Abstract
We used a selection of Arabidopsis thaliana mutants with knockouts in defence genes to demonstrate growth costs of trichome development and glucosinolate production. Four of the seven defence mutants had significantly higher size-standardized growth rates (SGRs) than the wild-type in early life, although this benefit declined as plants grew larger. SGR is known to be a good predictor of success under high-density conditions, and we confirmed that mutants with higher growth rates had a large advantage when grown in competition. Despite the lack of differences in flowering-time genes, the mutants differed in flowering time, a trait that strongly correlated with early growth rate. Aphid herbivory decreased plant growth rate and increased flowering time, and aphid population growth rate was closely coupled to the growth rate of the host plant. Small differences in early SGR thus had cascading effects on both flowering time and herbivore populations.
Keywords: herbivore defence, size-standardized growth rate, glucosinolates, trichomes, Arabidopsis
1. Introduction
Plants deter herbivores through physical structures such as spines, thorns and hairs that reduce damage to leaf tissue [1,2], and by producing toxic chemical compounds that reduce the growth rate or reproductive output of their enemies [3]. Such defences are assumed to be costly as they divert the plant's resources away from growth and reproduction [4–6]. However, experimental studies addressing fitness/defence trade-offs frequently fail to find the expected negative correlations [7–10], raising the question of whether such trade-offs are absent in many organisms (possibly through mechanisms that alleviate costs while maintaining resistance), or whether the methods employed to find them are inadequate [11].
Arabidopsis thaliana (L.) is attacked by a variety of pathogens [12] and herbivores, which include leaf-chewing caterpillars, sap-sucking aphids, flea beetles and leaf miners [13,14]. As defence against these herbivores, Arabidopsis produces leaf hairs, called trichomes, and glucosinolates, a group of secondary metabolites [13]. Glucosinolate compounds are produced by all species of the Brassicaceae [15], and plants show large variation for this trait in the field [16], probably as a consequence of differential selection by herbivore communities [17]. The majority of glucosinolates either have aliphatic or indolic side-chains [18]. Both types of glucosinolate negatively affect generalist leaf-chewing herbivores, while aliphatic glucosinolates tend to affect these herbivores more severely [19–22]. Phloem-feeding aphids are mainly impaired by indolic glucosinolates [23], although there is evidence from field studies that some aphid species are also impaired by aliphatic glucosinolates [24]. Previously, we demonstrated that the production of glucosinolate compounds appeared to be costly to the plant, as there was a negative correlation between plant growth rate and glucosinolate content [11]. We also showed that slow-growing plants suffered reduced herbivore damage. While suggestive, these correlations are not proof of causal relationships. Instead, the costs of defensive traits can be more directly estimated using knockout mutants, in which defence genes are disabled artificially. Ideally, knockout mutants differ from the wild-type only in target genes, and if mutant phenotypes are not more extreme than the phenotypes of naturally occurring variants, we believe that such mutants can be used to address ecological questions.
In this study, we compared the growth rate of mutants reduced in specific defence mechanisms with the wild-type. We conducted a multiple-harvest experiment and calculated size-standardized relative growth rates (SGRs) for a range of plant sizes (see also [11,25]). A reduction in early growth rate is a likely consequence of diverting resources to defence; however, it is possible that for isolated plants growing with no competition there will be no measurable reduction in the final seed output. This could occur because the resources diverted to defence compounds early in life can be later reclaimed and redirected to the seeds. However, under competitive conditions, a reduction in growth rate is likely to have severe fitness costs; for example, Fakheran et al. [26] showed that early growth rate was a very good predictor of success when a mixture of Arabidopsis genotypes were grown under high-density, competitive conditions. Yet when grown alone these same genotypes did not differ in their final biomass [11].
In this study, we compared the growth rates of nine mutants with the wild-type in the presence and absence of the generalist aphid Myzus persicae. We also compared the growth rate of the aphid population on each of the 10 genotypes and related this to the plant growth rate. Finally, we grew a subset of the genotypes in competition to test whether differences in early growth rates had greater fitness consequences under competitive conditions.
