Abstract
Dispersal modulates gene flow throughout a population’s spatial range. Gene flow affects adaptation at local spatial scales, and consequently impacts the evolution of reproductive isolation. A recent theoretical investigation has demonstrated that local adaptation along an environmental gradient, facilitated by the evolution of limited dispersal, can lead to parapatric speciation even in the absence of assortative mating. This and other studies assumed unconditional dispersal, so individuals start dispersing without regard to local environmental conditions. However, many species disperse conditionally; their propensity to disperse is contingent upon environmental cues, such as the degree of local crowding or the availability of suitable mates. Here, we use an individual-based model in continuous space to investigate by numerical simulation the relationship between the evolution of threshold-based conditional dispersal and parapatric speciation driven by frequency-dependent competition along environmental gradients. We find that, as with unconditional dispersal, parapatric speciation occurs under a broad range of conditions when reproduction is asexual, and under a more restricted range of conditions when reproduction is sexual. In both the asexual and sexual cases, the evolution of conditional dispersal is strongly influenced by the slope of the environmental gradient: shallow environmental gradients result in low dispersal thresholds and high dispersal distances, while steep environmental gradients result in high dispersal thresholds and low dispersal distances. The latter, however, remain higher than under unconditional dispersal, thus undermining isolation by distance, and hindering speciation in sexual populations. Consequently, the speciation of sexual populations under conditional dispersal is triggered by a steeper gradient than under unconditional dispersal. Enhancing the disruptiveness of frequency-dependent selection, more box-shaped competition kernels dramatically lower the speciation-enabling slope of the environmental gradient.
Keywords: Frequency-dependent selection, Sexual reproduction, Speciation, Evolutionary branching, Competition kernels
1. Introduction
Dispersal is a topic of central importance in ecology and evolutionary biology (Ronce, 2007), influencing spatial distributions of genetic diversity (Wright, 1969), adaptation to local environments (Gandon et al., 1996; Lenormand, 2002), and spatial population dynamics (Kendall et al., 2000). Dispersal mediates gene flow throughout a population’s spatial range and, through isolation by distance (Wright, 1943), thus affects the evolution of reproductive isolation (Barton, 2001; Eppstein et al., 2009).
Quantitative model-based studies have demonstrated that environmental gradients promote parapatric speciation driven by frequency-dependent competition: with limited dispersal, local adaptation and competition along the gradient cause disruptive selection (Doebeli and Dieckmann, 2003; Leimar et al., 2008). In contrast, long-range dispersal increases gene flow throughout the population, reduces local adaptation and frequency-dependent competition, and thus limits the possibility of parapatric speciation (Doebeli and Dieckmann, 2003).
In a recent study, Heinz et al. (2009) extended the model of Doebeli and Dieckmann (2003) by allowing for the evolution of dispersal distance. A key finding of their work (Heinz et al., 2009) is that short-range dispersal evolves in conjunction with parapatric speciation events. This leads to isolation by distance, providing an alternative mechanism to assortative mate preference for the evolution of reproductive isolation in parapatry (Wright, 1943).
Heinz et al. (2009) considered unconditional dispersal. Accordingly, individuals could not base their decision to disperse on salient environmental information, such as high local competition or low carrying capacity. Empirical evidence, however, suggests that in many species, an individual’s propensity to commence dispersing depends on the external environment (Ims and Hjermann, 2001), resulting in conditional dispersal. For example, pea aphids Acyrthosiphon pisum produce an increased proportion of winged dispersal morphs in the presence of an aphid alarm pheromone (Kunert et al., 2005); emigration rates in the collared flycatcher Ficedula albicollis increase when either the number or the condition of local offspring decrease (Doligez et al., 2002), and dispersive mutants of the nematode Caenorhabditis elegans increase in prevalence in response to the random destruction of patches in experimental metapopulations (Friedenberg, 2003).
The present study investigates the relationship between parapatric speciation and the evolution of conditional dispersal. We build upon a growing literature of theoretical models of conditional dispersal, which have considered a variety of dispersal functions and environmental cues. For example, Travis and Dytham (1999) considered a conditional dispersal strategy that was linearly dependent on patch density and allowed its slope and intercept to evolve. Bach et al. (2007) considered a sigmoidal density-dependent dispersal strategy and allowed its steepness and half-saturation point to evolve. Kun and Scheuring (2006), and later Travis et al. (2009), employed a general three-parameter density-dependent dispersal strategy able to capture numerous qualitatively different shapes. Metz and Gyllenberg (2001), and later Gyllenberg et al. (2008), utilized function-valued trait representations of conditional dispersal, which allowed for arbitrary functional forms. A common outcome of these models is the evolution of threshold-based dispersal, where dispersal propensity is low below some critical environmental cue and then high above it. These theoretical results are consistent with empirical evidence that dispersal strategies are threshold-based in some species (Hodgson, 2002).
