Abstract
During sexual imprinting, offspring learn parental phenotypes and then select mates who are similar to their parents. Imprinting has been thought to contribute to the process of speciation in only a few rare cases; this is despite imprinting's potential to generate assortative mating and solve the problem of recombination in ecological speciation. If offspring imprint on parental traits under divergent selection, these traits will then be involved in both adaptation and mate preference. Such ‘magic traits’ easily generate sexual isolation and facilitate speciation. In this study, we show that imprinting occurs in two ecologically divergent stickleback species (benthics and limnetics: Gasterosteus spp.). Cross-fostered females preferred mates of their foster father's species. Furthermore, imprinting is essential for sexual isolation between species; isolation was reduced when females were raised without fathers. Daughters imprinted on father odour and colour during a critical period early in development. These traits have diverged between the species owing to differences in ecology. Therefore, we provide the first evidence that imprinting links ecological adaptation to sexual isolation between species. Our results suggest that imprinting may facilitate the evolution of sexual isolation during ecological speciation, may be especially important in cases of rapid diversification, and thus play an integral role in the generation of biodiversity.
Keywords: ecological speciation, learning, mate recognition, reproductive isolation
1. Introduction
Ecological speciation occurs when two populations adapt to different environments and reproductive isolation evolves between them as a consequence of this divergence [1–3]. A challenge for ecological speciation is that gene flow and recombination break up associations between alleles for population-specific adaptive traits, mating traits and mate preference. Ecological speciation is facilitated by mechanisms that reduce recombination [4,5] such as when a single allele leads to assortative mating in both incipient species (‘one allele mechanisms’) [6] or when traits under divergent selection are also used in mate choice (‘magic traits’) [7]. Sexual imprinting generates mate preferences based on parental traits [8] and potentially may act as a one-allele mechanism that creates magic traits during speciation. When a population colonizes a novel habitat, divergent natural or sexual selection may lead to changes in parental traits. Imprinting can link that divergence to mate preference and generate assortative mating between ancestral and descendent populations. If the traits that imprinting is based on have diverged, then individuals will prefer mates of their own population. Thus, imprinting might allow populations to differentiate more easily than genetically determined preferences [9,10] and could effectively generate sexual isolation during ecological speciation. However, this remains unverified and there is currently little evidence that imprinting contributes to speciation [11].
Here, we ask if imprinting on divergent parental traits generates isolation in benthic and limnetic threespine sticklebacks (Gasterosteus spp.); two species, which have evolved under divergent natural and sexual selection [12–16]. In these species, fathers provide all parental care [17]. Furthermore, owing to differences in ecology, benthic and limnetic males differ in body size, nuptial colour and odour. Body size differences are the result of adaptation to different foraging niches [13]. Nuptial colour differences are due to adaptation of signalling and sensory systems to different light environments [15]. Odour differences may be owing to pleiotropic effects of adaptive differences in diet [18], habitat [12] and major histocompatibility complex (MHC) alleles selected by parasite differences [19,20]. These traits are all known to be important in mate choice and sexual isolation [15,21–24].
To examine whether imprinting on fathers underlies sexual isolation and if imprinting targets traits under divergent selection, we experimentally manipulated offspring exposure to fathers: offspring were raised without any father or cared for by a heterospecific foster father, a conspecific foster father or their biological (conspecific) father. When offspring matured, we measured mate preference of daughters and sons. We predicted that if imprinting occurred, offspring would prefer the caring father's species. If imprinting is important to maintain reproductive isolation in the stickleback species pairs, we predicted that sexual isolation should be strongest when offspring were raised by conspecific fathers. Furthermore, we tested how mate preference was altered by father traits, such as body size, nuptial colour and exposure to father odour mediated by care behaviour. We additionally examined the role of odour by raising some offspring with a conspecific father and exposing them to heterospecific odour during embryonic development. If offspring imprint on divergent father traits, it would forge a link between ecological divergence and sexual isolation.
2. Material and methods
(a). Father exposure
Benthic and limnetic sticklebacks originated from Paxton Lake, British Columbia. We placed offspring in a parental exposure within 22 h of fertilization. Offspring were reared by: their biological conspecific father, a foster father (conspecific or heterospecific) or without a parent (in an egg cup over an airstone [25]). Each father built a nest out of filamentous algae in a plastic container filled with sand, then fertilized eggs that a female deposited in their nest. If males parented their biological offspring, they cared for their fertilized eggs. If males raised foster offspring, we removed the nest container from the tank, removed the eggs from the male's nest using forceps, immediately replaced the eggs with other recently fertilized eggs, and replaced the nest in the tank (this process took less than 10 min). Replacement of eggs occurred 12.76 ± 0.45 h after fertilization (range = 1.57–21.90 h). The new eggs were either fertilized within another male's nest or by the sperm of a single male in a Petri dish (eggs from one to four females, mean = 1.11 ± 0.04). After egg replacement, we left the male undisturbed for 1 day before beginning care observations. We observed father care for the embryos once per day for 10 min on days 2–9. We recorded the rates of parental behaviours that might increase exposure of developing embryos to father visual cues: time spent fanning at the mouth of the nest to oxygenate the embryos and time spent near the nest. Olfactory cues may be transferred through fanning or through nest glue deposition [26]. Fathers secrete nest glue (spiggin) and deposit it by rubbing their body along the nest [17]. Either contact of the father's body with the nest or the secreted glue itself (which has antimicrobial properties [27]) could transfer male odour to the nest. Damaging the olfactory nerves of fathers reduces nest building and parental care, suggesting odour plays an important role in this process [28]. Therefore, we recorded rates of deposition of nest glue during care. After 18 days of care, we removed fathers. After we removed fathers from the tank, we left the nest intact in the home tank until fry were five months old.