2. Material and methods
(a). Knockout mutants
We used knockout mutants created in the genetic background of the Arabidopsis accession Columbia (Col-0; see electronic supplementary material, table S1 for a description of mutant phenotypes). One mutant (gl1-2) was originally created by X-ray mutagenesis and is deficient in trichome formation: the early leaves are entirely glabrous and there is greatly reduced trichome density on later leaves when compared with the wild-type [27]. The gl1-2 mutant also shows decreased phenolic defence expression (Daniel J. Kliebenstein 2004, unpublished data). All other mutants were originally created by T-DNA insertion. The mutants myb28, myb29 and myb28myb29 contain knockouts in transcription factors that decrease the expression of aliphatic glucosinolates [21,28], and the mutants cyp79B2, cyp79B3 and cyp79B2cyp79B3 contain enzyme knockouts that decrease or abolish the indolic glucosinolate and camalexin pathways [29]. The genes MYB28/MYB29 and CYP79B2/CYP79B3 are tandem duplicated genes within their respective cellular pathway and are traditionally considered redundant [28,29]. To control for non-target effects of transgenic plants such as the cost of expression of selection marker genes, we included two mutants with knockouts in genes not associated with defence and with no predicted fitness costs: ppi1-2 and nst1-2 [30,31].
Even though all mutants used here were originally created by artificial gene knockout, similar phenotypes can be found in natural accessions of Arabidopsis. For example, the accessions est-0 (NASC 1148) and wil-3 (NASC 1598) are both completely glabrous, and glucosinolate levels vary considerably among natural accessions [32].
(b). Experimental design
Plants were grown in a mixture of peat-based substrate (PP7, Tref Group, The Netherlands) and sand in a ratio of 1 : 1. Each pot (diameter = 40 mm, depth = 70 mm) was sown with five seeds and cold-stratified at 4°C for 48 h. The pots were then moved to a glasshouse with supplemental artificial light at a 16 L : 8 D and 26°C day/22°C night temperature. Plants were watered twice a week throughout the experiment and no additional nutrients were supplied. Five days after sowing, seedlings were thinned to leave only the most central seedling. Bolting (initiation of the flowering stem) was recorded for each plant to the nearest day. Six plants per genotype were harvested on days 5, 9, 13, 18, 23, 29 and 35 after germination. On day 5, the herbivore treatment was initiated by placing a single first instar aphid onto half of the remaining plants. The offspring of the introduced aphids (F1) were counted and removed at each harvest to keep herbivore pressure roughly constant among plant genotypes.
(c). Size-standardized growth rates of plants
We fitted an asymptotic regression model to log(aboveground biomass) through time:
| 2.1 |
where Mi,0 is the starting mass of genotype i at t = 0, Ai is the asymptotic mass as  and ri is the logarithm of the rate constant. The model was fitted with the function gnls in R [33] with genotype treated as a fixed effect. Models were compared based on their AIC values and SGRs were calculated with parameters taken from the best model. SGR is given by
and ri is the logarithm of the rate constant. The model was fitted with the function gnls in R [33] with genotype treated as a fixed effect. Models were compared based on their AIC values and SGRs were calculated with parameters taken from the best model. SGR is given by
| 2.2 |
where Mref is a reference mass (for derivation of equation (2.2) see electronic supplementary material, appendix S1 and [25]).
(d). Prediction intervals on size-standardized growth rates
The function gnls produces point estimates and confidence intervals for the two estimated model parameters, the rate constant ri and the asymptotic mass Ai. To estimate confidence intervals for SGR (a function of these two parameters), we generated population prediction intervals [34,35]. The method assumes that the distribution of the parameters is multivariate normal with a variance–covariance matrix given by the inverse of the information matrix. We used the function mvrnom, which selects multivariate normal random deviates, and the variance–covariance matrix given by the function vcov. We generated 1000 sets of parameters to calculate a distribution of differences between wild-type and mutant SGRs. The lower and upper 95 per cent quantiles of these distributions are the boundaries of the prediction intervals. Mutant SGRs are significantly different from wild-type SGR if the prediction interval does not include zero. Point estimates of SGR and prediction intervals were calculated at two reference masses (Mref, equation (2.2)): an early SGR using the average mass at age = 5 days and a late SGR using the average mass at age = 29 days.
(e). Aphid rate of reproduction
Aphid performance was analysed by fitting the same asymptotic model (equation (2.1)) to the log-transformed cumulative number of F1 aphids, thus generating an SGR of the aphid population. Estimates and prediction intervals of aphid SGR were calculated at two reference population sizes: 2 and 42 individuals, roughly corresponding to average offspring number on day 13 and day 29, respectively.
