Abstract
Ecological factors are known to cause evolutionary diversification. Recent work has shown that evolution in strongly interacting predator species has reciprocal impacts on ecosystems. These divergent impacts of predators may alter the selective landscape and cause the evolution of prey. Yet, this link between intraspecific variation and evolution is unexplored. We compared the life history of a species of zooplankton (Daphnia ambigua) from lakes in New England in which the dominant planktivorous predator, the alewife (Alosa pseudoharengus), differs in feeding traits and migratory behaviour. Anadromous alewife (seasonal migrants) exhibit larger gapes, gill-raker spacing and target larger prey than landlocked alewife (year-round freshwater resident). In ‘anadromous’ lakes, Daphnia are abundant in the spring but extirpated by alewife predation in summer. Daphnia are rare year-round in ‘landlocked’ lakes. We show that Daphnia from lakes with anadromous alewife grew faster, matured earlier but at the same size and produced more offspring than Daphnia from lakes with landlocked or no alewife across multiple temperature and resource treatments. Our results are inconsistent with a response to size-selective predation but are better explained as an adaptation to colder temperatures and shorter periods of development (countergradient variation) mediated by seasonal alewife predation.
Keywords: life-history evolution, local adaptation, temperature, countergradient variation
1. Introduction
It is well established that ecological factors cause evolutionary change often on contemporary timescales [1,2]. Recent work has shown that the converse is also true; evolutionary diversification in strongly interacting species, especially predators, can have pronounced impacts on communities and ecosystems [3–9]. The divergent effects of intraspecific variation in predators have the potential to drive evolution in their prey. It is known that the presence and absence of predators cause prey evolution [10,11], and additional work has demonstrated that coevolutionary predator–prey interactions vary across landscapes [12], but the link between intraspecific variation in predators and divergence in prey is far less known.
Alewife (Alosa pseudoharengus) and Daphnia sp. are keystone species in lakes. The alewife is a fish known for structuring the zooplankton community, including the presence or absence of Daphnia, in lakes across eastern North America [5,13]. Daphnia sp. are the dominant grazers on phytoplankton in most lakes and are a critical link for the transfer of nutrients to upper trophic levels [14–16]. The presence or absence of connections with the coastal ocean lead to two genetically distinct populations of alewives [17]: (i) lakes with permanent populations of landlocked alewives, and (ii) lakes with anadromous alewives that migrate seasonally between marine and freshwater environments. Alewives can become landlocked owing to natural causes or human introductions, and previous work showed that landlocked alewife diverged neutrally from a common anadromous ancestor as recently as 300 years ago [17]. More importantly, the migratory differences between landlocked and anadromous alewife promote a dynamic interaction between alewife duration of residence in freshwater, Daphnia abundances, and alewife morphology, which may ultimately shape evolution in Daphnia.
Adult anadromous alewives migrate into lakes to spawn during the spring (March–May), and young-of-the-year (YOY) alewives migrate from these lakes each autumn, which leads to a duration of residence of five to six months per year versus a year-round presence of landlocked alewives. Daphnia are thus highly abundant each spring in anadromous lakes, but are quickly extirpated by intense predation by YOY alewife in early summer and are absent from these lakes until populations re-establish from resting eggs the following winter (electronic supplementary material, figure S1) [5]. In landlocked lakes, year-round predation maintains a low biomass of Daphnia and a community dominated by small-bodied zooplankton (electronic supplementary material, figure S1) [5,13,18]. This constant exposure of landlocked alewives to predominantly small zooplankton has helped facilitate divergence in alewife foraging traits; landlocked alewives have smaller gape and narrower spacing between gill rakers than anadromous populations [5,17,18]. There are also correlated differences in prey selectivity as anadromous alewife target large zooplankton, while landlocked alewives are not size-selective [18]. Thus, differences in migratory behaviour facilitate contrasting zooplankton dynamics, which then feeds back on alewife feeding traits, although the extent to which changes in alewife traits are the result of evolutionary divergence, plasticity or maternal effects is unclear [19].
Here, we test the hypothesis that intraspecific variation in alewives moulds evolution in Daphnia. Variation in alewife feeding traits provides one potential mechanism of evolution. Age-/size-specific life-history theory predicts that: (i) increased predation on large size-classes of individuals favours the evolution of earlier maturation, a smaller size at maturation and increased reproductive effort (e.g. anadromous lakes) [20–25]; (ii) increased predation on small size-classes favours the opposite trends; and (iii) no evolutionary change will occur when predation uniformly increases mortality rates (e.g. landlocked lakes) [20–25]. However, predictions can depend upon specific model assumptions and whether impacts owing to density are considered [22–24,26]. For instance, a uniform increase in mortality can favour increased reproductive effort if density dependence impacts juveniles more than adults ([23], see also [24,26]).
An interaction between the duration of residence of alewives and the seasonality of lakes provides an additional link to evolution. New England lakes in the spring are colder and less productive than these same lakes during the summer and Daphnia from anadromous lakes are only present in lakes during these cold, low-resource conditions (electronic supplementary material, figure S1). This is important because variation in temperature and resources can exert selection on the evolution of life histories [11,27–29]. One possibility is that organisms will adapt to the temperature or resource level most frequently experienced [30,31]. In an environment characterized by low temperatures and productivity, reaction norms are shifted to a lower range of temperatures (or resources). Consequently, growth/development is increased at low temperatures, but reduced at higher temperatures (or resources). Alternatively, organisms may evolve to offset the negative (phenotypic) impacts of temperature on growth and development by shifting reaction norms vertically, such that individuals from a ‘colder environment’ evolve higher rates of growth or development irrespective of ambient temperatures when compared with individuals from a ‘warmer environment’ [32,33]. In this latter model, the end result is that genotypes are spatially distributed such that genetic and environmental influences are in opposition, and this phenomenon is thus called ‘countergradient variation’ [34].
We quantified genetic differences in life histories of Daphnia ambigua from four ‘landlocked’ lakes, three ‘anadromous’ lakes and four lakes without alewives (‘no alewife’) after three generations of common garden rearing. We used lakes that are located in eastern Connecticut and are all within a close physical proximity to one another (approx. tens of kilometres apart) (electronic supplementary material, table S1). To evaluate responses to spring versus summer conditions, our experiment included two-resource and two-temperature treatments, arrayed in a factorial design, which mimic the differences between spring and summer (electronic supplementary material, figure S1). Because anadromous alewife have larger gapes and gill-raker spacing and target large prey, we predict that Daphnia from anadromous lakes will exhibit earlier maturation, a smaller size at maturation and increased reproductive investment than Daphnia from landlocked or no-alewife lakes [20–25]. It is worth noting that uniform increases in mortality rates owing to predation by landlocked alewives could also favour earlier maturation and increased reproductive effort [23,26]. Furthermore, if Daphnia are adapted to the temperature and/or resource level they most commonly experience in nature, then we predict that the trait variation between lakes will be a function of contrasting food and/or temperature treatments [30,31]. Alternatively, if Daphnia evolve a countergradient response to the negative effect of temperature, then we would expect increased growth and/or development in individuals from anadromous lakes across all treatments [32,33].