Benthics and limnetics from Paxton Lake have documented differences in odour [23]. To examine the role of odour in the development of preference, 13 families were raised by their biological (conspecific) father and exposed them to heterospecific odour during the first 7 days post-fertilization. Exposure to heterospecific odour was by placing a wild-caught Paxton female 50 cm away from the nest (separated from it by a mesh divider) for 2 h per day (days 1–7 post-fertilization); there was no possibility for visual contact with the embryos and no effect of this exposure on father care (all p > 0.55). Females were used as odour sources owing to availability.
(b). Mate preference tests
We tested the mate preference of both daughters and sons from 89 families (46 benthic and 43 limnetic). Total number of families per exposure (benthic and limnetic): biological father = 12 (6, 6), conspecific foster father = 19 (10, 9), heterospecific foster father = 21 (12, 9), no father = 24 (13, 11), biological father exposed to heterospecific odour = 13 (5, 8). There were no differences between fish raised by their biological (conspecific) father and by a foster conspecific father in all tests (uncorrected p > 0.21), so we combined them into one group: raised by a conspecific father. Similar results are obtained if we include only those raised by foster fathers in the analyses.
For each family, two individuals of each sex were tested. In each choice test or set of no-choice tests, individuals had to select between a pair of potential mates (one benthic and one limnetic) who were unrelated and unfamiliar laboratory-raised fish. Each pair was only used once. Order was randomized for the first individual and the opposite order used for the second.
Sexually mature daughters were courted by males of each species in separate no-choice tests [25,29]. Our measure of preference was whether or not females examined the male's nest; this is a commonly used predictor of spawning [29–31]. Male courtship was measured in a dichotomous choice test [25,32]. We also measured the courtship behaviour of males raised by a conspecific father (foster or biological) when courting either a limnetic or a benthic female in separate no-choice trials (26 benthic and 32 limnetic males) [21]. For males, we calculated preference as the difference in the number of courtship behaviours to conspecific and heterospecific females out of the total number behaviours in a trial [25]. We measured three courtship behaviours (zigzags, bites and leads) and summed them to measure vigour [32].
(c). Statistical analysis
Female probability of examining was analysed using a generalized linear model with a binomial distribution and logit link function; family included as a repeated factor with unstructured covariance (allowing separate covariance within females between trials and between females within families) using SAS software v.9.1 (SAS Institute Inc, 2007). Significance was determined via likelihood ratio tests. We included as fixed factors the father's identity (conspecific, heterospecific, no father and conspecific with heterospecific odour), the species of mate seen (conspecific and heterospecific), the female's species (benthic and limnetic) and the interactions between these factors. We included throat redness of the male and absolute length difference from the female as covariates since they are known to affect preference [15,21,29]. Post hoc comparison p-values were adjusted using false discovery rate [33].
Male courtship preference was analysed in a mixed model ANCOVA. Family was included as a repeated factor with unstructured covariance. Father exposure and male species were included as fixed effects; length difference between the benthic and limnetic females was a covariate.
To determine if father behaviour (time near nest, fanning and gluing) affected female preference, we used Spearman rank correlations to look at the relationship between preference of daughters for their caring father's species and his behaviour using least-squares means for each father exposure in each species.
For any behaviour that influenced daughter preference, we attempted to determine if the mechanism was primarily olfactory or visual by comparing effects on preference across developmentally different time periods. In sticklebacks, the olfactory system forms near the end of day 3 post-fertilization and eyes begin to form on day 6 [34,35]. Therefore, we divided our parental care observations into three time periods: before olfactory development (days 2 and 3), after olfactory development but before the formation of the visual system (days 4 and 5), and after visual development (days 6–8). The effects of behaviour on preference were determined separately for days 4 and 5 and days 6–8. We analysed female probability of examining in a generalized linear model with a binomial distribution with family as a repeated factor.
To quantify the degree of divergence in male visual traits between species, we measured size (standard length) and nuptial colour in a large sample of males from Paxton Lake (50 Paxton benthic and 49 limnetics collected in 2008). Males were scored for throat redness, body hue (green to blue), body brightness (intensity of colour) and body darkness (melanism) by an observer before and after males courted females (using a standardized method developed by our laboratory group [15,36]). We also measured size, throat redness and body brightness for all fathers during care. We tested divergence between males of each species using t-tests for courting males and fathers separately, and for all sampled males. To compare the degree of divergence in different morphological traits between the species, we calculated the difference between species means after variables were standardized within species (mean = 0, standard deviation = 1).
To examine the effects of father cues on imprinted preferences, we looked at the relationship between daughter preference and caring father's throat redness, body brightness and body size, as well as father behaviour in a generalized linear model with a binomial distribution and logit link function with family as a repeated factor (daughters raised by both conspecific and heterospecific fathers: 51 families; 101 females). We tested the significance of interactions between father traits and mate species using likelihood ratio tests. Mate throat redness was included as a covariate. Non-significant interactions were removed to simplify the model.
To quantify total sexual isolation and how it might be influenced by imprinting, we calculated IPSI. This index compares the number of copulating pairs observed within and between species and accounts for the contribution of both species to isolation (positive values indicate assortative mating) [37]. We defined copulation as female examination of the male's nest and a pair as a female and her first male so all pairs were independent (76 within, 72 between species pairs; conspecific fathers: 32 benthic, 28 limnetic females; heterospecific fathers: 24, 18; no father: 24, 22; heterospecific odour treatment excluded owing to low sample size). We calculated IPSI and pair sexual isolation (PSI) estimates in JMating and tested their deviation from zero using bootstrapping [38]. We compared PSI estimates for conspecific pairs between father exposures (n = 6) in an ANOVA [39].
Additional details on methods and analyses are included in the electronic supplementary material.