(f). Early growth rate and competition
To determine whether differences in early growth rate affected the outcome of competition, we carried out a competition experiment with a subset of genotypes: myb28, myb29 and the wild-type. Plants were grown in 5 × 5.5 cm pots filled with germination soil and maintained under long-day (16 L : 8 D) conditions in a controlled-environment growth chamber. Prior to sowing, seeds were imbibed and cold-stratified at 4°C for 3 days. In each pot, nine seeds were arranged into a square with an area of 1 cm2, thus closely surrounding the central seed with eight neighbours. Mutant central seeds were either surrounded by their own genotype or by the wild-type, while wild-type central seeds were surrounded by myb28, myb29 or wild-type, resulting in a total of seven combinations. Each combination was replicated 12 times, half of which were harvested after three weeks and half after four weeks. There was some germination failure and only pots with more than five neighbour plants were kept, thus the sample size was decreased to 31 pots in week 3 and 28 pots in week 4. At day 18 for week 3 and day 25 for week 4, the rosette diameter of the central plant and two neighbours was recorded. Three days later, the same plants were harvested and fresh weight was measured. Fresh weight or rosette diameter was analysed as a function of target genotype, neighbour genotype and harvest week using linear models.
3. Results
(a). Size-standardized growth rates of plant genotypes
The final asymptotic regression model included effects of plant genotype and herbivory on the rate constant ri and the asymptotic mass Ai as judged by comparing AIC values (electronic supplementary material, table S2 and figure S1). There was no herbivory × plant genotype interaction. For the following analysis, only results from the control (without aphids) are shown.
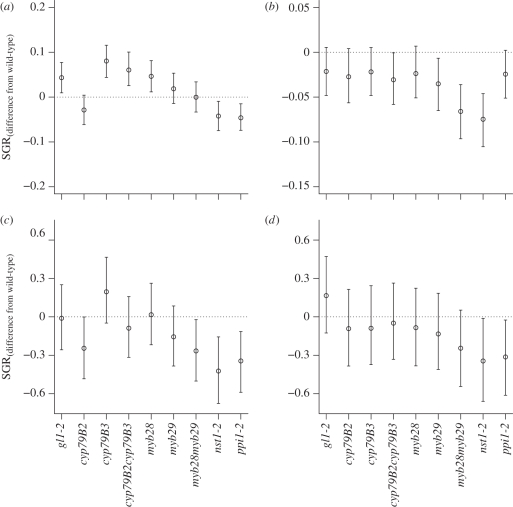
Six of the seven defence mutants had significantly higher values of the rate constant ri than wild-type, while the two mutants with knockouts in other genes did not differ from wild-type (table 1). In contrast, all mutants had lower values of the asymptotic mass Ai when compared with wild-type (electronic supplementary material, table S2). Early SGR was significantly higher than wild-type for the glabrous mutant gl1-2, the indole glucosinolate mutants cyp79B3 and cyp79B2cyp79B3, and the aliphatic glucosinolate mutant myb28 (figure 1a). In later life, mutants tended to have equal or lower SGRs than the wild-type (figure 1b).
Table 1.
Parameters from the asymptotic regression model and bolting age of plant genotypes. Parameters for the wild-type are absolute values while the parameter values of mutants are differences from the wild-type. Bolting ages are absolute values. ‘aphid’ gives the overall difference in parameter values or age at bolting in the presence of aphids. Significant differences are in boldface.
| plant genotype | rate constant (ri) | asymptotic mass (Ai) | bolting age |
|---|---|---|---|
| wild-type | −2.25 | 3.58 | 18.6 |
| gl1-2 | +0.12 | −0.27 | 17.7 |
| cyp79B2 | −0 | −0.26 | 19.8 |
| cyp79B3 | +0.18 | −0.30 | 18.0 |
| cyp79B2cyp79B3 | +0.16 | −0.36 | 16.4 |
| myb28 | +0.13 | −0.29 | 17.8 |
| myb29 | +0.10 | −0.37 | 17.3 |
| myb28myb29 | +0.12 | −0.64 | 20.1 |
| nst1-2 | +0.06 | −0.71 | 19.6 |
| ppi1-2 | −0.04 | −0.21 | 22.2 |
| aphid | −0.06 | −0.14 | +0.47 |
Figure 1.