2. Material and methods
(a). Study lakes
We examined evolutionary divergence in D. ambigua from 11 lakes in Connecticut. Our lakes included (see [5] for map): three anadromous lakes (Bride, Dodge, Gorton), four landlocked lakes (Amos, Long, Quonnipaug, Rogers) and four no-alewife lakes (Black, Gardner, Hayward, Linsley). The anadromous lakes are the only lakes with long-term anadromous populations within the same geographical region of the other lakes. We evaluated our lakes for systematic variation in lake characteristics and show that there are no significant differences in size, depth or productivity (electronic supplementary material, table S1). Data on a subset of our lakes revealed no differences in alewife biomass, although there is a trend towards an increase in the abundance of anadromous alewife (electronic supplementary material, table S1). Previous work showed that landlocked and anadromous lakes contain similar fish communities, with largemouth bass (Micropterus salmoides) and chain pickerel (Esox niger) as top predators, and bluegill (Lepomis macrochirus), yellow perch (Perca flavescens), golden shiners (Notemigonus crysoleucas) and pumpkinseed (Lepomis gibbosus), the most common non-alewife zooplanktivorous fishes [18]. The incidence of non-alewife planktivorous fishes is similar in landlocked and anadromous lakes but non-alewife zooplanktivores are more abundant in landlocked than in anadromous lakes [18]. Chaoborus sp., an invertebrate predator, is more abundant in landlocked than in anadromous lakes (electronic supplementary material, table S1).
(b). Common garden experiment
In August to September 2009, sediment samples were collected from areas of greater than 10 m water depth (when possible), using an Ekman grab. Distinct genotypes were established by hatching D. ambigua ephippia from sediment. At least 14 clones were established per lake. For the first generation, each clone was represented by a single post-ephippial female that was reared under common conditions (14 L : 10 D cycle, 25°C) in 90 ml jars containing COMBO medium [35] and fed non-limiting quantities of Scenedesmus obliquus (concentration: 0.6–1.0 mg C l−1 d−1). Media and algae were changed every other day. For the second laboratory generation, two to three neonates taken from the third clutch of each clone were reared under the same conditions (i.e. size of container, food concentration) as the previous generation.
Our common garden experiment compared the life histories of third-generation, laboratory-reared clones of D. ambigua from all focal lakes. To explore responses to contrasting ecological conditions observed between the spring and summer, this experiment used two resource levels and two temperatures. We used a ‘low-temperature’ treatment of 12°C that matches the water temperature observed during March and April in our lakes and a ‘high temperature’ of 25°C, which approximates peak summer water temperatures (electronic supplementary material, figure S1). Between spring and summer, there is an approximate threefold increase in algal biovolume, and we thus used ‘high’ and ‘low’ algal concentrations that mimicked these differences. We converted biovolume to carbon using established formula [36], and used concentrations of 0.6 and 0.2 mg C l−1 d−1 for our high- and low-food treatments, respectively.
The experiment was initiated by collecting eight neonates (approx. 12 h old) per clone from the second and subsequent clutches of the second-generation, laboratory-reared Daphnia. These neonates were photographed using Leima IM for measurement of length and area using ImageJ [37] and individually pipetted into 90 ml jars containing COMBO medium [35]. Each individual was assigned to one of the following treatments: (i) high food, high temperature, (ii) low food, high temperature, (iii) high food, low temperature, or (iv) low food, low temperature. Each clone was replicated twice per treatment and the experiment consisted of 14 clones per lake. The experimental conditions were the same as the prior generations and media and algae were replaced every other day. Pre-reproductive mortality was low and not biased towards any lake type (mortality rates: anadromous = 2.4%, landlocked = 2.5%, no alewife = 3.1%).
We compared rates of size-specific juvenile growth, age at maturation (release of the first clutch into the brood chamber), size at maturation, the number of embryos in clutches 1–4, interclutch interval and offspring size among lake types. Juvenile growth was measured by photographing all individuals on day 1 and day 4 and then converting this measurement to growth rate via: [ln(length on day 4) − ln(length on day 1)]/no. of days. Age at maturation was estimated by monitoring all Daphnia throughout development. Beginning on day 3, Daphnia on high-temperature treatments were examined for maturation every 3 h (between approx. 8.00 and 20.00). When the release of the first clutch was confirmed, age at maturation was recorded and each individual was photographed for estimates of size and fecundity. Since the low-temperature treatments matured at a slower rate, they were monitored less frequently; Daphnia reared at 12°C were evaluated for maturation starting on day 6, twice daily. After maturation, all individuals were examined every day for the production of clutches 2–4.
(c). Statistical analyses
Dependent variables were analysed with linear mixed models (SAS v. 9.1, Sas Institute, Cary, NC, USA) using restricted maximum-likelihood estimation. Lake type, temperature, resource level and all interactions were entered as fixed effects. Replicate lake populations nested within lake type and clone nested within lake were entered as random effects. We used between–within subjects degrees of freedom in all tests of fixed effects with the random effect of lake nested within lake type used as the subject. This method partitions residual degrees of freedom to between- and within-subject degrees of freedom to avoid overinflating degrees of freedom for the between-lake-type effect. The likelihood ratio test was employed for tests of significance of the random effects [38]. All dependent variables were either log-transformed (growth, age/size at maturation, interclutch interval, offspring size) or square-root-transformed (fecundity) to improve homoscedasticity and fits with normality. We first analysed the data using a multivariate analysis (linear mixed model) that included all dependent variables and subsequently analysed each dependent variable separately thereafter. When significant lake-type effects were observed for the univariate analyses, we evaluated the nature of the differences among lake types with post hoc Tukey tests.
(d). Discriminant function analysis
We used a discriminant function analysis to determine how well lakes can be separated and classified. The patterns of separation for juvenile growth, age at maturation, clutch size and interclutch interval were evaluated using lake type as a grouping variable. All trait values were based upon the least-square mean for each lake and all variables were standardized by subtracting the mean and dividing by the standard deviation. Significant differences among lakes were examined by quantifying Mahalanobis distances among centroids. Since the above analysis does not control for variation in temperature, we also evaluated how well lakes can be classified by performing separate discriminant analyses at each experimental temperature.