Dispersal is inherently risky. This is because any dispersal event either improves or worsens the environmental quality experienced by the disperser, without that individual having any chance to predict the outcome in advance of risking the dispersal event. Among the relevant factors influencing environmental quality are local competition and scarcity of resources. Dispersal may allow an individual to escape from intrinsic resource scarcity, but comes at the potential expense of moving to a location where environmental quality, in those two regards, is even worse. An additional risk of dispersal comes from the chance of moving to an environment where the individual is less well adapted. Moreover, the movement event itself imposes mortality risks, such as increased exposure to predation (Ims and Andreassen, 2000). The evolution of threshold-based dispersal strategies highlights the fundamental tension between these potential costs and benefits of dispersive behavior. The evolved dispersal threshold reflects the point at which the benefits begin to outweigh the costs (Parvinen et al., 2003).
Here, we use an individual-based model in continuous space to investigate by numerical simulation the evolution of threshold-based dispersal strategies in spatially extended populations subject to frequency-dependent competition along an environmental gradient. Systematically varying the environmental gradient and the phenotypic specificity of competition, we study the evolution of dispersal distances and thresholds, clarifying their impact on parapatric speciation. We investigate both asexual and sexual populations and competition kernels of different shapes, outline the parameter regions in which parapatric speciation occurs, and contrast these results with those obtained in the case of unconditional dispersal (Doebeli and Dieckmann, 2003; Heinz et al., 2009).
2. Methods
2.1. Model overview
We consider a spatially explicit, individual-based, stochastic model in continuous space and time, which extends the model of Heinz et al. (2009) to the case of conditional dispersal.
The environment is assumed to be two-dimensional and continuous. One direction is ecologically neutral, while an environmental gradient exists in the other: The ecological character that confers the best adaptation to the local resource, u0, varies linearly in space
| (1) |
where a is the slope of the environmental gradient (Roughgarden, 1972).
Individuals are described by their spatial location (x,y) in the unit square, an ecological character u, the threshold τ and distance δ defining their conditional dispersal, and – in the case of sexual populations – a mate search distance w. The ecological character u could describe a morphological, behavioral, or physiological trait, or a combination thereof. The bivariate character (τ,δ) is used to parametrize the individual’s conditional dispersal function, which we assume takes the form of a step function (Eq. (8)). The mate search distance w determines the probabilities of mate selection by spatial distance (Eq. (7)). Apart from the preference for spatially proximal individuals, no form of assortativity or mating preference is considered.
The population is described by its current abundance N and the traits and locations of all individuals. A list of all model variables is given in Table 1. The configuration of the population changes over time due to birth and death events, which occur with (probabilistic) rates depending on the current population configuration.
Table 1.
Model variables, their ranges, and initial values.
| Population size | 0 ≤ N | 300 |
| Location | 0 ≤ xi,yi ≤ 1 | Uniform in [0,1] |
| Ecological trait | 0 ≤ ui ≤ 1 | 0.5 |
| Dispersal threshold | 0 ≤ τi | 0.7 |
| Dispersal distance | 0 ≤ δi ≤ 1 | 0.2 |
| Mate search distance | 0 ≤ wi ≤ 1 | 0.2 |
2.2. Mortality
We assume a constant individual birth rate bi = b, but the death rate depends on the individual’s spatial location, phenotypic trait, and its competition for resources with all of the other individuals in the population. The intensity of both spatial and phenotypic competition increases as either spatial or phenotypic distance between any two individuals decreases. All these interactions are defined by kernels, which we now specify. Throughout, we use the following function (Roughgarden, 1974):
| (2) |
where
| (3) |
and Γ(x) is the gamma function. The kurtosis of Φ can be adjusted by varying n. For n = 2, the function is Gaussian. For n > 2, the function is platykurtic, with a broader peak and thinner tails, relative to the Gaussian. Independent of n, σ measures the function’s standard deviation.
The death rate di of an individual i is given by
| (4) |
where neff is the effective number of individuals with which individual i competes
| (5) |
and K(xi, ui) is the local carrying capacity, i.e., the density of individuals of type ui locally supported at location (xi, yi),
| (6) |
where K0 is the maximal carrying capacity and u0(xi) is the phenotype that maximizes carrying capacity at spatial position xi (Eq. (1)). The prefactor in Eq. (5) ensures that neff = K at demographic equilibrium in a monomorphic population with gradient a = 0, and hence di ≈ 1; in the Gaussian case (n = 2), it reduces to . The parameters σs and σc specify how quickly the strength of competition attenuates with spatial and phenotypic distance, respectively. In Eq. (5), we consider both Gaussian (n = 2) and platykurtic (n = 3) competition kernels, the latter of which being known to facilitate adaptive divergence (Doebeli et al., 2006; Leimar et al., 2008). However, in Eq. (6) we will use the Gaussian function Φσ,2(x) with mean x and standard deviation σ. Carrying capacity (Eq. (6)) thus decreases with phenotypic distance from its maximum at u0(xi) according to a Gaussian function with standard deviation σK.
We can define the fitness of an individual at every instant as the difference between its current birth and death rate, fi = b − di. The population-level birth and death rates are given by B = bN and , respectively. Thus, the population-level event rate is E = B + D.
2.3. Mating and inheritance
For birth events, we consider both asexual and sexual reproduction. In the asexual case, the phenotype (u,τ,δ) is inherited nearly faithfully from parent to offspring, subject, at each birth event, to small mutations that displace the offspring phenotype by a random increment drawn from a Gaussian distribution with mean zero and standard deviation σm.