Differences in size-standardized relative growth rates (SGRs) of mutant plants from wild-type in (a,b) and (c,d) population SGRs of aphids feeding on mutant plants. For plants, early SGR is calculated for average mass (a) at age = 5 days and (b) at age = 29 days, while for aphids, SGR is calculated at the average population size (c) when plant age = 13 days and (d) when plant age = 29 days. Dotted lines represent zero difference from wild-type in SGR, error bars show 95% prediction intervals.
As an unexpected result, we found that across the 10 genotypes early SGR is an excellent predictor of mean bolting age (r = −0.813; F1,8 = 15.63, p = 0.004); that is, fast-growing genotypes flowered earlier. This demonstrates that changes in early growth rate can influence flowering time, despite the fact that the mutant genotypes in question did not contain altered flowering genes. This apparently direct link between early growth rate and flowering time is confirmed by the aphid treatment: aphid feeding also decreased growth rate but increased bolting age in all genotypes (table 1).
(b). Aphid rate of reproductive output
The asymptotic regression model included effects of plant genotype on the rate constant ri and the asymptotic mass Ai (electronic supplementary material, figure S2 and table S3). With the exceptions of ppi1-2 and nst1-2, none of the aphid SGRs calculated from this model was significantly different from wild-type (figure 1c,d). However, the aphid rate of reproductive output on the different plant genotypes was strongly correlated with the plant SGR at early stages (r = 0.877, F1,8 = 26.67, p = 0.0009), and this correlation, even though weakened, was still present at the end of the experiment (r = 0.630, F1,8 = 5.26, p = 0.051). Thus, aphid populations performed better on fast-growing genotypes.
(c). Size-standardized growth rates and competition
Based on measurements of early SGR, we would predict that myb28 should outcompete the wild-type, whereas myb29 and wild-type should be equal competitors. In the analysis of fresh weight, neighbour genotype had a significant effect on the target genotype in week 4 (F2,23 = 5.74, p = 0.010). myb28 target plants weighed 0.14 (±0.06, 1 s.e.) g when surrounded by other myb28 plants, but weighed 0.41 (±0.07, 1 s.e.) g when surrounded by wild-type plants. Wild-type plants surrounded by wild-type neighbours weighed on average 0.25 (±0.07, 1 s.e.) g, while wild-type plants surrounded by myb28 neighbours weighed only 0.15 (±0.07, 1 s.e.) g. The weight of myb29 was not significantly affected by neighbour identity. The direction of the effects in week 3 and for rosette diameter in both weeks was similar but non-significant. Thus, it seems that the observed significant difference in early growth rate between myb28 and wild-type has fitness consequences when the plants are grown in competition.
4. Discussion
Six of the seven genotypes with knockouts in defence genes had a higher rate constant (ri) than the wild-type, but the asymptotic mass (Ai) was lower for all mutants. As SGR is a function of both parameters, this meant that only four defence mutants had significantly higher early growth rate than the wild-type, and this difference decreased with increasing plant size. The observed differences in early growth rate were relatively small, but these differences had large effects on target plant size when growing in competition. For example, myb28 has a higher initial growth rate than wild-type and thus should be able to outcompete it when the two genotypes are grown together. In support of this, myb28 was more than twice as large with wild-type as with myb28 neighbours and, similarly, wild-type individuals were larger with wild-type than with myb28 neighbours. In contrast, the early growth rate of myb29 (which was only grown with either wild-type or myb29 neighbours in the competition experiment) is similar to wild-type and it was unaffected by neighbour identity when grown under competition. The large advantage observed under competitive conditions is not unexpected under scramble competition for resources, as a difference in early growth rate will lead to unequal resource uptake, and with a finite pool of resources the plant with the higher uptake rate will gain a greater share of the total. In a recent study, Fakheran et al. [26] also showed that early growth rate was the best predictor of success in high-density competitive landscapes. Differences in growth rates among genotypes are thus also likely to be the underlying mechanism creating the sometimes ambiguous results from studies looking at kinship effects on the competitive ability of plants (e.g. [36,37]).