(e). Intrinsic rate of increase
We combined estimates of age at maturation, clutch size and interclutch interval to calculate intrinsic rates (r) for each lake at 12°C and 25°C [39]. We calculated r as: r = ln(R0)/G, where R0 is the net reproductive rate (summation of fecundity × survivorship) and G is generation time (average age of the parents of all offspring produced by a single cohort). Differences in r were evaluated using a linear mixed model, with lake type and temperature entered as fixed effects and lake (nested within lake type) entered as a random effect. We then simulated changes in population size at 12°C over a two month period using the formula: Nt = N0ert (Nt is the population size at time t, N0 is the initial population size, r is the intrinsic rate of increase and t is time in days).
3. Results
The results of a multivariate analysis revealed significant effects of lake type (F2,8 = 7.74, p = 0.014), temperature (F1,8 = 842.7, p < 0.001), resource level (F1,8 = 105.5, p < 0.001) and a significant interaction between the temperature and resource level (F1,8 = 35.7, p = 0.0003). We subsequently evaluated trends in the results with univariate analyses.
(a). Lake-type effects
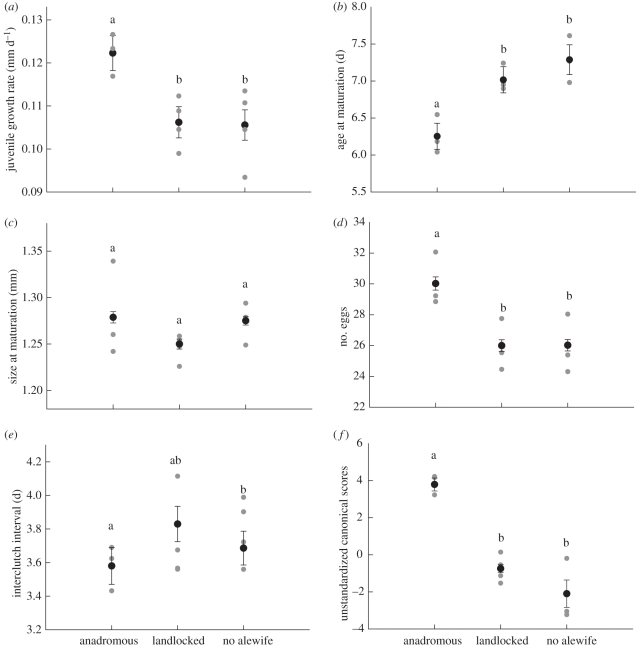
We observed significant (p < 0.05) life-history differences among lake types; Daphnia from lakes with anadromous alewife grew 16 per cent faster, matured 16 per cent earlier and produced larger clutches (16% increase) than Daphnia from either no alewife or landlocked lakes (figure 1, table 1 and electronic supplementary material, table S2). Post hoc Tukey tests revealed significant differences between Daphnia from anadromous and landlocked lakes for all three traits. This combination of faster growth and earlier maturation in Daphnia from anadromous lakes is correlated with small, non-significant differences in size at maturation (table 1 and figure 1). Daphnia from anadromous lakes produced clutches that developed at a rate that was 7 per cent faster than the other lake types, although these differences were not significant (table 1). However, removal of the non-significant lake effect revealed marginally significant differences among lake types for interclutch interval (F2,151 = 2.97, p = 0.054). There were minor, non-significant differences in offspring size (mean length in mm ± 1 s.e.: anadromous = 0.607 ± 0.003, landlocked = 0.611 ± 0.003, no alewife = 0.623 ± 0.003; table 1).
Figure 1.
Life-history differences among lakes. (a) juvenile growth, (b) age at maturation, (c) size at maturation, (d) clutch size, (e) interclutch interval, and (f) unstandardized scores from the discriminant function analysis. Closed circles, lake-type means; grey circles, individual lake means. Letters denote significant differences among lake types based upon post hoc Tukey tests. Error = ±1 s.e.
Table 1.
Analyses of life-history traits. (Linear mixed models were used, with lake type, temperature and resource level entered as fixed effects and lake (nested within lake type) and clone (nested within lake) entered as random effects. Entries for the fixed effects are F statistics, while entries for random effects are Wald Z-values from a likelihood ratio test. The denominator degrees of freedom for all traits was 8.) NSp > 0.1; † 0.05 < p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001.
| factor | no. d.f. | juvenile growth | age at maturation | size at maturation | no. of eggs | interclutch interval | offspring size |
|---|---|---|---|---|---|---|---|
| fixed effects | F | ||||||
| lake type | 2 | 10.94** | 10.08** | 1.59NS | 7.33* | 1.12NS | 1.06NS |
| temperature | 1 | 2334.0*** | 7193.7*** | 10.1* | 56.53*** | 8643.7*** | 65.5*** |
| resource | 1 | 54.5*** | 66.62*** | 19.5** | 155.32*** | 12.73** | 16.1** |
| lake type × temperature | 2 | 1.03NS | 3.85† | 0.31NS | 0.15NS | 1.12NS | 2.56NS |
| lake type × resource | 2 | 2.5NS | 0.55NS | 0.69NS | 1.21NS | 0.2NS | 0.1NS |
| temperature × resource | 1 | 51.48*** | 23.1** | 7.9† | 53.03*** | 4.67† | 0.79NS |
| lake type × temperature × resource | 2 | 0.17NS | 1.77NS | 0.97NS | 0.42NS | 1.24NS | 0.59NS |
| random effects | Wald Z | ||||||
| lake (lake type) | 1 | 1.1NS | 0.77NS | 1.26NS | 0.95NS | 1.32† | 1.4† |
| clone (lake) | 1 | 6.48*** | 4.99*** | 6.58*** | 6.2*** | 2.14* | 5.08*** |
(b). Food effects
There was a significant effect of food level on the expression of all life-history traits (table 1). Lower food levels resulted in a 15 per cent decline in juvenile growth, a 9 per cent increase in age at maturation, a 14 per cent decline in clutch size, a 5 per cent increase in interclutch interval and a 2 per cent increase in offspring size (electronic supplementary material, table S2 and figure S2).
(c). Temperature effects
Rearing temperature significantly influenced all life-history traits (table 1). Daphnia exhibited a rate of growth that was 65 per cent faster at high temperatures and this was correlated with a 65 per cent reduction in the timing of maturation (table 1). Clutch size and interclutch interval was reduced by 10 and 67 per cent at high temperatures, respectively (electronic supplementary material, table S2 and figure S2).
