Abstract
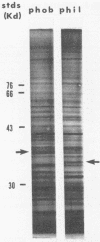
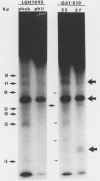
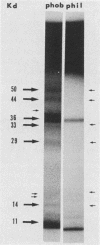
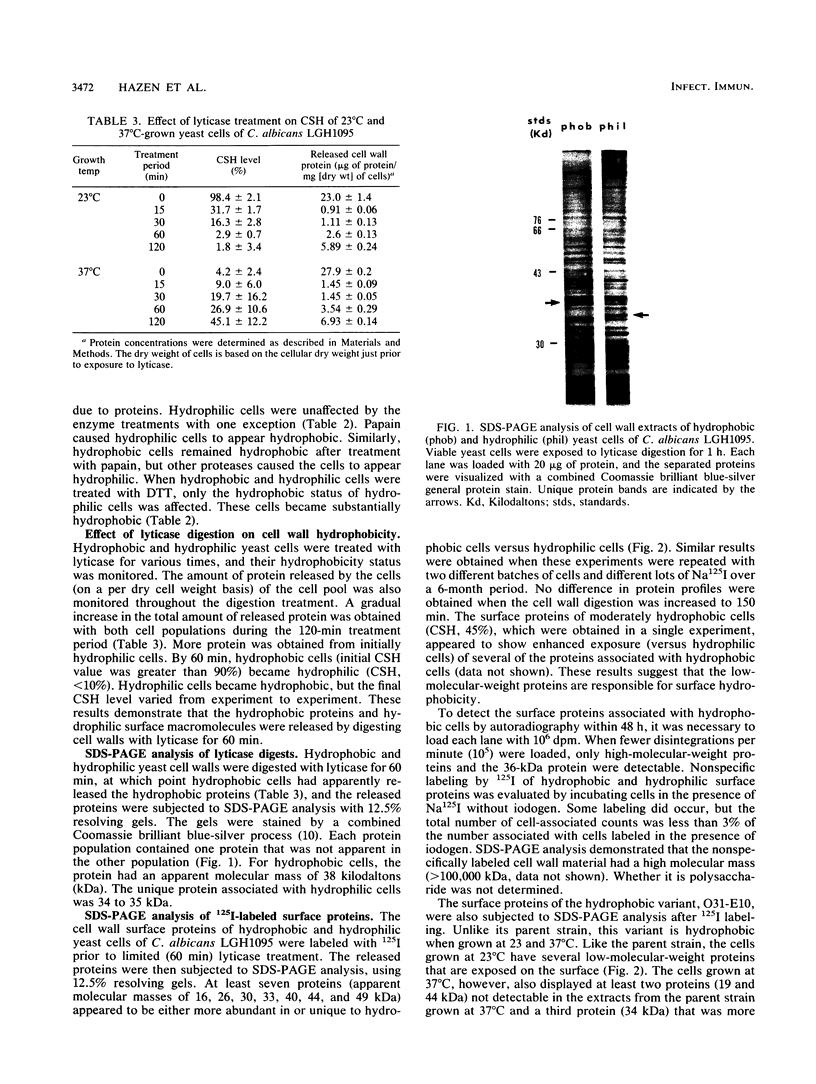
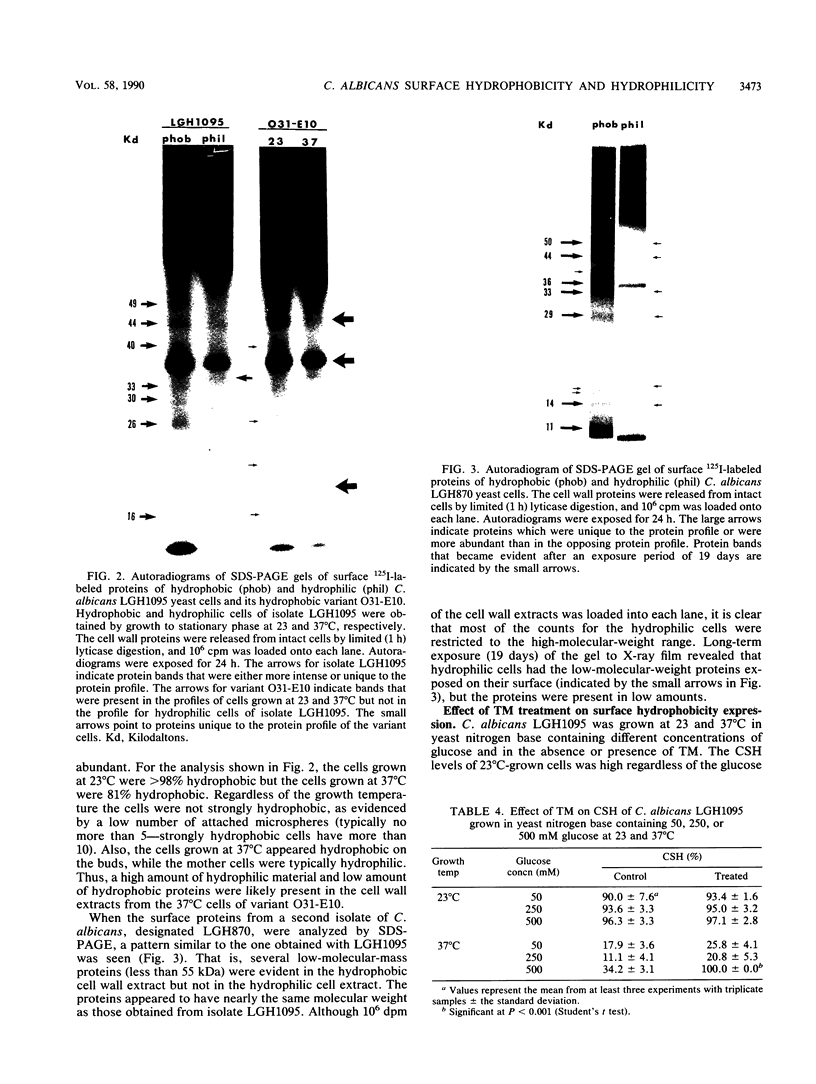
Hydrophobic yeast cells of Candida albicans are more virulent than hydrophilic yeast cells in mice. Results of experiments performed in vitro suggest that surface hydrophobicity contributes to virulence in multiple ways. Before definitive studies in vivo concerning the contribution of fungal surface hydrophobicity to pathogenesis can be performed, biochemical, physiological, and immunochemical characterization of the macromolecules responsible for surface hydrophobicity must be accomplished. This report describes our initial progress toward this goal. When hydrophobic and hydrophilic yeast cells of C. albicans were exposed to various enzymes, only proteases caused any change in surface hydrophobicity. Hydrophobic cell surfaces were sensitive to trypsin, chymotrypsin, pronase E, and pepsin. This indicates that surface hydrophobicity is due to protein. Papain, however, had no significant effect. The hydrophobicity of hydrophilic cells was altered only by papain. The proteins responsible for surface hydrophobicity could be removed by exposure to lyticase, a beta 1-3 glucanase, for 30 to 60 min. When 60-min lyticase digests of hydrophobic and hydrophilic cell walls were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 12.5% resolving gel, each protein population contained a single unique protein that was not evident in the other protein population. However, when the cell wall surface proteins of hydrophobic and hydrophilic cells were first labeled with 125I and then removed by lyticase and analyzed by SDS-PAGE, at least four low-molecular-mass (less than 65 kilodaltons) proteins associated with hydrophobic cells were either absent or much less abundant in the hydrophilic cell digests. This result was seen for both C. albicans strains that we tested. When late-exponential-phase hydrophilic cells were treated with tunicamycin, high levels of surface hydrophobicity were obtained by stationary phase. These results indicate that the surface hydrophobicity of C. albicans reflects changes in external surface protein exposure and that protein mannosylation may influence exposure of hydrophobic surface proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antley P. P., Hazen K. C. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infect Immun. 1988 Nov;56(11):2884–2890. doi: 10.1128/iai.56.11.2884-2890.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Cell surface and intracellular expression of two Candida albicans antigens during in vitro and in vivo growth. Microb Pathog. 1987 Apr;2(4):249–257. doi: 10.1016/0882-4010(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Calderone R., Wadsworth E. Characterization with crossed immunoelectrophoresis of some antigens differentiating a virulent Candida albicans from its derived, avirulent strain. Proc Soc Exp Biol Med. 1987 Jul;185(3):325–334. doi: 10.3181/00379727-185-42552. [DOI] [PubMed] [Google Scholar]
- Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol. 1989;3:248–314. doi: 10.1007/978-1-4612-3624-5_10. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L., Stocco D. M. Cell wall proteins of Candida albicans. Can J Microbiol. 1983 Oct;29(10):1438–1444. doi: 10.1139/m83-220. [DOI] [PubMed] [Google Scholar]
- Critchley I. A., Douglas L. J. Isolation and partial characterization of an adhesin from Candida albicans. J Gen Microbiol. 1987 Mar;133(3):629–636. doi: 10.1099/00221287-133-3-629. [DOI] [PubMed] [Google Scholar]
- Cutler J. E., Brawner D. L., Hazen K. C., Jutila M. A. Characteristics of Candida albicans adherence to mouse tissues. Infect Immun. 1990 Jun;58(6):1902–1908. doi: 10.1128/iai.58.6.1902-1908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moreno M. R., Smith J. F., Smith R. V. Silver staining of proteins in polyacrylamide gels: increased sensitivity through a combined Coomassie blue-silver stain procedure. Anal Biochem. 1985 Dec;151(2):466–470. doi: 10.1016/0003-2697(85)90206-4. [DOI] [PubMed] [Google Scholar]
- Diedrich D. L., Mendel S. M., Lawrence R. M. Apparent extracellular glycoprotein turnover product from Candida albicans. Mycopathologia. 1984 May 30;86(2):65–70. doi: 10.1007/BF00436488. [DOI] [PubMed] [Google Scholar]
- Douglas L. J. Adhesion of Candida species to epithelial surfaces. Crit Rev Microbiol. 1987;15(1):27–43. doi: 10.3109/10408418709104446. [DOI] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Elorza M. V., Murgui A., Sentandreu R. Dimorphism in Candida albicans: contribution of mannoproteins to the architecture of yeast and mycelial cell walls. J Gen Microbiol. 1985 Sep;131(9):2209–2216. doi: 10.1099/00221287-131-9-2209. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hazen B. W., Hazen K. C. Dynamic expression of cell surface hydrophobicity during initial yeast cell growth and before germ tube formation of Candida albicans. Infect Immun. 1988 Sep;56(9):2521–2525. doi: 10.1128/iai.56.9.2521-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen B. W., Hazen K. C. Isolation of hydrophobic and hydrophilic variants of Candida albicans. FEMS Microbiol Lett. 1989 Jan 15;48(2):167–171. doi: 10.1111/j.1574-6968.1989.tb03293.x. [DOI] [PubMed] [Google Scholar]
- Hazen B. W., Hazen K. C. Modification and application of a simple, surface hydrophobicity detection method to immune cells. J Immunol Methods. 1988 Mar 16;107(2):157–163. doi: 10.1016/0022-1759(88)90214-1. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Hazen B. W. Temperature-modulated physiological characteristics of Candida albicans. Microbiol Immunol. 1987;31(6):497–508. doi: 10.1111/j.1348-0421.1987.tb03112.x. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., LeMelle W. G. Improved assay for surface hydrophobic avidity of Candida albicans cells. Appl Environ Microbiol. 1990 Jun;56(6):1974–1976. doi: 10.1128/aem.56.6.1974-1976.