Early growth rate was also a very good predictor of flowering time, a trait that varied by several days among genotypes, despite identical flowering genes. Aphid herbivory also reduced early growth rate and increased flowering time, again indicating a possible causal link between early growth rate and the initiation of flowering. Small differences in early growth rate are therefore biologically relevant, leading to a disadvantage in competition and to delayed flowering. Hence the production of defensive traits and the consequent reduction in growth rate are likely to be costly to the plant. This supports findings from field experiments that show that both trichomes and glucosinolates have a visible fitness cost if herbivores are eliminated (e.g. [13]). It also supports theoretical work that assumes such a trade-off between defence and fitness.
Surprisingly, genotypes with knockouts in the homologous gene pairs MYB28/MYB29 and CYP79B2/CYP79B3 had relatively large differences in their growth rate. cyp79B2 grew more slowly than cyp79B3 and the double mutant cyp79B2cyp79B3, and myb28 grew faster than myb29 and the double mutant myb28myb29. MYB28 and MYB29 are not completely functionally redundant and there is evidence of an incoherent feed-forward loop involving these two genes that complicates our ability to place them in a linear pathway [38]. Likewise, CYP79B2 and CYP79B3 are not completely functionally redundant, with the genes having quantitative preferences to the camalexin versus indole glucosinolate pathways. How the fluxes are reshuffled in the single mutants is not currently understood and, as such, the double mutant cyp79B2cyp79B3 is a cleaner background to directly interpret [39]. These data suggest that the genes MYB28/MYB29 and CYP79B2/CYP79B3 are involved in nonlinear pathways that are not completely understood and will require further research to parse. This does suggest that single gene mutants in any background may be more complicated to interpret than is traditionally considered.
Defence mutants benefited from the lack of defensive traits in early life but, as plants grew larger, this benefit apparently disappeared. In contrast, the two mutants with knockouts in other (non-defence-related) genes performed worse than the wild-type at all sizes—a phenomenon that was not observed previously; hence these mutants were thought to be neutral [30,31]. The poor performance of the two non-defence-related mutants in our study may be owing to the growing conditions: our plants were grown in small pots in a sand–soil mixture with no additional nutrients, and this could be a more stressful environment than that normally used for genetic work. That all mutants had poorer performance at larger sizes is possibly due to pleiotropic effects, as disabling a gene usually affects several functions. It could also be due to the expression of selection marker genes, which might have associated costs (although this would not explain the poor performance of gl1-2, which is not a transgenic).
According to optimal defence theory [40], plants should follow different defence strategies before and after bolting, hence the decline in mutant SGRs with respect to wild-type could also represent a change in the value of defensive traits. Prior to bolting, growth is mass-dependent and removal of leaf tissue by herbivores should be particularly costly, thus plants should invest heavily in leaf defences. Mutant plants, unable to produce such defensive traits, then have additional resources available for growth. After bolting, the inflorescence becomes the most valuable plant organ. However, at least part of the defensive compounds in the inflorescence are relocated from rosette leaves [18]; wild-type plants might thus synthesize less glucosinolates de novo during the post-flowering period, hence decreasing the relative advantage of knockout mutants.
All plant genotypes were similarly susceptible to aphid herbivory and aphid performance was not generally better on genotypes with knockouts in defence genes. However, if aphids remove a constant fraction of the plant's resources, we still expect faster-growing plants to support higher aphid population growth (see [41] for a similar situation with a parasitic plant, Rhinanthus alectorolophus). This was indeed the case, as aphid population growth rate was strongly correlated with plant SGR. The relatively small differences in aphid population size on wild-type and mutant plants in our study is probably partly a result of keeping aphid densities low by constantly removing offspring. Low herbivore densities might in turn be unable to trigger a full defensive response by the plants, as part of the defence response of Arabidopsis is induced only by herbivore feeding [23,42,43]. That high concentration of certain glucosinolate compounds can affect aphid feeding has been shown by Kim & Jander [23], who demonstrated that indolic (but not aliphatic) glucosinolates deterred M. persicae when applied in artificial diets. However, Kim et al. [44], too, failed to show increased aphid reproduction on the cyp79B2cyp79B3 double-knockout mutant and demonstrated only decreased reproduction on a mutant overexpressing indolic glucosinolates. The specific mechanism involved in plant defence against aphids thus remains unclear, while the relevance of glucosinolates in defence against leaf-chewing herbivores has been demonstrated repeatedly [19–22].