(d). Statistical interactions
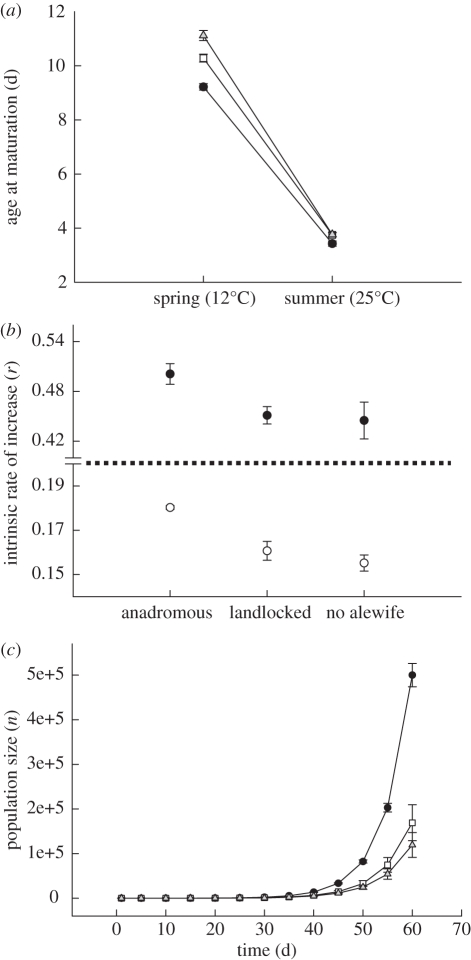
The interaction between lake type and temperature for age at maturation was marginally significant (table 1 and figure 2a). We observed small differences (less than 1 day) in the timing at maturation among lakes at 25°C, but Daphnia from anadromous lakes matured 1–2 days earlier than landlocked or no alewife lakes at 12°C.
Figure 2.
Daphnia age at maturation and population growth rate as a function of temperature. (a) Age at maturation. The lake type × temperature interaction was marginally non-significant (F2,8 = 3.85, p = 0.068). Closed circles, anadromous; open squares, landlocked; grey triangles, no alewife. (b) Intrinsic rates of increase (r) at 12°C and 25°C. Closed circles, 25°C; open circles, 12°C. (c) Changes in population size at 12°C over a two month period that approximates the typical period of population growth in anadromous lakes. Closed circles, anadromous; open squares, landlocked; grey triangles, no alewife. Error = ±1 s.e.
We obtained significant interactions between food and temperature for growth, age at maturation and clutch size (table 1; bottom of electronic supplementary material, table S2). The differences in trait values between temperatures were reduced under low-food conditions for growth and age at maturation, but the trend was opposite for fecundity.
(e). Discriminant function analysis
We first performed a discriminant function analysis based upon the lake mean for each trait. The first component distinguished among lake types (figure 1f) as two of 11 lakes were misclassified. The first discriminant function accounted for 97.2 per cent of the variance, and the factor structure coefficients demonstrate that this function differentiates between Daphnia characterized by fast growth, early maturation, high fecundity and fast offspring development and those that exhibit slow growth, late maturation, low fecundity and slow offspring development (figure 1f and electronic supplementary material, table S3). The second discriminant function accounted for 2.8 per cent of the variance. An analysis of the centroid distances demonstrated that Daphnia from anadromous lakes were significantly different from Daphnia from no alewife lakes (F4,5 = 9.7, p = 0.014) and landlocked lakes (F4,5 = 5.9, p = 0.039). Daphnia from landlocked and no alewife lakes did not differ significantly (F4,5 = 0.87, p = 0.54).
The above analysis did not control for variation in the temperature or resource level. Yet, none of our temperature or food by lake-type interactions was significant at the 0.05 level. Such a result indicates that we would not expect classification frequencies to be altered by a consideration of temperature or food. However, we performed two additional analyses to determine how well the lakes can be classified at each experimental temperature. Classification frequencies were similar at each temperature; at 12°C, all lakes were correctly classified and at 25°C, 75 per cent of the lakes were correctly classified.
(f). Intrinsic rate of increase
We observed significant differences in the rate of intrinsic increase (r) among lakes (linear mixed model: F2,8 = 4.63, p = 0.046) as Daphnia from lakes with anadromous alewife exhibited an r that was 14 and 12 per cent higher than Daphnia from landlocked or no alewife lakes at 12°C and 25°C, respectively (figure 2b; lake type × temperature: F2,8 = 1.33, p = 0.32). Our simulation of changes in population size at 12°C showed that Daphnia from lakes with anadromous alewife attained a population size that was threefold larger than Daphnia from the other lakes after 60 days (figure 2c).
4. Discussion
Our results revealed strong genetic differences in life-history traits of Daphnia between lakes with contrasting alewife phenotypes. Daphnia from lakes with anadromous alewife matured significantly (p < 0.05) earlier, grew faster and thereby differed little in size at maturation than Daphnia from landlocked and no alewife lakes (figure 1). They also produced larger clutches that developed faster (figure 1). This combination of earlier maturation, increased fecundity and shorter interclutch interval in Daphnia from anadromous lakes facilitates significantly higher intrinsic rates of increase (figure 2b). Growth rate simulations show that Daphnia from anadromous lakes attain a population size that is approximately three times larger than those observed in landlocked and no alewife lakes during the spring (figure 2c). These changes in Daphnia help to explain why Daphnia naturally reach densities in the spring in anadromous lakes that are as great as or greater than that witnessed in summer in lakes without alewife [5].
Evolutionary divergence in Daphnia is not consistent with a response to variation in alewife morphology and prey selectivity. Anadromous alewife target large prey and age-/size-specific life-history theory predicts that increased predation on large size-classes selects for earlier maturation, a smaller size at maturation and increased reproductive effort [20–25]. There are many examples consistent with these predictions in nature (e.g. [10,11,40]). In our study, Daphnia from anadromous lakes matured earlier and exhibited increased reproductive investment than Daphnia from landlocked lakes, which is consistent with predator-mediated divergence. Yet, there were no significant differences in size at maturation among lakes and Daphnia from anadromous lakes exhibited a slightly larger size at maturation than Daphnia from landlocked lakes (table 1 and figure 1). Thus, variation in size-specific predation between lakes is unlikely to explain divergence in Daphnia.