3. Results
(a). Imprinted mate preferences and sexual isolation
We found that females imprinted on the species of their caring father (figure 1; electronic supplementary material, table S1). Females raised by a conspecific father preferred con over heterospecific males (con versus het mates = 1.89 ± 0.38, 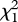 = 22.68, p < 0.0001, pFDR = 0.0004) in contrast to females raised by heterospecific fathers, who preferred heterospecific mates (con versus het mates = − 0.78 ± 0.30,
= 22.68, p < 0.0001, pFDR = 0.0004) in contrast to females raised by heterospecific fathers, who preferred heterospecific mates (con versus het mates = − 0.78 ± 0.30, 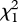 = 6.54, p = 0.011, pFDR = 0.018; con versus het fathers = 2.59 ± 0.49, Z = 3.43, p < 0.0001, pFDR = 0.0004). Furthermore, those raised without a father showed reduced conspecific preference (con versus het mates = 0.26 ± 0.50,
= 6.54, p = 0.011, pFDR = 0.018; con versus het fathers = 2.59 ± 0.49, Z = 3.43, p < 0.0001, pFDR = 0.0004). Furthermore, those raised without a father showed reduced conspecific preference (con versus het mates = 0.26 ± 0.50, 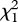 = 0.27, p = 0.61, pFDR = 0.66; con versus no father = 1.60 ± 0.64, Z = 2.46, p = 0.014, pFDR = 0.024). Thus, imprinting can reverse preferences and is essential for strong conspecific preference in females.
= 0.27, p = 0.61, pFDR = 0.66; con versus no father = 1.60 ± 0.64, Z = 2.46, p = 0.014, pFDR = 0.024). Thus, imprinting can reverse preferences and is essential for strong conspecific preference in females.
Figure 1.
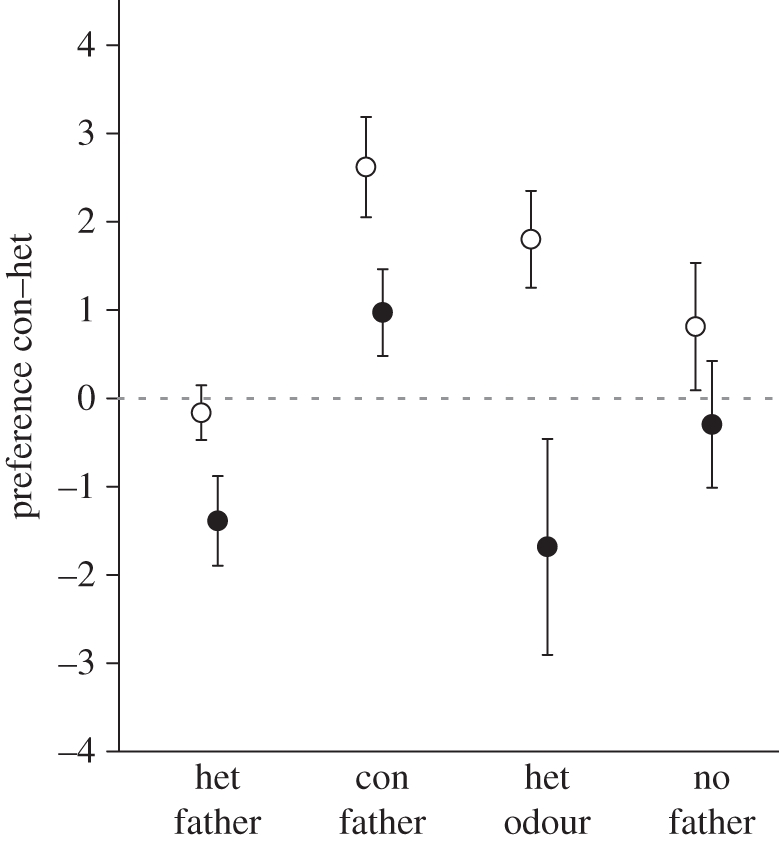
Female mate preference. Least-squares mean (±s.e.m.) probability of examining conspecific over heterospecific mates (logit-transformed values shown) plotted by father exposure. Limnetics indicated by open symbols, benthics by filled. Females raised by conspecific fathers preferred conspecific mates more than those raised by heterospecific fathers (con versus het fathers: limnetics pFDR = 0.0005, benthics pFDR = 0.0021). Exposure to heterospecific odour decreased preference for conspecifics in benthics only (con father versus het odour: limnetics pFDR = 0.35, benthics pFDR = 0.0125).
We found sex differences in the strength of imprinting; the effect fathers had on conspecific mate preference of daughters was not observed in sons. The species of the caring father had little influence on which species males courted in choice tests (all F3,77 < 1.78, p > 0.16; electronic supplementary material, table S2). Males in no-choice tests also did not prefer conspecifics (all paired two-tailed t57 < 0.94, p > 0.17).
Imprinted preferences in females affected the strength of sexual isolation between species. Sexual isolation was strongest in females raised by conspecific fathers and near zero in those raised by heterospecifics (conspecific fathers: IPSI = 0.75 ± 0.14, p = 0.0002; heterospecific fathers: IPSI = − 0.29 ± 0.34, p = 0.39). In those without fathers, sexual isolation was reduced and not significantly different than zero (IPSI = 0.43 ± 0.25, p = 0.14). Between father exposures, PSI estimates for conspecific pairs differed significantly (ANOVA: F2,3 = 87.93, p = 0.0022; con versus no father 0.31 ± 0.09, t5 = 3.43, p = pFDR = 0.042; con versus het father: 1.16 ± 0.09, t5 = 12.81, p = 0.001, pFDR = 0.003). Thus, significant sexual isolation was observed only when females were raised by conspecific fathers.