In summary, mutants with knockouts in defence genes generally grew faster at small sizes than the wild-type. This enhanced early growth rate gave them an advantage in competition and allowed them to flower earlier. Combined with earlier work demonstrating a negative correlation between glucosinolate concentrations and growth rates, this study supports the hypothesis that the defence traits of Arabidopsis are costly to the plant. While knockout mutants helped to reveal these costs, such mutants can exhibit growth disadvantages, particularly in later life, and especially when grown under nutrient-poor conditions, and hence should be used with caution.
Acknowledgements
This work was funded by the Forschungskredit of the University of Zürich (grant 57230502) to T.Z. and by Swiss National Science Foundation Grant 31-107531 to L.A.T. Several colleagues kindly provided seeds (electronic supplementary material, table S1).
References
- 1.Levin D. A. 1973. The role of trichomes in plant defense. Q. Rev. Biol. 48, 3–15 10.1086/407484 (doi:10.1086/407484) [DOI] [Google Scholar]
- 2.Grubb P. J. 1992. A positive distrust in simplicity: lessons from plant defences and from competition among plants and among animals. J. Ecol. 80, 585–610 10.2307/2260852 (doi:10.2307/2260852) [DOI] [Google Scholar]
- 3.Fraenkel G. S. 1959. The raison d'être of secondary plant substances. Science 129, 1466–1470 10.1126/science.129.3361.1466 (doi:10.1126/science.129.3361.1466) [DOI] [PubMed] [Google Scholar]
- 4.Bazzaz F. A., Chiariello N. R., Coley P. D., Pitelka L. F. 1987. Allocating resources to reproduction and defense. Bioscience 37, 58–67 10.2307/1310178 (doi:10.2307/1310178) [DOI] [Google Scholar]
- 5.Coley P. D., Bryant J. P., Chapin F. S. 1985. Resource availability and plant antiherbivore defense. Science 230, 895–899 10.1126/science.230.4728.895 (doi:10.1126/science.230.4728.895) [DOI] [PubMed] [Google Scholar]
- 6.Herms D. A., Mattson W. J. 1992. The dilemma of plants: to grow or defend. Q. Rev. Biol. 67, 283–335 10.1086/417659 (doi:10.1086/417659) [DOI] [Google Scholar]
- 7.Koricheva J. 2002. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83, 176–190 10.1890/0012-9658(2002)083[0176:MAOSOV]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[0176:MAOSOV]2.0.CO;2) [DOI] [Google Scholar]
- 8.Bergelson J., Purrington C. B. 1996. Surveying patterns in the cost of resistance in plants. Am. Nat. 148, 536–558 10.1086/285938 (doi:10.1086/285938) [DOI] [Google Scholar]
- 9.Almeida-Cortez J. S., Shipley B., Arnason J. T. 1999. Do plant species with high relative growth rates have poorer chemical defences? Funct. Ecol. 13, 819–827 10.1046/j.1365-2435.1999.00383.x (doi:10.1046/j.1365-2435.1999.00383.x) [DOI] [Google Scholar]
- 10.Almeida-Cortez J. S., Shipley W. 2002. No significant relationship exists between seedling relative growth rate under nutrient limitation and potential tissue toxicity. Funct. Ecol. 16, 122–127 10.1046/j.0269-8463.2001.00598.x (doi:10.1046/j.0269-8463.2001.00598.x) [DOI] [Google Scholar]
- 11.Paul-Victor C., Züst T., Rees M., Kliebenstein D. J., Turnbull L. A. 2010. A new method for measuring relative growth rate can uncover the costs of defensive compounds in Arabidopsis thaliana. New Phytol. 187, 1102–1111 10.1111/j.1469-8137.2010.03325.x (doi:10.1111/j.1469-8137.2010.03325.x) [DOI] [PubMed] [Google Scholar]
- 12.Tian D., Traw M. B., Chen J. Q., Kreitman M., Bergelson J. 2003. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423, 74–77 10.1038/nature01588 (doi:10.1038/nature01588) [DOI] [PubMed] [Google Scholar]
- 13.Mauricio R. 1998. Costs of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana. Am. Nat. 151, 20–28 10.1086/286099 (doi:10.1086/286099) [DOI] [PubMed] [Google Scholar]