Ecological differences that are correlated with the presence of anadromous alewife represent an unlikely causal mechanism of Daphnia evolution. Our lakes are located just tens of kilometres apart, and do not differ significantly in size, depth, productivity or alewife biomass (electronic supplementary material, table S1). Previous inquiry did observe a higher abundance of non-alewife zooplanktivores in landlocked lakes [18]. Such differences have the potential to increase predation on Daphnia in landlocked lakes. Also, since these species of fish are not gape-limited, their increased abundance is unlikely to favour delayed maturation and decreased reproductive effort, although specific predictions depend upon model assumptions [20–25]. Invertebrate predators, such as Chaoborus, can also impose selection on Daphnia. A selection experiment showed that Chaoborus predation selected for earlier maturation, increased fecundity and a larger body size in Daphnia [41]. Yet, Chaoborus are more abundant in landlocked and no alewife lakes than in anadromous lakes (electronic supplementary material, table S1), providing little insight into our results.
Our experiment revealed limited evidence that Daphnia from anadromous lakes are adapted to the temperature or resource level they most frequently experience. No lake type-by-treatment interactions were significant and Daphnia from anadromous lakes exhibited higher rates of population growth than Daphnia from the other lakes at both experimental temperatures (figure 2b). However, adaptation to a specific temperature is not the only mechanism by which contrasting thermal regimes can influence adaptation.
Countergradient variation is an alternative model of thermal adaptation commonly observed in organisms that are located across latitudinal or altitudinal gradients (reviewed in [32,33]). To compensate for a colder environment and a shorter growing season at higher latitudes or altitudes, many organisms have evolved intrinsically higher rates of growth or development. Such changes in individuals from a colder environment are apparently irrespective of ambient temperature and are thus not context specific. The underlying cause of countergradient variation is often hypothesized to be time constraints and not temperature per se. This is because organisms frequently need to attain a size large enough to survive winter or complete development [32,33]. Though, impacts of temperature and seasonality are not mutually exclusive as these forces can act synergistically or in opposition [42,43].
Countergradient variation may apply to evolution in Daphnia. Daphnia from anadromous lakes are only present in lakes during the spring (approx. April–June) because they are eliminated by intense alewife predation each summer (electronic supplementary material, figure S1). Consequently, Daphnia from anadromous lakes develop, on average, under colder conditions and experience a shorter growing season (the duration in which Daphnia are present in lakes) than Daphnia from lakes with landlocked or no alewives. Our results show that Daphnia from anadromous lakes exhibited faster rates of growth and development and produced larger clutches than Daphnia from the other lake types across two temperatures that mimicked naturally occurring differences in water temperature between the spring and summer months (electronic supplementary material, figure S1). This divergence mimics many studies of countergradient variation [32,33] and may represent an adaptation to a colder environment and/or a shorter growing season. We propose that cold water temperatures and the shorter period of development in lakes with anadromous alewife favour rapid growth and early maturation. Daphnia clones that develop rapidly and produce lots of offspring prior to the arrival of YOY anadromous alewife will differentially contribute to the egg bank in lake sediment. Support for this conclusion comes from the observation that production of resting eggs by Daphnia, which signals a deteriorating environment, peaks in late spring in anadromous lakes [44]; a period of otherwise favourable conditions for reproduction. Also, since selection acts primarily on growth or development, divergence in size is not necessarily expected as a countergradient response to temperature or growing season (as in the current study).
There is also reason to expect that temperature per se could impact life-history evolution in Daphnia. Laboratory selection experiments that manipulated temperature have yielded results that mimic the life-history differences observed in Daphnia between anadromous lakes and the other lakes. Populations of Drosophila that were reared under a cold temperature for multiple generations evolved faster rates of growth and development than the warm-temperature lines ([27,28], but see [45]). This is important because Daphia from anadromous lakes experience an environment that is, on average, colder than Daphnia from the other lake types and may thus experience similar temperature-mediated selection. It has also been hypothesized that organisms with long development times relative to season length are more likely to experience time constraints than organisms with rapid development and multiple generations per year, such as Daphnia [42,46]. In our study, the maximum period from hatching until release of the first clutch was 16 days, while the period from ice-out until the onset of anadromous alewife predation is at least 60 days. Such a difference may relax any selection associated with seasonal population cycles and thus indicates temperature as the more likely mechanism of divergence in Daphnia. Regardless of the mechanism, countergradient variation provides the best fit between theory and evolution in Daphnia.
One question that arises naturally is: since Daphnia in landlocked and no alewife lakes develop, in part, during the spring, then why is there no evidence for countergradient selection in these populations? One reason is because there is no growing season effect in either landlocked or no alewife lakes. Alewives and non alewife planktivorous fishes are always present in landlocked and no alewife lakes. Second, there are differences in water temperature among lakes from the perspective of Daphnia. Daphnia from anadromous lakes only experience the cold spring conditions and are absent from these lakes in the summer and autumn. By contrast, Daphnia in landlocked and no alewife lakes are present throughout the spring, summer and autumn and experience similar seasonal temperature fluctuations. There could be temperature-mediated selection on clones during the spring; however, any such selection will probably be balanced by selection on clones that are reproducing during the summer. For these reasons, we would not expect there to be evidence of countergradient variation in Daphnia from landlocked or no alewife lakes, nor would we expect countergradient selection to drive diversification between Daphnia from these lakes.
(a). Life-history trade-offs?
Our results show that Daphnia from anadromous lakes exhibited faster growth, earlier maturation, increased fecundity and comparable size at maturation compared with Daphnia from landlocked or no alewife lakes. Thus, there was no apparent cost associated with adaptation to an environment with anadromous alewife. If evolution in Daphnia from anadromous lakes is not accompanied by additional costs, then clones from anadromous lakes would eventually invade and proliferate in all habitats. Yet, the evolution of such a ‘Darwinian demon’ is unlikely because trade-offs are expected to accompany shifts in life-history traits [47]. There are two classes of trade-offs: (i) when performance in one environment trades off with performance in another environment (genotype-by-environment interaction), and (ii) when the performance of one trait trades off with the expression of another trait [33]. Our results revealed little evidence for the first class of trade-offs because fitness did not differ among lake types as a function of experimental treatments. Although, it is possible that a greater spectrum of food and temperature treatments would reveal such interactions. The only evidence for the latter class of trade-offs was the production of smaller offspring by Daphnia from anadromous lakes (electronic supplementary material, table S2), but such differences were minor. However, trade-offs can involve many traits; previous inquiry has revealed trade-offs between rapid growth/development and competitive ability [48], immune function [49,50], longevity [51], energy acquisition and allocation [52], starvation endurance [53,54], oxidative stress [55], physiological performance [56] and risk of predation [57]. An alternative explanation for the lack of apparent trade-off is that growth efficiency increases or maintenance costs decline with decreasing temperature [58].