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989 Jul;57(7):1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Plotkin B. J., Klimas D. M. Influence of growth conditions on cell surface hydrophobicity of Candida albicans and Candida glabrata. Infect Immun. 1986 Oct;54(1):269–271. doi: 10.1128/iai.54.1.269-271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Evidence for N-terminal exposure of the protein IA subclass of Neisseria gonorrhoeae protein I. Infect Immun. 1986 Nov;54(2):408–414. doi: 10.1128/iai.54.2.408-414.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J. Adhesion and association mechanisms of Candida albicans. Curr Top Med Mycol. 1988;2:73–169. doi: 10.1007/978-1-4612-3730-3_4. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Zajic J. E. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985 Oct;50(1):97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. C., King R. D. Characterization of Candida albicans adherence to human vaginal epithelial cells in vitro. Infect Immun. 1983 Sep;41(3):1024–1030. doi: 10.1128/iai.41.3.1024-1030.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagi S., Miyake Y., Inagaki K., Tsuru H., Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect Immun. 1985 Jan;47(1):11–14. doi: 10.1128/iai.47.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton J., Jones J. M. Analysis of cell wall extracts of Candida albicans by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot techniques. Infect Immun. 1986 Sep;53(3):565–572. doi: 10.1128/iai.53.3.565-572.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton J., Jones J. M. Identification of two germ-tube-specific cell wall antigens of Candida albicans. Infect Immun. 1986 Dec;54(3):864–868. doi: 10.1128/iai.54.3.864-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D., Hopwood V., Vernes A. Antigenic variability of Candida albicans. Crit Rev Microbiol. 1985;12(3):223–270. doi: 10.3109/10408418509104430. [DOI] [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Lefebvre B., Husson M. O. Antigenic variability between Candida albicans blastospores isolated from healthy subjects and patients with Candida infection. Sabouraudia. 1982 Sep;20(3):173–177. [PubMed] [Google Scholar]
- Rotrosen D., Calderone R. A., Edwards J. E., Jr Adherence of Candida species to host tissues and plastic surfaces. Rev Infect Dis. 1986 Jan-Feb;8(1):73–85. doi: 10.1093/clinids/8.1.73. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Muller G., Buckley H. R. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun. 1984 Jun;44(3):576–580. doi: 10.1128/iai.44.3.576-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P. M., Kenny G. E. Enzymatic release of germ tube-specific antigens from cell walls of Candida albicans. Infect Immun. 1985 Sep;49(3):609–614. doi: 10.1128/iai.49.3.609-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R., Senet J. M. Adherence of Candida albicans germ tubes to plastic: ultrastructural and molecular studies of fibrillar adhesins. Infect Immun. 1988 Aug;56(8):1987–1993. doi: 10.1128/iai.56.8.1987-1993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]