(b). Imprinting on divergent odour cues
In daughters raised by conspecific fathers but exposed to heterospecific odour, we saw a striking difference in conspecific preference between benthics and limnetics ( − 3.48 ± 1.33, Z = −2.61, p = 0.009, pFDR = 0.018). Exposure to heterospecific odour had little effect on limnetics; they behaved similarly to those raised by conspecific fathers and retained their conspecific preference. However, there was a large effect on benthics who switched to preferring heterospecific males (figure 1). Thus in benthics, olfactory exposure to heterospecific cues on days 1–7 post-fertilization was sufficient to develop a preference for heterospecific males.
Additionally, we found that preference of daughters for their caring father's species was correlated only with one parental behaviour: mean rate of glue deposition on the nest (figure 2; other behaviours: all Spearman r < 0.49, p > 0.33). Therefore, gluing behaviour appears to have increased exposure to the father's cues either through the odour of the glue itself or the father's contact with the nest.
Figure 2.
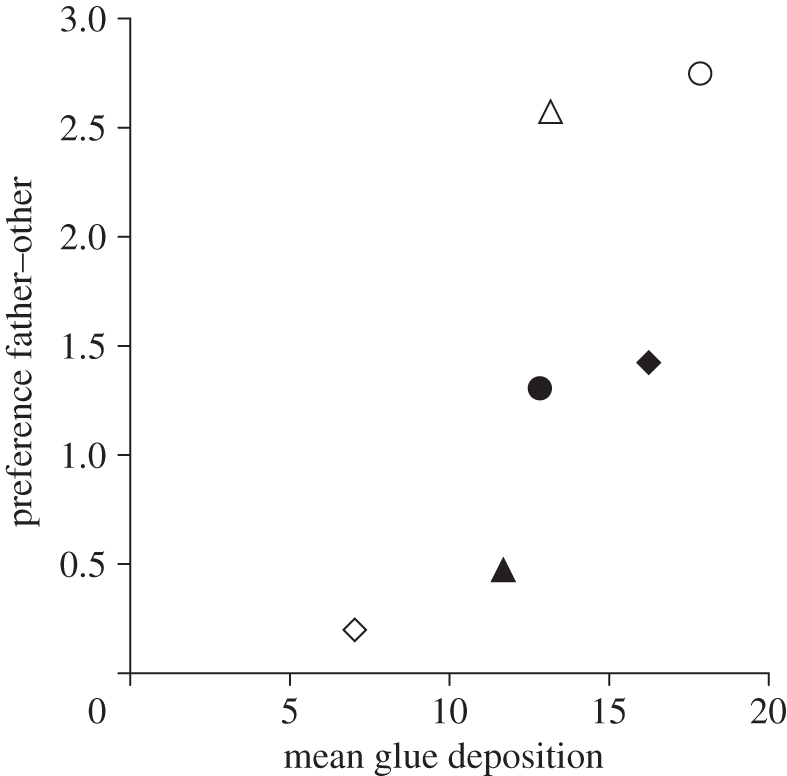
Father glue deposition and daughter preference. Least-squares mean probability of examining mates of father species over other (non-father) species (logit-transformed values shown) plotted against least-squares mean number of glue depositions by fathers. Symbols, limnetics (open), benthics (filled), heterospecific foster father (diamonds), conspecific foster father (circles) and biological father (triangles). Spearman rank correlation: r = 0.943, n = 6, p = 0.0048.
We narrowed the effect of gluing behaviour to a critical period early in development immediately after the formation of the olfactory system. Rates of gluing did not differ across developmental time periods (F2,60 = 0.04, p = 0.96). However, we found that father gluing only on days 4 and 5 post-fertilization significantly increased the probability of daughters raised by conspecific fathers examining conspecific over heterospecific nests (days 4 and 5: 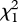 = 4.16, p = 0.03, βCon = 0.10 ± 0.07, βHet = −0.10 ± 0.07; days 6–8:
= 4.16, p = 0.03, βCon = 0.10 ± 0.07, βHet = −0.10 ± 0.07; days 6–8: 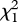 = 1.38, p = 0.24). The effect of gluing on days 4 and 5 remained significant when daughters raised by heterospecific fathers were included in the analysis (51 families; 101 females;
= 1.38, p = 0.24). The effect of gluing on days 4 and 5 remained significant when daughters raised by heterospecific fathers were included in the analysis (51 families; 101 females; 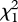 = 5.91, p = 0.015; figure 3a). Furthermore, sexual isolation indices were strong and significant in daughters whose conspecific fathers glued at high rates on days 4 and 5 (IPSI = 0.84 ± 0.17, p = 0.006; 25 females), whereas those in daughters whose fathers glued at low rates were not different from zero (IPSI = 0.50 ± 0.29, p = 0.12; 34 females; fathers classified as high- or low-based on being above or below the species-specific median). Since embryos could smell but not see on days 4 and 5 [34,35], we propose that embryos are exposed to the odour of their father during this time via gluing and they imprint on this odour.
= 5.91, p = 0.015; figure 3a). Furthermore, sexual isolation indices were strong and significant in daughters whose conspecific fathers glued at high rates on days 4 and 5 (IPSI = 0.84 ± 0.17, p = 0.006; 25 females), whereas those in daughters whose fathers glued at low rates were not different from zero (IPSI = 0.50 ± 0.29, p = 0.12; 34 females; fathers classified as high- or low-based on being above or below the species-specific median). Since embryos could smell but not see on days 4 and 5 [34,35], we propose that embryos are exposed to the odour of their father during this time via gluing and they imprint on this odour.
Figure 3.