(b). Landlocked versus no alewife lakes
Our results revealed modest life-history divergence between Daphnia from landlocked and no alewife lakes. This was unanticipated because the presence of landlocked alewives has strong negative impacts on the abundances of Daphnia [5], and much work has shown that variation in predation intensity is a potent mechanism of life-history evolution (e.g. [10,11]). There are several potential explanations for this surprising result. First, landlocked alewives may not alter size-specific mortality. Theoretical predictions depend upon the size-classes that are preyed upon. If predators uniformly elevate mortality rates, then no life-history evolution may occur ([23], but see [59]). More complex models that consider interactions between uniform increases in predation and ecological complexities such as density dependence yield scenarios where increases in predation do not cause life-history evolution [22,23,26]. Thus, a range of models indicate that increases in predation may not cause evolution.
Second, as described above, shifts in life-history traits incur costs and selection by landlocked alewives may favour changes in other components of fitness. Under the constant threat of predation in landlocked lakes, shifts in behaviour may outweigh any benefits associated with faster growth or development. Several studies have shown that Daphnia subjected to higher fish predation have evolved an increased propensity to migrate vertically towards deepwater refuges or horizontally towards littoral refuges [60–62]. These refuges offer increased safety but are presumably less productive. Increased horizontal migration may be important because landlocked alewives forage exclusively in the pelagic habitat [18].
Finally, enhanced gene flow between landlocked and no alewife lakes could mitigate divergence in Daphnia. However, our lakes are randomly scattered across the landscape, and there is no a priori reason to expect rates of gene flow to vary among lake types. Yet, this hypothesis warrants consideration.
5. Conclusions
We used phenotypic and migratory differences among populations of alewives and demonstrated that variation within a predator species can facilitate evolutionary divergence in the life history of zooplankton prey. Evolution in Daphnia is best explained as an adaptation to a truncated growing season and a colder environment (i.e. countergradient variation) that are a by-product of seasonal predation by anadromous alewives. As observed in alewives in this same system [5,7], phenotypic divergence can have potent ecological consequences. Given that Daphnia exert top-down control on phytoplankton abundance [14–16], the observed evolutionary changes in Daphnia life-history traits could have resultant ecological impacts. Such eco-evolutionary interactions across multiple species represent an important future direction.
Acknowledgements
We thank Suzanne Alonzo, Steve Stearns and Dave Strayer for use of equipment, and Jakob Brodersen, Torrance Hanley, Elizabeth Hatton, Jennifer Howeth and Andrew Jones for help in the field or laboratory. We thank Kim LaPierre and Linda Puth for providing biovolume data. Comments by three anonymous reviewers greatly improved this paper. The National Science Foundation and the Yale Institute for Biospheric Studies provided funding.
References
- 1.Hendry A. P., Kinnison M. T. 1999. The pace of modern life: measuring rates of microevolution. Evolution 53, 1637–1653 10.2307/2640428 (doi:10.2307/2640428) [DOI] [PubMed] [Google Scholar]
- 2.Reznick D. N., Ghalambor C. K. 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112–113, 183–198 10.1023/A:1013352109042 (doi:10.1023/A:1013352109042) [DOI] [PubMed] [Google Scholar]
- 3.Schweitzer J. A., Bailey J. K., Rehill B. J., Martinsen G. D., Hart S. C., Lindroth R. L., Keim P., Whitham T. G. 2004. Genetically based trait in a dominant tree affects ecosystem processes. Ecol. Lett. 7, 127–134 10.1111/j.1461-0248.2003.00562.x (doi:10.1111/j.1461-0248.2003.00562.x) [DOI] [Google Scholar]
- 4.Whitham T. G. 2006. A framework for community and ecosystem genetics: from genes to ecosystems. Nat. Rev. Genet. 7, 510–523 10.1038/nrg1877 (doi:10.1038/nrg1877) [DOI] [PubMed] [Google Scholar]
- 5.Post D. M., Palkovacs E. P., Schielke E. G., Dodson S. I. 2008. Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology 89, 2019–2032 10.1890/07-1216.1 (doi:10.1890/07-1216.1) [DOI] [PubMed] [Google Scholar]
- 6.Harmon L. J., Matthews B., Des Roches S., Chase J. M., Shurin J. B., Schluter D. 2009. Evolutionary diversification in sticklebacks affects ecosystem function. Nature 458, 1167–1170 10.1038/nature07974 (doi:10.1038/nature07974) [DOI] [PubMed] [Google Scholar]
- 7.Palkovacs E. P., Post D. M. 2009. Experimental evidence that phenotypic divergence in predators drives community divergence in prey. Ecology 90, 300–305 10.1890/08-1673.1 (doi:10.1890/08-1673.1) [DOI] [PubMed] [Google Scholar]
- 8.Palkovacs E. P., Marshall M. C., Lamphere B. A., Lynch B. R., Weese D. J., Fraser D. F., Reznick D. N., Pringle C. M., Kinnison M. T. 2009. Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Phil. Trans. R. Soc. B 364, 1617–1628 10.1098/rstb.2009.0016 (doi:10.1098/rstb.2009.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassar R. D., et al. 2010. Local adaptation in Trinidadian guppies alters ecosystem processes. Proc. Natl Acad. Sci. USA 107, 3616–3621 10.1073/pnas.0908023107 (doi:10.1073/pnas.0908023107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reznick D., Bryga H., Endler J. A. 1990. Experimentally induced life history evolution in a natural population. Nature 346, 357–359 10.1038/346357a0 (doi:10.1038/346357a0) [DOI] [Google Scholar]
- 11.Walsh M. R., Reznick D. N. 2008. Interactions between the direct and indirect effects of predators determine life history evolution in a killifish. Proc. Natl Acad. Sci. USA 105, 594–599 10.1073/pnas.0710051105 (doi:10.1073/pnas.0710051105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson J. N. 2005. The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press [Google Scholar]