In the sexual case, when an individual i is chosen for reproduction it selects a mate j ≠ i based on spatial proximity, with probability
| (7) |
Thus, mate choice is solely dependent upon spatial location, and does not involve any form of assortment or sexual selection. However, the standard deviation of the mate search area is wi, which is an evolvable trait. The parents i and j produce an offspring k, which inherits phenotypic trait values (uk,τk,δk,wk) from its parents by drawing from a Gaussian distribution with mean equal to the mid-parental values and with standard deviations equal to . This captures the effects of segregation and recombination simultaneously, and allows for a direct comparison with the results of Heinz et al. (2009), who introduced this specific offspring distribution with the argument that it preserves the variance of an existing Gaussian trait distribution in the well-mixed population. Experimentation with Gaussian distributions of constant width produced results that were statistically indistinguishable from those reported herein (paired t-test, p > 0.01).
2.4. Conditional dispersal
In both the asexual and sexual case, the inherited dispersal characters (τk,δk) affect how an offspring’s spatial position is displaced relative to that of parent i. We thus consider natal dispersal, so individuals only move once in their lifetime and this movement occurs immediately after birth. The distance an offspring disperses is conditioned on local environmental quality, evaluated in terms of the individual’s death rate dk. This allows for the simultaneous assessment of both local competition and resource availability (Eq. (4)). Conditional dispersal is assumed to take the form of a step function (also known as bang–bang control). The step function’s threshold is given by τ and its height by δ (Fig. 1). Thus, an individual k experiencing a death rate dk and having dispersal characters (τk,δk) will take a dispersal step (Δx,Δy) drawn from a Gaussian distribution with mean zero and standard deviation
| (8) |
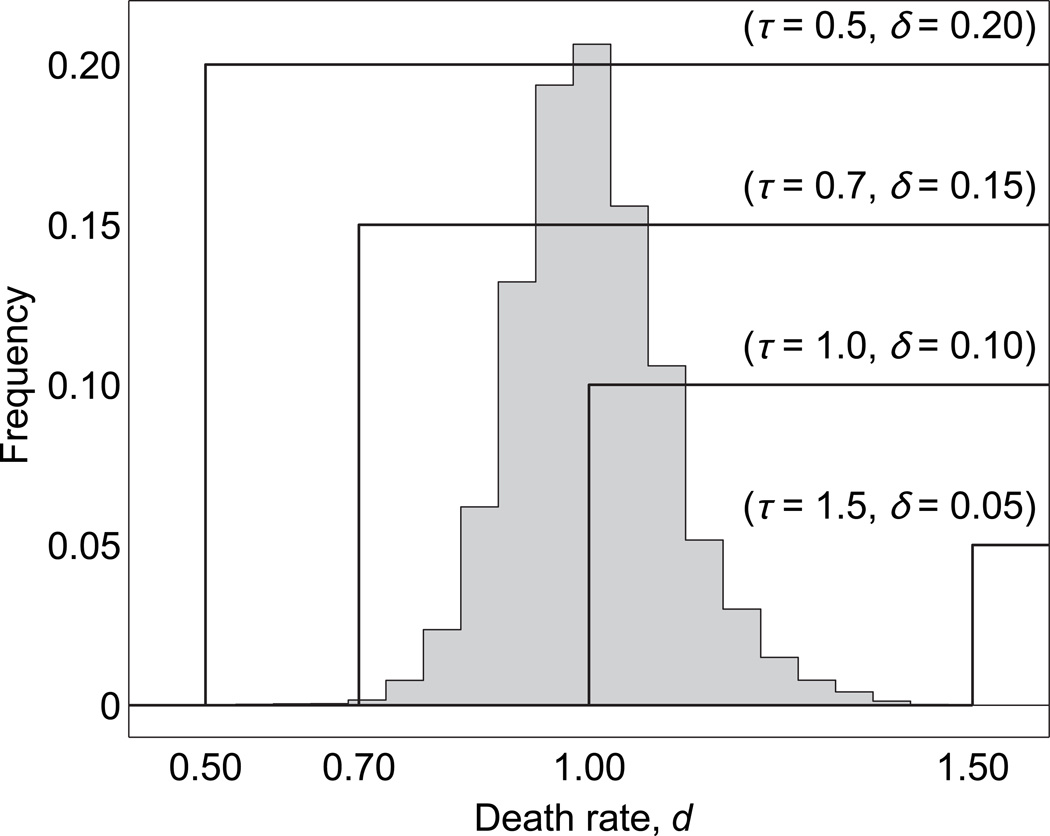
Fig. 1.
Schematic illustration of four possible conditional dispersal strategies, assumed to take the form of a step function. As a measure of environmental quality, these strategies are conditioned on an individual’s death rate d, and are encoded as a (τ,δ) pair, where τ denotes the dispersal threshold and δ the dispersal distance (see Eq. (8)). The gray bars depict the death-rate distribution of a representative population, illustrating the frequency and domain of experienced environmental cues.
