204 Cambridge, MA: MIT Press [Google Scholar]
- 10.Carey S., Spelke E. S. 1996. Science and core knowledge. Philos. Sci. 63, 515–533 10.1086/289971 (doi:10.1086/289971) [DOI] [Google Scholar]
- 11.Hespos S., Baillargeon R. 2008. Young infants' actions reveal their developing knowledge of support variables: converging evidence for violation-of-expectation findings. Cognition 107, 304–316 10.1016/j.cognition.2007.07.009 (doi:10.1016/j.cognition.2007.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y., Kaufman L., Baillargeon R. 2009. Young infants' reasoning about physical events involving inert and self-propelled objects. Cogn. Psychol. 58, 441–486 10.1016/j.cogpsych.2008.11.001 (doi:10.1016/j.cogpsych.2008.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spelke E. S. 2000. Core knowledge. Am. Psychol. 55, 1233–1243 10.1037/0003-066X.55.11.1233 (doi:10.1037/0003-066X.55.11.1233) [DOI] [PubMed] [Google Scholar]
- 14.Call J. 2007. Apes know that hidden objects can affect the orientation of other objects. Cognition 105, 1–25 10.1016/j.cognition.2006.08.004 (doi:10.1016/j.cognition.2006.08.004) [DOI] [PubMed] [Google Scholar]
- 15.Kundey S. M. A., De Los Reyes A., Taglang C., Baruch A., German R. 2009. Domesticated dogs' (Canis familiaris) use of the solidity principle. Anim. Cogn. 13, 497–505 10.1007/s10071-009-0300-6 (doi:10.1007/s10071-009-0300-6) [DOI] [PubMed] [Google Scholar]
- 16.Pattison K. F., Miller H. C., Rayburn-Reeves R., Zentall T. 2010. The case of the disappearing bone: dogs' understanding of the physical properties of objects. Behav. Process. 85, 278–282 10.1016/j.beproc.2010.06.016 (doi:10.1016/j.beproc.2010.06.016) [DOI] [PubMed] [Google Scholar]
- 17.Santos L. R., Hauser M. D. 2002. A nonhuman primate's understanding of solidity: dissociations between seeing and acting. Dev. Sci. 5, F1–F7 10.1111/1467-7687.t01-1-00216 (doi:10.1111/1467-7687.t01-1-00216) [DOI] [Google Scholar]
- 18.Bird C. D., Emery N. J. 2010. Rooks perceive physical support relations like 6-month old human babies. Proc. R. Soc. B 277, 147–151 10.1098/rspb.2009.1456 (doi:10.1098/rspb.2009.1456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollok B., Prior H., Güntürkün O. 2000. Development of object permanence in food-storing magpies (Pica pica). J. Comp. Psychol. 114, 148–157 10.1037/0735-7036.114.2.148 (doi:10.1037/0735-7036.114.2.148) [DOI] [PubMed] [Google Scholar]
- 20.Zucca P., Milos N., Vallortigara G. 2007. Piagetian object permanence and its development in Eurasian Jays (Garrulus glandarius). Anim. Cogn. 10, 243–258 10.1007/s10071-006-0063-2 (doi:10.1007/s10071-006-0063-2) [DOI] [PubMed] [Google Scholar]
- 21.Wiesel T. 1982. The postnatal development of the visual cortex and the influence of environment. Nobel Lecture, 8 December 1981. Biosci. Rep. 2, 351–377 10.1007/BF01119299 (doi:10.1007/BF01119299) [DOI] [PubMed] [Google Scholar]
- 22.Landau B., Lakusta L. 2009. Spatial representation across species: geometry, language, and maps. Curr. Opin. Neurobiol. 19, 12–19 10.1016/j.conb.2009.02.001 (doi:10.1016/j.conb.2009.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platt M. L., Spelke E. S. 2009. What can developmental and comparative cognitive neuroscience tell us about the adult human brain? Curr. Opin. Neurobiol. 19, 1–5 10.1016/j.conb.2009.06.002 (doi:10.1016/j.conb.2009.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrew R. J. 1991. Neural and behavioural plasticity: the use of the domestic chick as a model. Oxford, UK: Oxford University Press [Google Scholar]
- 25.Haun D. B. M., Jordan F. M., Vallortigara G., Clayton N. S. 2010. Origins of spatial, temporal and numerical cognition: insights from comparative psychology. Trends Cogn. Sci. 14, 552–560 10.1016/jj.tics.2010.09.006 (doi:10.1016/jj.tics.2010.09.006) [DOI] [PubMed] [Google Scholar]
- 26.Mascalzoni E., Regolin L., Vallortigara G. 2010. Innate sensitivity for self-propelled causal agency in newly-hatched chicks. Proc. Natl Acad. Sci. USA 107, 4483–4485 10.1073/pnas.0908792107 (doi:10.1073/pnas.0908792107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallortigara G., Sovrano V. A., Chiandetti C. 2009. Doing Socrates experiment right: controlled rearing studies of geometrical knowledge in animals. Curr. Opin. Neurobiol. 19, 20–26 [DOI] [PubMed] [Google Scholar]
- 28.Vallortigara G., Andrew R. J. 1991. Lateralization of response by chicks to change in a model partner. Anim. Behav. 41, 187–194 10.1016/S0003-3472(05)80470-1 (doi:10.1016/S0003-3472(05)80470-1) [DOI] [Google Scholar]
- 29.Rogers L. J. 1995. The development of brain and behaviour in the chicken. Wallingford, UK: CAB International [Google Scholar]
- 30.Fellows B. J. 1967. Chance stimulus sequence for discrimination tasks. Psychol. Bull. 67, 87–92 10.1037/h0024098 (doi:10.1037/h0024098) [DOI] [PubMed] [Google Scholar]
- 31.Aguiar A., Baillargeon R. 1999. 2.5-month-old infants' reasoning when objects should and should not be occluded. Cognitive Psychology 39, 116–157 [DOI] [PubMed] [Google Scholar]
- 32.Valenza E., Leo I., Gava L., Simion F. 2006. Perceptual completion in newborn human infants. Child Dev. 77, 1810–1821 10.1111/j.1467-8624.2006.00975.x (doi:10.1111/j.1467-8624.2006.00975.x) [DOI] [PubMed] [Google Scholar]
- 33.Regolin L., Vallortigara G. 1995. Perception of partly occluded objects by young chicks. Percept. Psychophys. 57, 971–976 [DOI] [PubMed] [Google Scholar]
- 34.Spelke E. S., Breinlinger K., Macomber J., Jacobson K. 1992. Origins of knowledge. Psychol. Rev. 99, 605–632 10.1037/0033-295X.99.4.605 (doi:10.1037/0033-295X.99.4.605) [DOI] [PubMed] [Google Scholar]
- 35.Luo Y., Baillargeon R. 2005. When the ordinary seems unexpected: evidence for incremental physical knowledge in young infants. Cognition 95, 297–328 10.1016/j.cognition.2004.01.010 (doi:10.1016/j.cognition.2004.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]