- 13.Brooks J. L., Dodson S. I. 1965. Predation, body size, and the composition of plankton. Science 150, 28–35 10.1126/science.150.3692.28 (doi:10.1126/science.150.3692.28) [DOI] [PubMed] [Google Scholar]
- 14.Carpenter S. R., et al. 1987. Regulation of lake primary productivity by food web structure. Ecology 68, 1863–1876 10.2307/1939878 (doi:10.2307/1939878) [DOI] [PubMed] [Google Scholar]
- 15.Elser J. J., Elser M. M., MacKay N. A., Carpenter S. R. 1988. Zooplankton mediated transitions between N and P limited algal growth. Limnol. Ocean. 33, 1–14 10.4319/lo.1988.33.1.0001 (doi:10.4319/lo.1988.33.1.0001) [DOI] [Google Scholar]
- 16.Carpenter S. R., Cottingham K. L., Schindler D. E. 1992. Biotic feedbacks in lake phosphorus cycles. Trends Ecol. Evol. 7, 332–336 10.1016/0169-5347(92)90125-U (doi:10.1016/0169-5347(92)90125-U) [DOI] [PubMed] [Google Scholar]
- 17.Palkovacs E. P., Dion K. B., Post D. M., Cacconne A. 2008. Independent evolutionary origins of landlocked alewife populations and rapid parallel evolution of phenotypic traits. Mol. Ecol. 17, 582–597 10.1111/j.1365-294X.2007.03593.x (doi:10.1111/j.1365-294X.2007.03593.x) [DOI] [PubMed] [Google Scholar]
- 18.Palkovacs E. P., Post D. M. 2008. Eco-evolutionary interactions between predators and prey: can predator-induced changes to prey communities feed back to shape predator foraging traits? Evol. Ecol. Res. 10, 699–720 [Google Scholar]
- 19.Post D. M., Palkovacs E. P. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629–1640 10.1098/rstb.2009.0012 (doi:10.1098/rstb.2009.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadgil M., Bossert P. W. 1970. Life history consequences of natural selection. Am. Nat. 104, 1–24 10.1086/282637 (doi:10.1086/282637) [DOI] [Google Scholar]
- 21.Law R. 1979. Optimal life histories under age-specific predation. Am. Nat. 114, 399–417 10.1086/283488 (doi:10.1086/283488) [DOI] [Google Scholar]
- 22.Michod R. E. 1979. Evolution of life histories in response to age-specific mortality factors. Am. Nat. 113, 531–550 10.1086/283411 (doi:10.1086/283411) [DOI] [Google Scholar]
- 23.Charlesworth B. 1980. Evolution in age-structured populations, 1st edn. Cambridge, UK: Cambridge University Press [Google Scholar]
- 24.Gardmark A., Dieckmann U. 2006. Disparate maturation adaptations to size dependent mortality. Proc. R. Soc. B 273, 2185–2192 10.1098/rspb.2006.3562 (doi:10.1098/rspb.2006.3562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor B. E., Gabriel W. 1992. To grow or not to grow: optimal resource allocation for Daphnia. Am. Nat. 139, 248–266 10.1086/285326 (doi:10.1086/285326) [DOI] [Google Scholar]
- 26.Abrams P., Rowe L. 1996. The effects of predation on the age and size of maturity of prey. Evolution 50, 1052–1061 10.2307/2410646 (doi:10.2307/2410646) [DOI] [PubMed] [Google Scholar]
- 27.Partridge L., Barrie B., Fowler K., French V. 1994. Thermal evolution of pre-adult life history traits in Drosophila melanogaster. J. Evol. Biol. 7, 645–663 10.1046/j.1420-9101.1994.7060645.x (doi:10.1046/j.1420-9101.1994.7060645.x) [DOI] [PubMed] [Google Scholar]
- 28.Partridge L., Barrie B., Barton N. H., Fowler K., French V. 1995. Rapid laboratory evolution of adult life history traits in Drosophila melanogaster in response to temperature. Evolution 49, 538–544 10.2307/2410277 (doi:10.2307/2410277) [DOI] [PubMed] [Google Scholar]
- 29.Mueller L. D. 1997. Theoretical and empirical examination of density-dependent selection. Annu. Rev. Ecol. Evol. Syst. 28, 269–288 10.1146/annurev.ecolsys.28.1.269 (doi:10.1146/annurev.ecolsys.28.1.269) [DOI] [Google Scholar]
- 30.Stearns S. C., Koella J. C. 1986. The evolution of phenotypic plasticity in life history traits: predictions of reaction norms for age and size at maturity. Evolution 40, 893–913 10.2307/2408752 (doi:10.2307/2408752) [DOI] [PubMed] [Google Scholar]
- 31.Levinton J. S. 1983. The latitudinal compensation hypothesis: growth data and a model of latitudinal growth differentiation based upon energy budgets. I. Interspecific comparison of Ophryotrocha (Polychaeta: Dorvilleidae). Biol. Bull. 165, 686–698 10.2307/1541471 (doi:10.2307/1541471) [DOI] [PubMed] [Google Scholar]
- 32.Conover D. O., Schultz E. T. 1995. Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol. Evol. 10, 248–252 10.1016/S0169-5347(00)89081-3 (doi:10.1016/S0169-5347(00)89081-3) [DOI] [PubMed] [Google Scholar]
- 33.Conover D. O., Duffy T. A., Hice L. A. 2009. The covariance between genetic and environmental influences across ecological gradients: reassessing the evolutionary significance of countergradient and cogradient variation. The year in evolutionary biology 2009. Ann. NY Acad. Sci. 1168, 100–129 10.1111/j.1749-6632.2009.04575.x (doi:10.1111/j.1749-6632.2009.04575.x) [DOI] [PubMed] [Google Scholar]
- 34.Levins R. 1969. Termal acclimation and heat resistance in Drosophila species. Am. Nat. 103, 483–499 10.1086/282616 (doi:10.1086/282616) [DOI] [Google Scholar]
- 35.Kilham S. S., Kreeger D. A., Lynn S. G., Goulden C. E., Herrera L. 1998. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377, 147–159 10.1023/A:1003231628456 (doi:10.1023/A:1003231628456) [DOI] [Google Scholar]
- 36.Rocha O., Duncan A. 1981. The relationship between cell carbon and cell volume in freshwater algal species used in zooplankton studies. J. Plankton Res. 7, 279–294 10.1093/plankt/7.2.279 (doi:10.1093/plankt/7.2.279) [DOI] [Google Scholar]
- 37.Abramoff M. D., Magelhaes P. J., Ram S. J. 2004. Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 38.Littell R. C., Milliken G. A., Stroup W. W., Wolfinger R. D. 1996. SAS system for mixed models. Cary, NC: SAS Institute, Inc [Google Scholar]
- 39.Gotelli N. J. 1998. A primer of ecology, 4th edn. Sunderland, MA: Sinauer Associates [Google Scholar]