- 14.Mauricio R., Rausher M. D. 1997. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 51, 1435–1444 10.2307/2411196 (doi:10.2307/2411196) [DOI] [PubMed] [Google Scholar]
- 15.Fahey J. W., Zalcmann A. T., Talalay P. 2001. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56, 5–51 10.1016/S0031-9422(00)00316-2 (doi:10.1016/S0031-9422(00)00316-2) [DOI] [PubMed] [Google Scholar]
- 16.Mithen R., Raybould A. F., Giamoustaris A. 1995. Divergent selection for secondary metabolites between wild populations of Brassica oleracea and its implications for plant–herbivore interactions. Heredity 75, 472–484 10.1038/hdy.1995.164 (doi:10.1038/hdy.1995.164) [DOI] [Google Scholar]
- 17.Newton E. L., Bullock J. M., Hodgson D. J. 2009. Glucosinolate polymorphism in wild cabbage (Brassica oleracea) influences the structure of herbivore communities. Oecologia 160, 63–76 10.1007/s00442-009-1281-5 (doi:10.1007/s00442-009-1281-5) [DOI] [PubMed] [Google Scholar]
- 18.Brown P. D., Tokuhisa J. G., Reichelt M., Gershenzon J. 2003. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62, 471–481 10.1016/S0031-9422(02)00549-6 (doi:10.1016/S0031-9422(02)00549-6) [DOI] [PubMed] [Google Scholar]
- 19.Burow M., Müller R., Gershenzon J., Wittstock U. 2006. Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J. Chem. Ecol. 32, 2333–2349 10.1007/s10886-006-9149-1 (doi:10.1007/s10886-006-9149-1) [DOI] [PubMed] [Google Scholar]
- 20.Lankau R. A. 2007. Specialist and generalist herbivores exert opposing selection on a chemical defense. New Phytol. 175, 176–184 10.1111/j.1469-8137.2007.02090x (doi:10.1111/j.1469-8137.2007.02090x) [DOI] [PubMed] [Google Scholar]
- 21.Beekwilder J., et al. 2008. The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis. 3, e2068. 10.1371/journal.pone.0002068 (doi:10.1371/journal.pone.0002068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlaeppi K., Bodenhausen N., Buchala A., Mauch F., Reymond P. 2008. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J. 55, 774–786 10.1111/j.1365-313X.2008.03545.x (doi:10.1111/j.1365-313X.2008.03545.x) [DOI] [PubMed] [Google Scholar]
- 23.Kim J. H., Jander G. 2007. Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J. 49, 1008–1019 10.1111/j.1365-313X.2006.03019.x (doi:10.1111/j.1365-313X.2006.03019.x) [DOI] [PubMed] [Google Scholar]
- 24.Newton E., Bullock J. M., Hodgson D. 2009. Bottom-up effects of glucosinolate variation on aphid colony dynamics in wild cabbage populations. Ecol. Entomol. 34, 614–623 10.1111/j.1365-2311.2009.01111.x (doi:10.1111/j.1365-2311.2009.01111.x) [DOI] [Google Scholar]
- 25.Rose K. E., Atkinson R. L., Turnbull L. A., Rees M. 2009. The costs and benefits of fast living. Ecol. Lett. 12, 1379–1384 10.1111/j.1461-0248.2009.01394.x (doi:10.1111/j.1461-0248.2009.01394.x) [DOI] [PubMed] [Google Scholar]
- 26.Fakheran S., Paul-Victor C., Heichinger C., Schmid B., Grossniklaus U., Turnbull L. A. 2010. Adaptation and extinction in experimentally fragmented landscapes. Proc. Natl Acad. Sci. USA 107, 19 120–19 125 10.1073/pnas.1010846107 (doi:10.1073/pnas.1010846107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esch J. J., Oppenheimer D. G., Marks M. D. 1994. Characterization of a weak allele of the GL1 gene of Arabidopsis thaliana. Plant Mol. Biol. 24, 203–207 10.1007/BF00040586 (doi:10.1007/BF00040586) [DOI] [PubMed] [Google Scholar]