The disperser is then given the spatial coordinates (xi + Δx,yi + Δy). Therefore, is the expected (root-mean-square) dispersal distance, given the decision to disperse.
Our model assumes that there is no explicit cost to dispersal. However, an environmental gradient results in an implicit cost, since individuals run the risk of moving to a spatial location in which they are poorly adapted. Increasing the slope of the gradient increases this risk, while decreasing the slope has the opposite effect.
2.5. Boundary conditions
When a dispersal step would lead outside the unit square, we follow Heinz et al. (2009) and reset the x-location to 0 or 1, respectively, and the y-location to 1+y or y − 1, thus implementing impermeable boundaries in the direction of the gradient, and periodic boundaries in the ecologically neutral direction. Competitive interactions stretch across the periodic, but not across the impermeable boundaries.
2.6. Implementation
Time proceeds in increments drawn from an exponential distribution with mean E−1. At each time step, either a birth or death event is chosen, with probabilities B/E and D/E, respectively, which makes generations overlapping. After the event type is chosen, individual i is selected with probability bi/B or di/D, respectively. According to the event type, individual i then either reproduces or dies. In the latter case, it is removed from the population; in the former case, a new individual is introduced into the population as described in Sections 2.3 and 2.4. The theoretical background to this scheduling procedure is presented in detail by Gillespie (1976).
In a population regulated by frequency-dependent competition, as considered herein, average individual fitness (f̄) is zero at equilibrium, and therefore b̄ = d̄ = 1. This results in a distribution of environmental cues, more than 99% of which is comprised in the domain 0.7 ≤ d ≤ 1.4 (Fig. 1). Therefore, in all realizations we initialize the dispersal character τ with 0.7, which is on the fringe of the death-rate distribution, but still under selective pressure (Fig. 1). Initializing τ outside of this range results in virtually vanishing selection pressures on τ and thus in the mere genetic drifting of τ. Specifically, if τ is initialized well below 0.7, then individuals unconditionally disperse according to δ and all selective pressure falls on δ. In this case, our results reduce to those reported by Heinz et al. (2009). If τ is initialized well above 1.4, then individuals never disperse and our results reduce to those of Doebeli and Dieckmann (2003) for the case of zero mobility. These two cases highlight an important aspect of the conditional dispersal function used in this study: it also allows for unconditional dispersal to evolve.
2.6.1. Speciation
In our model, speciation is considered to have occurred when an initially monomorphic population has split into two or more separate phenotypes. For asexual populations, we use the term speciation to mean evolutionary branching, in line with Heinz et al. (2009).
We identify speciation as follows (Heinz et al., 2009). For asexual populations, the initially unimodal phenotype must branch, and remain branched, into a bi- or multimodal distribution for at least 500 generations. For sexual populations, we additionally demand that virtually no hybrids occur between these branches, enforcing the strict requirement that the modes in the phenotype distribution are sharply delineated from one another.
2.6.2. Parameters
The model is described by two dimensionless parameters: the scaled width of the phenotypic competition function c = σc/σK and the scaled slope of the environmental gradient s = aσs/σK. Here, we systematically vary these two parameters in the range 0.3 ≤ c ≤ 2.0 and 0 ≤ s ≤ 1 (as in Heinz et al., 2009), while the numerator of the scaled dispersal distance δ/σs (Doebeli and Dieckmann, 2003) is allowed to evolve. The other parameters considered in this study are presented in Table 2. For each combination of c and s, either 1 or 100 independent realizations were performed, depending on the experiment. In each realization, we allow the population to evolve for 105 generations and measure the evolutionary dynamics of the phenotypic traits. We keep all phenotypic traits in the range [0,1], except for the dispersal character τ, whose upper limit is left unbounded.
Table 2.
Model parameters and their values, chosen to facilitate direct comparison with Heinz et al. (2009).
| Birth rate | b | 1 |
| Maximal carrying capacity density | K0 | 300 |
| Standard deviation of carrying capacity density | σK | 0.3 |
| Standard deviation of competition function | σs | 0.2 |
| Standard deviation of mutation steps | σm | 0.001 |
3. Results
We begin our analysis with asexual populations, to provide a frame of reference for the subsequent analysis of sexual populations.
3.1. Asexual populations
When reproduction is asexual, the joint evolution of the ecological character u and the conditional-dispersal character (τ,δ) results in the two qualitatively distinct evolutionary outcomes shown in Fig. 2a, b: (i) conditional dispersal without speciation and (ii) conditional dispersal with speciation. This occurs under both Gaussian (Fig. 2a) and platykurtic (Fig. 2b) competition, although the parameter region in which speciation does not occur is slightly enlarged in the platykurtic case.