- 40.Wellborn G. A. 1994. Size-biased predation and prey life histories: a comparative study of freshwater amphipod populations. Ecology 75, 2104–2117 10.2307/1941614 (doi:10.2307/1941614) [DOI] [Google Scholar]
- 41.Spitze K. 1991. Predation and life history evolution in Daphnia pulex: temporal pattern of population diversity, fitness, and mean life history. Evolution 45, 82–92 10.2307/2409484 (doi:10.2307/2409484) [DOI] [PubMed] [Google Scholar]
- 42.Blanckenhorn W. D., Demont M. 2004. Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr. Comp. Biol. 44, 413–424 10.1093/icb/44.6.413 (doi:10.1093/icb/44.6.413) [DOI] [PubMed] [Google Scholar]
- 43.Yamahira K., Kawajiri M., Takeshi K., Irie T. 2007. Intra- and interpopulation variation in thermal reaction norms for growth rate: evolution of latitudinal compensation in ectotherms with a genetic constraint. Evolution 61, 1577–1589 10.1111/j.1558-5646.2007.00130.x (doi:10.1111/j.1558-5646.2007.00130.x) [DOI] [PubMed] [Google Scholar]
- 44.Hanley T. 2009. The independent and interactive effects of resource availability and predation on Daphnia life history and stoichiometry. PhD dissertation, Yale University, New Haven, CT, USA [Google Scholar]
- 45.Van Doorslaer W., Stoks R., Duvivier C., Bednarska A., De Meester L. 2009. Population dynamics determine genetic adaptation to temperature in Daphnia. Evolution 63, 1867–1878 10.1111/j.1558-5646.2009.00679.x (doi:10.1111/j.1558-5646.2009.00679.x) [DOI] [PubMed] [Google Scholar]
- 46.Chown S. L., Gaston K. J. 1999. Exploring links between physiology and ecology at macro-scales: the role of respiratory metabolism in insects. Biol. Rev. 74, 87–120 10.1017/S000632319800526X (doi:10.1017/S000632319800526X) [DOI] [Google Scholar]
- 47.Roff D. A. 1992. The evolution of life histories: theory and analysis. New York, NY: Chapman and Hall [Google Scholar]
- 48.James A. C., Partridge L. 1998. Geographic variation in competitive ability in Drosophila melanogaster. Am. Nat. 151, 530–537 10.1086/286138 (doi:10.1086/286138) [DOI] [PubMed] [Google Scholar]
- 49.Smoker W. W. 1986. Variability of embryo developmental rate, fry growth, and disease susceptibility in hatchery stocks of chum salmon. Aquaculture 57, 219–226 10.1016/0044-8486(86)90200-0 (doi:10.1016/0044-8486(86)90200-0) [DOI] [Google Scholar]
- 50.De Block M., Slos S., Johansson F., Stoks R. 2008. Integrating life history and physiology to understand latitudinal size variation in a damselfly. Ecography 31, 115–123 10.1111/j.2007.0906-7590.05313.x (doi:10.1111/j.2007.0906-7590.05313.x) [DOI] [Google Scholar]
- 51.Jonsson B., L'Abee-Lund J. H., Heggberget T. G., Jensen A. J., Johnsen B. J., Naesje T. F., Saettem L. M. 1992. Longevity, body size, and growth in anadromous brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 48, 1838–1845 10.1139/f91-217 (doi:10.1139/f91-217) [DOI] [Google Scholar]
- 52.Stoks R., De Block M., McPeek M. A. 2006. Physiological costs of compensatory growth in a damselfly. Ecology 87, 1566–1574 10.1890/0012-9658(2006)87[1566:PCOCGI]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1566:PCOCGI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 53.Gotthard K., Nylin S., Wiklund C. 1994. Adaptive variation in growth rate: life history costs and consequences in the speckled wood butterfly, Pararge aegeria. Oecologia 99, 281–289 10.1007/BF00627740 (doi:10.1007/BF00627740) [DOI] [PubMed] [Google Scholar]
- 54.Scharf I., Filin I., Ovadia O. 2009. A trade-off between growth and starvation endurance in a pit-building antlion. Oecologia 153, 453–460 10.1007/s00442-009-1316-y (doi:10.1007/s00442-009-1316-y) [DOI] [PubMed] [Google Scholar]
- 55.De Block M., Stoks R. 2008. Compensatory growth and oxidative stress in a damselfly. Proc. R. Soc. B 275, 781–785 10.1098/rspb.2007.1515 (doi:10.1098/rspb.2007.1515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Billerbeck J. M., Lankford T. E., Jr, Conover D. O. 2001. Evolution of intrinsic growth and energy acquisition rates. I. Trade-offs with swimming performance in Menidia menidia. Evolution 55, 1883–1872 10.1111/j.0014-3820.2col.tb00835.x (doi:10.1111/j.0014-3820.2col.tb00835.x) [DOI] [PubMed] [Google Scholar]
- 57.Lankford T. E., Jr, Billerbeck J. M., Conover D. O. 2001. Evolution of intrinsic growth and energy acquisition rates. II. Trade-offs with vulnerability to predation in Menidia menidia. Evolution 55, 1673–1681 10.1111/j.0014-3820.2001.tb00836.x (doi:10.1111/j.0014-3820.2001.tb00836.x) [DOI] [PubMed] [Google Scholar]
- 58.James A. C., Partridge L. 1995. Thermal evolution of rate of larval development in Drosphophila melanogaster in laboratory and field populations. J. Evol. Biol. 8, 315–330 10.1046/j.1420-9101.1995.8030315.x (doi:10.1046/j.1420-9101.1995.8030315.x) [DOI] [Google Scholar]
- 59.Reznick D. N., Butler M. J., IV, Rodd F. H., Ross P. 1996. Life history evolution in guppies (Poecilia reticulata). 6. Differential mortality as a mechanism for natural selection. Evolution 50, 1651–1660 10.2307/2410901 (doi:10.2307/2410901) [DOI] [PubMed] [Google Scholar]
- 60.De Meester L. 1996. Evolutionary potential and local genetic differentiation in a phenotypically plastic trait of a cyclical parthenogen, Daphnia magna. Evolution 50, 1293–1298 10.2307/2410669 (doi:10.2307/2410669) [DOI] [PubMed] [Google Scholar]
- 61.Cousyn C., De Meester L., Colbourne J. K., Brenock L., Verschuren D., Volckaert F. 2001. Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc. Natl Acad. Sci. USA 98, 6256–6260 10.1073/pnas.111606798 (doi:10.1073/pnas.111606798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michels H., Amsinck S. L., Jeppesen E., De Meester L. 2007. Interclonal variation in diel horizontal migration behaviour of the water flea Daphnia magna: searching for a signature of adaptive evolution. Hydrobiologia 594, 117–129 10.1007/s10750-007-9086-1 (doi:10.1007/s10750-007-9086-1) [DOI] [Google Scholar]