- 28.Sønderby I. E., Hansen B. G., Bjarnholt N., Ticconi C., Halkier B. A., Kliebenstein D. J. 2007. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS ONE 2, e1322. 10.1371/journal.pone.0001322 (doi:10.1371/journal.pone.0001322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y. D., Hull A. K., Gupta N. R., Goss K. A., Alonso J., Ecker J. R., Normanly J., Chory J., Celenza J. L. 2002. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 16, 3100–3112 10.1101/gad.1035402 (doi:10.1101/gad.1035402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitsuda N., Iwase A., Yamamoto H., Yoshida M., Seki M., Shinozaki K., Ohme-Takagi M. 2007. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 19, 270–280 10.1105/tpc.106.047043 (doi:10.1105/tpc.106.047043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anzi C., Pelucchi P., Vazzola V., Murgia I., Gomarasca S., Piccoli M. B., Morandini P. 2008. The proton pump interactor (Ppi) gene family of Arabidopsis thaliana: expression pattern of Ppi1 and characterisation of knockout mutants for Ppi1 and 2. 10, 237–249 10.1111/j.1438-8677.2007.00022.x (doi:10.1111/j.1438-8677.2007.00022.x) [DOI] [PubMed] [Google Scholar]
- 32.Kliebenstein D. J., Kroymann J., Brown P., Figuth A., Pedersen D., Gershenzon J., Mitchell-Olds T. 2001. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 126, 811–825 10.1104/pp.126.2.811 (doi:10.1104/pp.126.2.811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 34.Lande R., Engen S., Sæther E. 2003. Stochastic population dynamics in ecology and conservation. Oxford, UK: Oxford University Press [Google Scholar]
- 35.Bolker B. M. 2008. Ecological models and data in R. Princeton, NJ: Princeton University Press [Google Scholar]
- 36.Tonsor S. J. 1989. Relatedness and intraspecific competition in Plantago lanceolata. Am. Nat. 134, 897–906 10.1086/285020 (doi:10.1086/285020) [DOI] [Google Scholar]
- 37.Masclaux F., Hammond R. L., Meunier J., Gouhier-Darimont C., Keller L., Reymond P. 2010. Competitive ability not kinship affects growth of Arabidopsis thaliana accessions. New Phytol. 185, 322–331 10.1111/j.1469-8137.2009.03057.x (doi:10.1111/j.1469-8137.2009.03057.x) [DOI] [PubMed] [Google Scholar]
- 38.Sønderby I. E., Burow M., Rowe H. C., Kliebenstein D. J., Halkier B. A. 2010. A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiol. 153, 348–363 10.1104/pp.109.149286 (doi:10.1104/pp.109.149286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kliebenstein D. J., Rowe H. C., Denby K. J. 2005. Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J. 44, 25–36 10.1111/j.1365-313X.2005.02508.x (doi:10.1111/j.1365-313X.2005.02508.x) [DOI] [PubMed] [Google Scholar]
- 40.Rhoades D. F. 1979. Evolution of plant chemical defense against herbivores. In Herbivores: their interaction with secondary metabolites (eds Rosenthal G. A., Janzen D. H.), pp. 3–54 New York, NY: Academic Press [Google Scholar]
- 41.Hautier Y., Hector A., Vojtech E., Purves D., Turnbull L. A. 2010. Modelling the growth of parasitic plants. J. Ecol. 98, 857–866 10.1111/j.1365-2745.2010.01657.x (doi:10.1111/j.1365-2745.2010.01657.x) [DOI] [Google Scholar]
- 42.Gigolashvili T., Berger B., Mock H. P., Müller C., Weisshaar B., Flügge U. I. 2007. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 50, 886–901 10.1111/j.1365-313X.2007.03099.x (doi:10.1111/j.1365-313X.2007.03099.x) [DOI] [PubMed] [Google Scholar]
- 43.Mewis I., Tokuhisa J. G., Schultz J. C., Appel H. M., Ulrichs C., Gershenzon J. 2006. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry 67, 2450–2462 10.1016/j.phytochem.2006.09.004 (doi:10.1016/j.phytochem.2006.09.004) [DOI] [PubMed] [Google Scholar]
- 44.Kim J. H., Lee B. W., Schroeder F. C., Jander G. 2008. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J. 54, 1015–1026 10.1111/j.1365-313X.2008.03476.x (doi:10.1111/j.1365-313X.2008.03476.x) [DOI] [PubMed] [Google Scholar]