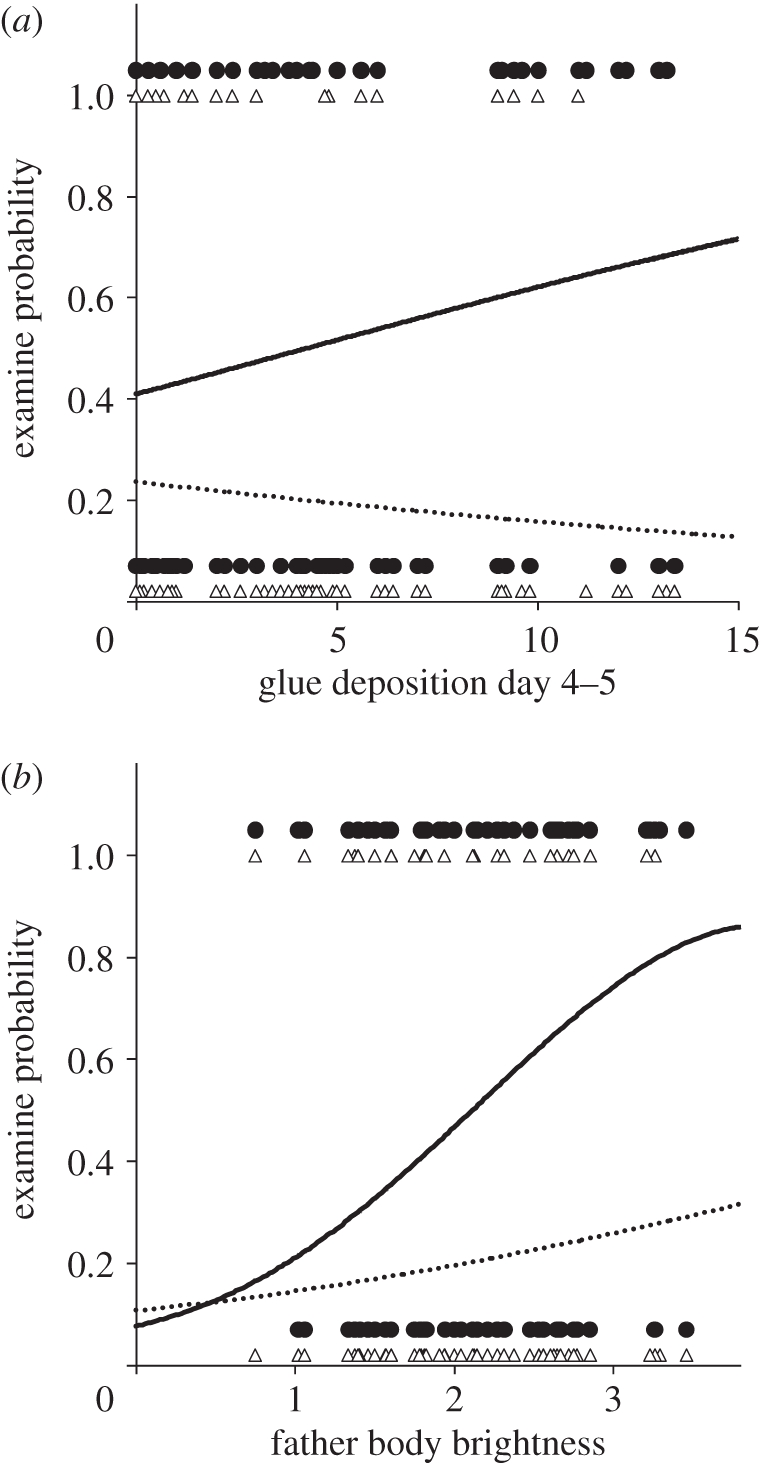
Influence of father olfactory and visual traits on daughter preference. Daughter preference plotted against father glue deposition and body brightness. Relationship shown for mates of the father's species (filled circles and solid line) and mates of the other species (open triangles and dashed line). Lines indicate predicted probability from the generalized linear model. Backtransformed values are shown. Data points jittered to improve visualization. (a) Glue deposition rate on days 4 and 5: βFather = 0.09 ± 0.06; βOther = − 0.05 ± 0.06. (b) Father body brightness score: βFather = 1.18 ± 0.43; βOther = 0.36 ± 0.37.
(c). Imprinting on divergent visual cues
Benthic and limnetic father populations differed significantly only in size and body colour (benthic versus limnetic: size two-tailed t97 = 9.54, p < 0.0001; throat redness t97 = 0.76, p = 0.44; body hue t91 = −7.68, p < 0.0001; body brightness t97 = −3.60, p = 0.0005; body darkness t97 = 5.88, p < 0.0001; electronic supplementary material, table S3). Benthics were larger than limnetics. Benthic bodies were greener and duller with many melanophores; limnetics were blue and bright with few melanophores. As body brightness increased, divergence in hue increased: brighter benthics were greener and brighter limnetics were bluer (species by brightness interaction F1,92 = 6.17, p = 0.015). Patterns were similar between courting and caring males but body brightness increased during care (brightness in the same male after courtship versus after care: paired two-tailed t19 = 6.53, p < 0.0001) while throat redness did not (t19 = 1.41, p = 0.17).
Daughter preference was influenced by the father's nuptial colour, but not father size. Father body brightness increased daughter preference for the caring father's species (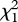 = 5.17, p = 0.023; figure 3b; electronic supplementary material, table S4); but father throat redness decreased preference for the father's species (
= 5.17, p = 0.023; figure 3b; electronic supplementary material, table S4); but father throat redness decreased preference for the father's species (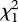 = 13.33, p = 0.0003; βFather = −0.56 ± 0.19). Father size had no significant influence on preference for the father's species; using size difference from the other species yielded similar results (all
= 13.33, p = 0.0003; βFather = −0.56 ± 0.19). Father size had no significant influence on preference for the father's species; using size difference from the other species yielded similar results (all 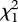 < 1.41, p > 0.24). As father body brightness increases, the father may be more noticeable to offspring or brightness may accentuate species differences in body hue (which increased with brightness), body darkness or male shape. By contrast, father throat redness did not differ between the species nor did it lead daughters to prefer their father's species.
< 1.41, p > 0.24). As father body brightness increases, the father may be more noticeable to offspring or brightness may accentuate species differences in body hue (which increased with brightness), body darkness or male shape. By contrast, father throat redness did not differ between the species nor did it lead daughters to prefer their father's species.