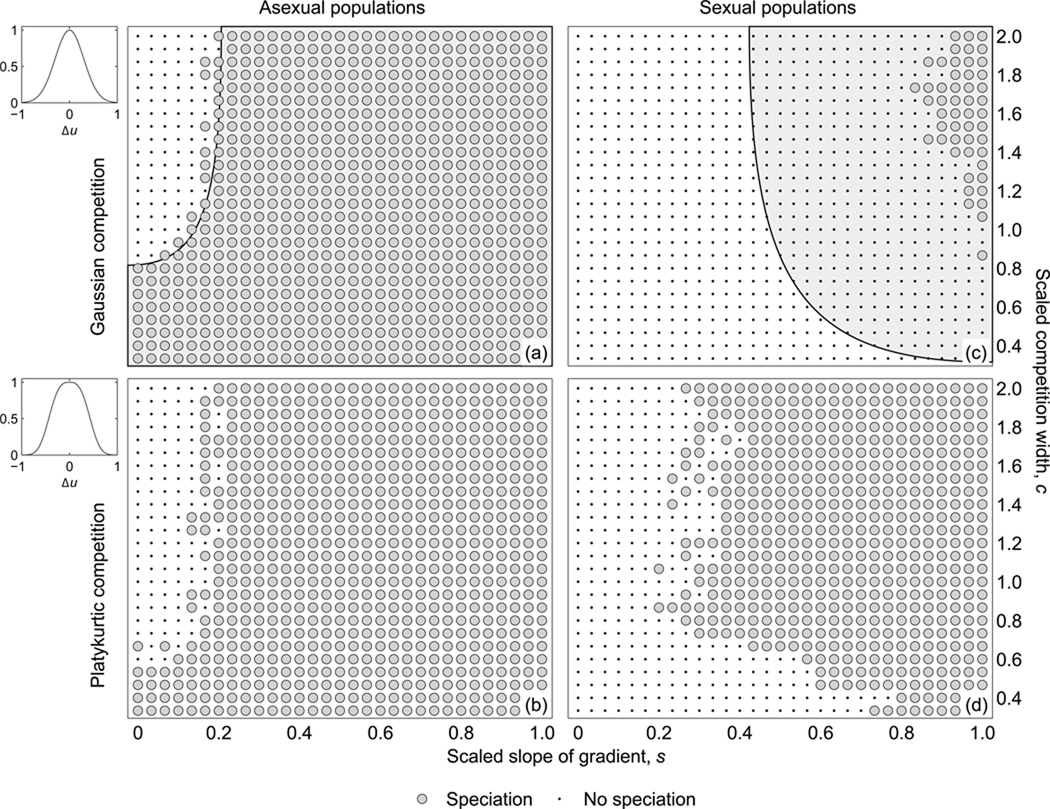
Fig. 2.
Evolutionary outcomes as a function of the scaled slope of the environmental gradient s and the scaled competition width c for (a) asexual populations with Gaussian competition, (b) asexual populations with platykurtic competition, (c) sexual populations with Gaussian competition, and (d) sexual populations with platykurtic competition. In (a) and (c), the lightly shaded region to the right indicates where Heinz et al. (2009) obtained speciation with unconditional dispersal. Whereas in asexual populations, the switch from unconditional to conditional dispersal leads to virtually indistinguishable results, in sexual populations conditional dispersal appreciably reduces the scope for speciation. Small panels on the left illustrate the two different kernel shapes for identical standard deviations.
In the first evolutionary regime (Fig. 2a, b, dot symbols), where competition kernels are wide and environmental gradients are shallow, speciation does not occur. This result is consistent with Doebeli and Dieckmann (2003), where speciation is not found under Gaussian competition for c ≥ 1 unless accompanied by steep environmental gradients and low mobility. This observation also corroborates the results reported by Heinz et al. (2009), who found that dispersal evolution does not always lead to the reduced mobility required for speciation.
In the second evolutionary regime (Fig. 2a, b, circle symbols), speciation occurs for both shallow environmental gradients accompanied by narrow competition kernels and for steep environmental gradients accompanied by arbitrarily wide competition kernels. This result is again consistent with Doebeli and Dieckmann (2003), since speciation is expected under Gaussian competition when the gradient is sufficiently steep and/or the competition kernel is sufficiently narrow (c < 1). This is also consistent with the results reported by Heinz et al. (2009), who furthermore found that increasing the slope of the gradient led to a marked decrease in the evolved dispersal distance. Here, we observe a similar trend, but threshold-based conditional dispersal allows the dispersal distance to remain at relatively high values even for steep gradients.