4. Discussion
Imprinting on ecologically divergent traits of odour and body coloration produces sexual isolation in benthic and limnetic sticklebacks. As odour or body coloration diverged owing to species differences in ecology, imprinting could have rapidly driven divergence in mate preference and sexual isolation between species would have resulted. Therefore, imprinting has turned odour and nuptial coloration into magic traits. Previous work has identified other components of pre- and post-mating isolation in sticklebacks which are ecologically dependent [13,15,21]. These mechanisms combine with imprinting to produce strong isolation between these incipient species. Speciation in benthics and limnetics appears to be driven by ecological divergence in multiple dimensions [40]. Imprinting may also play a role in other stickleback species pairs and the adaptive radiation of sticklebacks worldwide [41].
Our results provide new insight into the role of imprinting in speciation, providing an explanation for the conflicting evidence from previous theoretical and empirical studies of imprinting [9,10,42]. It is not imprinting per se that generates sexual isolation but imprinting that is linked to ecological differences, such as when parental traits underlying mate preference are subject to ecologically divergent selection. For example, in Darwin's finches, sexual isolation is due to offspring imprinting on father song and song has diverged in part owing to natural selection on beak shape [43,44]. Additionally, if offspring simply imprint on ecological cues, sexual isolation based on ecological differences can also be produced, such as when salmon imprint on natal stream odour [45] and indigo birds imprint on host song [11].
Thus, imprinting's ability to facilitate the evolution of sexual isolation depends critically on divergence in parental traits between populations. Adaptation to different ecological niches may be a particularly common mechanism driving parental trait divergence. However, parental traits could diverge through sexual selection or genetic drift and imprinting could still produce assortative mating [42]. Lake Victoria cichlid species differ in sexually selected nuptial colour and offspring may imprint on these colour differences [46,47]. At present, we need to determine how imprinting interacts with the forces leading to parental trait divergence and how imprinting affects speed of divergence. Furthermore, we do not know if the association between imprinting and isolation is merely opportunistic. Potentially, imprinting could itself be under selection or could evolve to target traits under divergent selection.
Clearly, learning is an important component of stickleback conspecific mate preference, but effects differ substantially between the sexes. Only females imprinted on their father. Previous work found that experience with foster heterospecific siblings led to increased conspecific mate preference in males but decreased preference in limnetic females (benthic females were unaffected) [25]. Thus, it appears that males learn more from experience with siblings than from their father. However, in females, father experience appears to have a stronger overall effect in both species than sibling experience. In our current study, we did not detect any effects of conspecific siblings but females also did not select between wild-caught males (which have accentuated species differences relative to laboratory-raised ones). Interestingly, cichlids show a similar learning pattern between sexes: females imprint on their mother and males imprint on their siblings [46,48]. In benthics and limnetics, we know females have stronger conspecific mate preference [31]. Thus, these sex differences in learning may be due to stronger selection on females to reject heterospecific mates during courtship.
The importance of father odour cues in imprinting potentially explains the difference between our study and an earlier one testing imprinting in sticklebacks, which did not allow fathers to contact or glue their nests and found little effect of the father [29]. In addition, gluing behaviour may also explain the difference between the species when daughters were raised by conspecific fathers but exposed to heterospecific odour in our study. In limnetics, 75 per cent of fathers (six of eight) deposited glue on days 4 and 5 and daughters did not imprint on the heterospecific odour. However, only 40 per cent of benthic fathers (two of five) glued on days 4 and 5; heterospecific odour may have been the dominant odour during this time and daughters misimprinted. Previous work has found the genes for spiggin (the primary component of nest glue) show signs of positive selection across populations of sticklebacks [49]. Thus, an intriguing direction for future work is determining what component of father odour daughters imprint on. In addition to spiggin, father MHC alleles may be a probable candidate. MHC odours of nest tending males are particularly salient to females [50]. The relative contribution of olfactory and visual cues during imprinting in sticklebacks could also be the subject of further research.
In summary, we show that stickleback daughters imprinted on their fathers, this imprinting was based on traits under divergent natural and sexual selection, and imprinting was essential for sexual isolation. Therefore, sexual imprinting that is coupled to adaptive ecological divergence can generate reproductive isolation as a by-product, facilitating ecological speciation. Sexual imprinting may be an unrecognized, but potentially powerful, ecologically dependent form of isolation that can drive speciation.
Acknowledgements
Institutional Animal Care and Use Committee at University of Wisconsin approved all experimental procedures (protocol no. L00317).
Funded by NSF Doctoral Dissertation Research Improvement Grant (G.M.K., J.W.B.), NSF Graduate Fellowship (G.M.K.), American Association of University Women Fellowship (G.M.K.), NSF Grant (J.W.B), Association for the Study of Animal Behaviour Research Grant (M.L.H). J. Koop provided ideas and input on project design. A. Lackey collected data on Paxton male colour. M. Barnes, M. Hartes, K. Hagemann, C. Long, P. Kell, M. Manes and M. Rounds assisted in data collection. D. Schluter, A. Lackey, I. Cooper, R. Gilman, A. Peters, H. Rundle and two anonymous reviewers provided helpful comments on the manuscript.