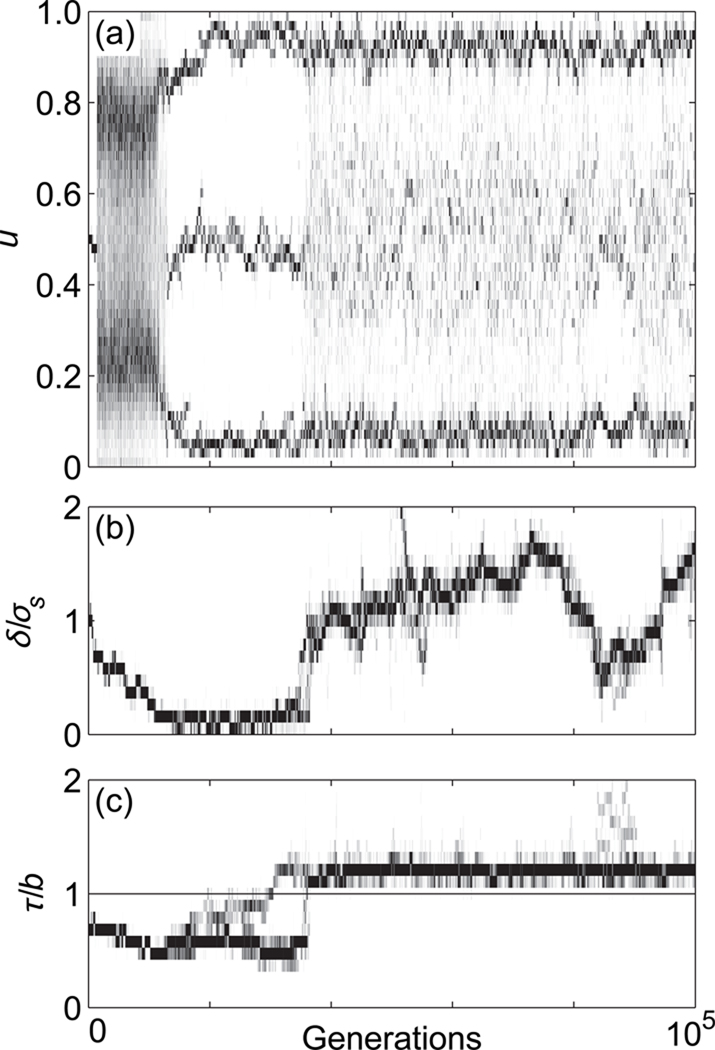
To illustrate the relationship between conditional dispersal and speciation in asexual populations, we depict in Fig. 3 the evolutionary dynamics of the ecological character and the conditional dispersal characters under Gaussian competition, using a parameter combination for which Heinz et al. (2009) observed short-range dispersal in conjunction with speciation. In Fig. 3a, speciation occurs rapidly, with divergence of the ecological character into two discrete morphs within 5000 generations, and into three discrete morphs within 25,000 generations. In contrast to the case of unconditional dispersal studied in Heinz et al. (2009), this happens not through a reduction in dispersal distance, but rather through an increased reluctance to disperse. This reluctance is achieved through the evolution of an increased dispersal threshold (Fig. 3c), which settles around a value slightly greater than the average death rate. The corresponding dispersal distances drift considerably during their evolution, but remain consistently and significantly above zero (Fig. 3b).
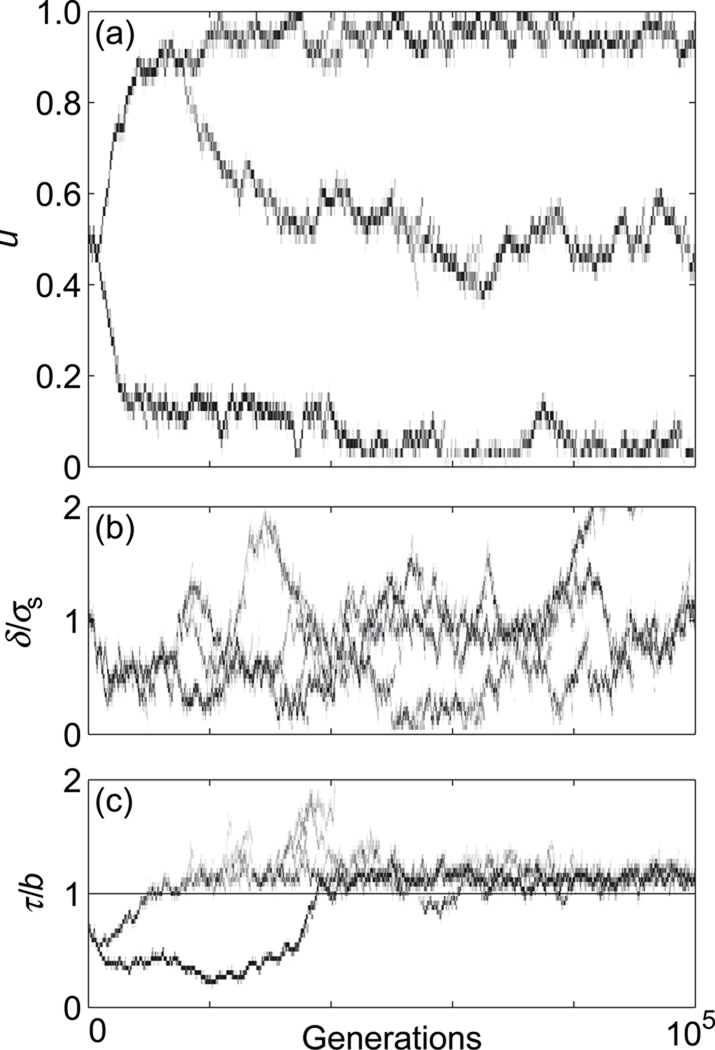
Fig. 3.
Evolutionary dynamics of the (a) ecological character u, (b) scaled dispersal distance δ/σs, and (c) scaled dispersal threshold τ/b in asexual populations with Gaussian competition, for a scaled competition width c = 2.0 and a scaled slope of the environmental gradient s = 0.6. The horizontal line in (c) indicates the average death rate, d = 1.0.