References
- 1.Schluter D. 2001. Ecology and the origin of species. Trends. Ecol. Evol. 16, 372–380 (doi:10.1016/S0169-5347(01)02198-X) [DOI] [PubMed] [Google Scholar]
- 2.Schluter D. 2009. Evidence for ecological speciation and its alternative. Science 323, 737–741 (doi:10.1126/science.1160006) [DOI] [PubMed] [Google Scholar]
- 3.Sobel J. M., Chen G. F., Watt L. R., Schemske D. W. 2010. The biology of speciation. Evolution 64, 295–315 (doi:10.1111/j.1558-5646.2009.00877.x) [DOI] [PubMed] [Google Scholar]
- 4.Otto S. P., Servedio M. R., Nuismer S. L. 2008. Frequency-dependent selection and the evolution of assortative mating. Genetics 179, 2091–2112 (doi:10.1534/genetics.107.084418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Servedio M. R. 2009. The role of linkage disequilibrium in the evolution of premating isolation. Heredity 102, 51–56 (doi:10.1038/hdy.2008.98) [DOI] [PubMed] [Google Scholar]
- 6.Felsenstein J. 1981. Skepticism towards Santa Rosalia, or why are there so few kinds of animals. Evolution 35, 124–138 (doi:10.2307/2407946) [DOI] [PubMed] [Google Scholar]
- 7.Gavrilets S. 2004. Fitness landscapes and the origin of species. Princeton, NJ: Princeton University Press [Google Scholar]
- 8.Immelmann K. 1972. Sexual and other long-term aspects of imprinting in birds and other species. Adv. Study Behav. 4, 147–174 (doi:10.1016/S0065-3454(08)60009-1) [Google Scholar]
- 9.Verzijden M. N., Lachlan R. F., Servedio M. R. 2005. Female mate-choice behavior and sympatric speciation. Evolution 59, 2097–2108 (doi:10.1111/j.0014-3820.2005.tb00920.x) [PubMed] [Google Scholar]
- 10.Servedio M., Saether S., Saetre G. 2009. Reinforcement and learning. Evol. Ecol. 23, 109–123 (doi:10.1007/s10682-007-9188-2) [Google Scholar]
- 11.Sorenson M. D., Sefc K. M., Payne R. B. 2003. Speciation by host switch in brood parasitic indigobirds. Nature 424, 928–931 (doi:10.1038/nature01863) [DOI] [PubMed] [Google Scholar]
- 12.McPhail J. D. 1992. Ecology and evolution of sympatric sticklebacks (Gasterosteus)—evidence for a species-pair in Paxton Lake, Texada Island, British Columbia. Can. J. Zool. 70, 361–369 (doi:10.1139/z92-054) [Google Scholar]
- 13.Schluter D. 1993. Adaptive radiation in sticklebacks—size, shape, and habitat use efficiency. Ecology 74, 699–709 (doi:10.2307/1940797) [Google Scholar]
- 14.Rundle H. D., Nagel L., Boughman J. W., Schluter D. 2000. Natural selection and parallel speciation in sympatric sticklebacks. Science 287, 306–308 (doi:10.1126/science.287.5451.306) [DOI] [PubMed] [Google Scholar]
- 15.Boughman J. W. 2001. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411, 944–948 (doi:10.1038/35082064) [DOI] [PubMed] [Google Scholar]
- 16.Boughman J. W., Rundle H. D., Schluter D. 2005. Parallel evolution of sexual isolation in sticklebacks. Evolution 59, 361–373 (doi:10.1111/j.0014-3820.2005.tb00995.x) [PubMed] [Google Scholar]
- 17.van Iersel J. J. A. 1953. An analysis of the parental behaviour of the male three-spined stickleback (Gasterosteus aculeatus L.). Behav. Suppl. 3, 1–159 [Google Scholar]
- 18.Schluter D., McPhail J. D. 1992. Ecological character displacement and speciation in sticklebacks. Am. Nat. 140, 85–108 (doi:10.1086/285404) [DOI] [PubMed] [Google Scholar]
- 19.Matthews B., Harmon L. J., M'Gonigle L., Marchinko K. B., Schaschl H. 2010. Sympatric and allopatric divergence of MHC genes in threespine stickleback. PLoS ONE 5, e10948 (doi:10.1371/journal.pone.0010948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacColl A. D. C. 2009. Parasite burdens differ between sympatric three-spined stickleback species. Ecography 32, 153–160 (doi:10.1111/j.1600-0587.2008.05486.x) [Google Scholar]
- 21.Nagel L., Schluter D. 1998. Body size, natural selection, and speciation in sticklebacks. Evolution 52, 209–218 (doi:10.2307/2410936) [DOI] [PubMed] [Google Scholar]
- 22.Reusch T. B. H., Haberli M. A., Aeschlimann P. B., Milinski M. 2001. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature 414, 300–302 (doi:10.1038/35104547) [DOI] [PubMed] [Google Scholar]
- 23.Rafferty N. E., Boughman J. W. 2006. Olfactory mate recognition in a sympatric species pair of three-spined sticklebacks. Behav. Ecol. 17, 965–970 (doi:10.1093/beheco/arl030) [Google Scholar]
- 24.Snowberg L. K., Bolnick D. I. 2008. Assortative mating by diet in a phenotypically unimodal but ecologically variable population of stickleback. Am. Nat. 172, 733–739 (doi:10.1086/591692) [DOI] [PubMed] [Google Scholar]
- 25.Kozak G. M., Boughman J. W. 2009. Learned conspecific mate preference in a species pair of sticklebacks. Behav. Ecol. 20, 1282–1288 (doi:10.1093/beheco/arp134) [Google Scholar]
- 26.McLennan D. A. 2003. The importance of olfactory signals in the gasterosteid mating system: sticklebacks go multimodal. Biol. J. Linn. Soc. 80, 555–572 (doi:10.1111/j.1095-8312.2003.00254.x) [Google Scholar]
- 27.Little T. J., Perutz M., Palmer M., Crossan C., Braithwaite V. A. 2008. Male three-spined sticklebacks Gasterosteus aculeatus make antibiotic nests: a novel form of parental protection? J. Fish Biol. 73, 2380–2389 (doi:10.1111/j.1095-8649.2008.02086.x) [Google Scholar]
- 28.Segaar J., Debruin J. P. C., Vandermeche A. P., Vandermechejacobi M. E. 1983. Influence of chemical receptivity on reproductive-behavior of the male three-spined stickleback (Gasterosteus aculeatus L): an ethological analysis of cranial nerve functions regarding nest fanning activity and the zigzag dance. Behaviour 86, 100–166 (doi:10.1163/156853983X00598) [Google Scholar]
- 29.Albert A. Y. K. 2005. Mate choice, sexual imprinting and speciation: a test of a one-allele isolating mechanism in sympatric sticklebacks. Evolution 59, 927–931 (doi:10.1111/j.0014-3820.2005.tb01767.x) [PubMed] [Google Scholar]
- 30.McKinnon J. S., Mori S., Blackman B. K., David L., Kingsley D. M., Jamieson L., Chou J., Schluter D. 2004. Evidence for ecology's role in speciation. Nature 429, 294–298 (doi:10.1038/nature02556) [DOI] [PubMed] [Google Scholar]
- 31.Kozak G. M., Reisland M., Boughmann J. W. 2009. Sex differences in mate recognition and conspecific preference in species with mutual mate choice. Evolution 63, 353–365 (doi:10.1111/j.1558-5646.2008.00564.x) [DOI] [PubMed] [Google Scholar]
- 32.Albert A. Y. K., Schluter D. 2004. Reproductive character displacement of male stickleback mate preference: reinforcement or direct selection? Evolution 58, 1099–1107 (doi:10.1111/j.0014-3820.2004.tb00443.x) [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 [Google Scholar]
- 34.Ekstrom P., Borg B., Vanveen T. 1983. Ontogenetic development of the pineal organ, parapineal organ, and retina of the three-spined stickleback, Gasterosteus aculeatus L (Teleostei)—development of photoreceptors. Cell Tissue Res. 233, 593–609 (doi:10.1007/BF00212227) [DOI] [PubMed] [Google Scholar]
- 35.Honkanen T., Ekstrom P. 1991. An immunocytochemical study of the development of the olfactory system in the three-spined stickleback (Gasterosteus aculeatus L, Teleostei). Anat. Embryol. 184, 469–477 (doi:10.1007/BF01236053) [DOI] [PubMed] [Google Scholar]
- 36.Lewandowski E., Boughman J. 2008. Effects of genetics and light environment on colour expression in threespine sticklebacks. Biol. J. Linn. Soc. 94, 663–673 (doi:10.1111/j.1095-8312.2008.01021.x) [Google Scholar]
- 37.Rolan-Alvarez E., Caballero M. 2000. Estimating sexual selection and sexual isolation effects from mating frequencies. Evolution 54, 30–36 (doi:10.1554/0014-3820(2000)054[0030:ESSASI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 38.Carvajal-Rodriguez A., Rolan-Alvarez E. 2006. JMATING: a software for the analysis of sexual selection and sexual isolation effects from mating frequency data. BMC Evol. Biol. 6, 40 (doi:10.1186/1471-2148-6-40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coyne J. A., Elwyn S., Rolan-Alvarezm E. L. 2005. Impact of experimental design on Drosophila sexual isolation studies: direct effects and comparison to field hybridization data. Evolution 59, 2588–2601 (doi:10.1111/j.0014-3820.2005.tb00971.x) [PubMed] [Google Scholar]
- 40.Nosil P., Harmon L. J., Seehausen O. 2009. Ecological explanations for (incomplete) speciation. Trends Ecol. Evol. 24, 145–156 (doi:10.1016/j.tree.2008.10.011) [DOI] [PubMed] [Google Scholar]
- 41.Mckinnon J. S., Rundle H. D. 2002. Speciation in nature: the threespine stickleback model systems. Trends Ecol. Evol. 17, 480–488 (doi:10.1016/S0169-5347(02)02579-X) [Google Scholar]
- 42.Laland K. N. 1994. On the evolutionary consequences of sexual imprinting. Evolution 48, 477–489 (doi:10.2307/2410106) [DOI] [PubMed] [Google Scholar]
- 43.Podos J. 2001. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature 409, 185–188 (doi:10.1038/35051570) [DOI] [PubMed] [Google Scholar]
- 44.Huber S. K., Podos J. 2006. Beak morphology and song features covary in a population of Darwin's finches (Geospiza fortis). Biol. J. Linn. Soc. 88, 489–498 (doi:10.1111/j.1095-8312.2006.00638.x) [Google Scholar]
- 45.Hendry A. P., Wenburg J. K., Bentzen P., Volk E. C., Quinn T. P. 2000. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science 290, 516–518 (doi:10.1126/science.290.5491.516) [DOI] [PubMed] [Google Scholar]
- 46.Verzijden M. N., ten Cate C. 2007. Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biol. Lett. 3, 134–136 (doi:10.1098/rsbl.2006.0601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seehausen O., et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–626 (doi:10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- 48.Verzijden M. N., Korthof R. E. M., ten Cate C. 2008. Females learn from mothers and males learn from others. The effect of mother and siblings on the development of female mate preferences and male aggression biases in Lake Victoria cichlids, genus Mbipia. Behav. Ecol. Sociobiol. 62, 1359–1368 (doi:10.1007/s00265-008-0564-x) [Google Scholar]
- 49.Kawahara R., Nishida M. 2006. Multiple occurrences of spiggin genes in sticklebacks. Gene 373, 58–66 (doi:10.1016/j.gene.2006.01.008) [DOI] [PubMed] [Google Scholar]
- 50.Milinski M., Griffiths S. W., Reusch T. B. H., Boehm T. 2010. Costly major histocompatibility complex signals produced only by reproductively active males, but not females, must be validated by a ‘maleness signal’ in three-spined sticklebacks. Proc. R. Soc. B 277, 391–398 (doi:10.1098/rspb.2009.1501) [DOI] [PMC free article] [PubMed] [Google Scholar]