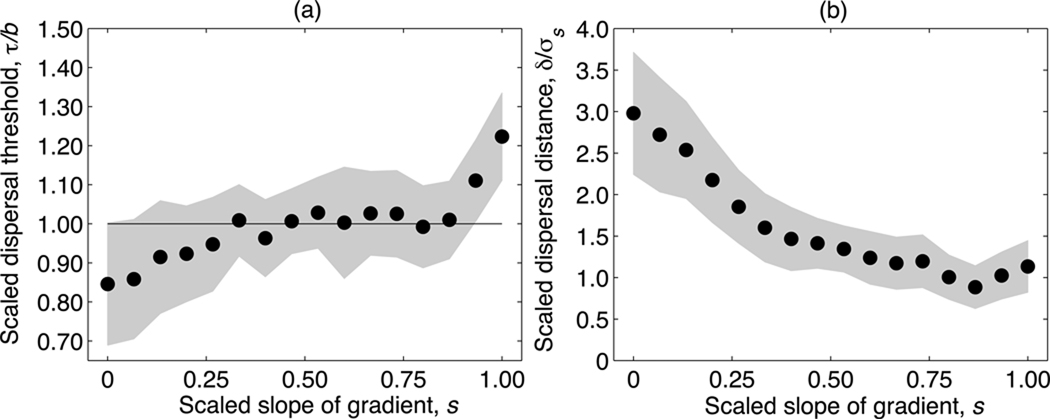
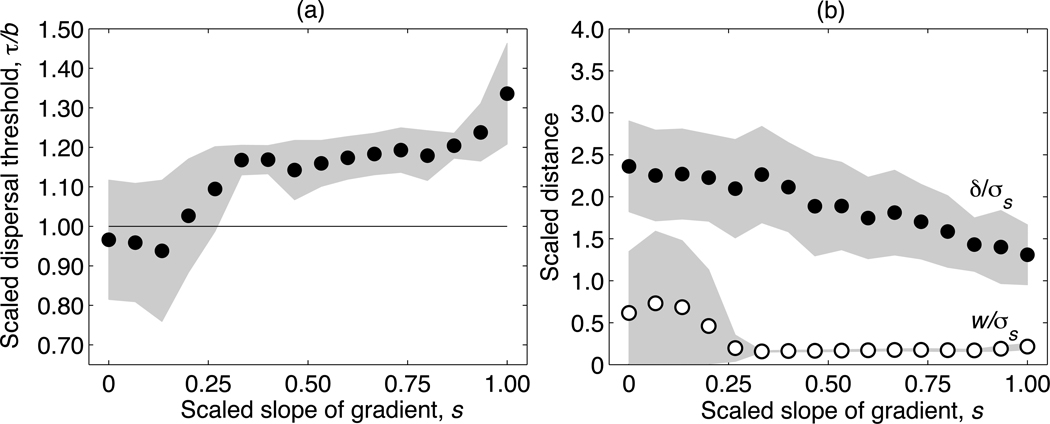
The evolved conditional dispersal strategy is affected by the steepness of the environmental gradient (Fig. 4), but is relatively insensitive to the shape (Gaussian or platykurtic) and the scaled width c of the competition kernel. (Consequently, in Fig. 4 we present data only for the representative case c = 1.) The dispersal threshold τ increases with the scaled gradient slope s (Fig. 4a), causing an increased reluctance to disperse as the environmental gradient steepens. For shallow gradients, the dispersal threshold τ evolves to a value below the population-level average death rate (d = 1, Fig. 4a, horizontal line), whereas for steep gradients, τ evolves to a value above this average. Thus, when environmental gradients are shallow, dispersal is selectively favored even in environments where the death rate is below average. This most likely results from kin competition, as decreased dispersal increases the spatial clustering of related individuals.
Fig. 4.
Evolved dispersal strategies in asexual populations with Gaussian competition, shown as a function of the scaled slope of the environmental gradient s, for the scaled competition width c = 1.0. The scaled threshold of the dispersal strategy τ/b is shown in (a) and the scaled dispersal distance δ/σs is shown in (b). The horizontal line in (a) indicates the average death rate d = 1.0. Filled circles show the average of the final 5000 generations of 100 independent realizations, and the gray-shaded areas represent the respective standard deviations across realizations.
For steep environmental gradients, the risk of dispersing to an area in which the organism is not well adapted is higher than the risk of remaining in an area with above-average death rates; accordingly, higher dispersal thresholds are selectively favored. The scaled movement distance δ/σs decreases with increasing gradient slope s, since the inherent cost of dispersal increases with s (Fig. 4b). The reduction of dispersal distance for steep gradients was also observed by Heinz et al. (2009). However, the case of unconditional dispersal considered therein led to dispersal distances evolving toward zero as the gradient became increasingly steep. Our results demonstrate that when dispersal is conditional, movement distances always remain well above zero, regardless of the gradient.
3.2. Sexual populations
In sexual populations, speciation occurs under more restrictive conditions. Specifically, speciation under Gaussian competition was observed only for steep gradients and wide competition kernels (Fig. 2c). This result bears a close resemblance to the observations made by Heinz et al. (2009), except that speciation is now found in conjunction with conditional dispersal, as opposed to unconditional, short-range dispersal. Under platykurtic competition, the speciation region dramatically widens toward more intermediate gradients and narrower competition kernels (Fig. 2d). This result agrees with the observations made by Leimar et al. (2008) for asexual populations with low mobility.
To illustrate how the speciation process can be frustrated by conditional dispersal, we depict in Fig. 5 an illustrative example of the evolutionary dynamics of the ecological character (Fig. 5a) and the dispersal characters (Fig. 5b, c) using a parameter combination for which speciation was observed in the case of unconditional dispersal (Heinz et al., 2009). After about 20,000 generations, the population has segregated into three distinct phenotypes (Fig. 5a). This results from the evolution of the scaled dispersal distance δ/σs (Fig. 5b), which is quickly driven toward zero. Simultaneously, the dispersal threshold τ (Fig. 5c) evolves toward higher values. As τ surpasses the population-level average death rate at around 40,000 generations (Fig. 5c, horizontal line), the scaled dispersal distance δ/σs responds with a rapid increase. The resulting conditional dispersal breaks up the discrete phenotypic clusters that had previously evolved, impeding the evolution of reproductive isolation (Fig. 5a). In contrast, when dispersal distances evolve toward zero under unconditional dispersal, phenotypic clusters stabilize and thus result in parapatric speciation (Heinz et al., 2009).
Fig. 5.
Evolutionary dynamics of the (a) phenotypic character u, (b) scaled dispersal distance δ/σs, and (c) scaled dispersal threshold τ/b in sexual populations with Gaussian competition, for a scaled competition width c = 2.0, and a scaled slope of the environmental gradient s = 0.6. The horizontal line in (c) denotes the average death rate, d = 1.0.
The relationships between the dispersal characters τ and δ and the scaled slope of the environmental gradient s (Fig. 6) are qualitatively similar to the asexual case (Fig. 4), although the scaled dispersal distances δ/σs are generally higher in the sexual case. In contrast to Heinz et al. (2009), the evolved mating distances w (open circles in Fig. 6b) are consistently driven to small values as s increases, for all scaled competition widths c. This is because non-vanishing dispersal distances make it selectively advantageous to keep the mate search local, so as to avoid producing maladaptive offspring.
Fig. 6.
Evolved dispersal and mating strategies in sexual populations with Gaussian competition, shown as a function of the environmental gradient s, for the scaled competition width c = 1.0. The scaled threshold of the dispersal strategy τ/b is shown in (a) and both the scaled dispersal distance δ/σs and scaled mating distance w/σs are shown in (b). The horizontal line in (a) indicates the average death rate, d = 1.0. Filled circles show the average of the final 5000 generations of 100 independent realizations, and the gray-shaded areas represent the respective standard deviations across realizations.
4. Discussion
Our results demonstrate that the evolution of conditional dispersal has a significant impact on parapatric speciation along environmental gradients. It is worth highlighting that even though dispersal evolution may lead to a form of isolation by distance, the ensuing speciation process remains driven by frequency-dependent competition, rather than by the gradual accumulation of reproductive incompatibilities.
In asexual populations, speciation can occur for a wide range of parameters, and is always accompanied by conditional dispersal. Both the dispersal threshold and dispersal distance of the conditional dispersal strategy are influenced by the steepness of the environmental gradient, with shallow gradients resulting in lower thresholds and higher dispersal distances, and steeper gradients resulting in higher thresholds and lower dispersal distances. In sexual populations, a similar result is obtained for the dispersal thresholds and distances. However, speciation occurs under a more restricted range of conditions. Specifically, speciation is only observed when the gradient is sufficiently steep and the competition kernel is sufficiently wide. Enhancing the disruptiveness of frequency-dependent selection, more box-shaped competition kernels dramatically lower the speciation-enabling slope of the environmental gradient. For species to emerge on more shallow gradients, some form of assortative mate preference is most likely required, as was found in the original formulation of this model (Dieckmann and Doebeli, 1999; Doebeli and Dieckmann, 2003).
For well-mixed populations, speciation via frequency-dependent disruptive selection is facilitated by narrow phenotypic competition kernels (Dieckmann and Doebeli, 1999). For populations structured along sufficiently steep environmental gradients, correlations arise between spatial position and ecological character. Because of the spatial component of the competition kernel (Eq. (5)), an environmental gradient thus induces frequency-dependent disruptive selection, which therefore occurs even when phenotypic competition kernels are wide (Doebeli and Dieckmann, 2003).
For sexual populations, speciation additionally requires reproductive isolation between phenotypic clusters (which along a gradient tend to correspond to spatial clusters). We only consider isolation by distance; therefore, for sexual populations speciation becomes easier with increasing spatial distance between these clusters, and more difficult with decreasing distance between them. While the distance between clusters is in principle determined by the width of both the spatial and the phenotypic component of the competition kernel, it is the narrower component that essentially determines this distance in practice (see also Eq. (5) in Leimar et al., 2008). Therefore, as the phenotypic component becomes wide, the distance is mostly defined by the (fixed) width of the spatial component; however, as it becomes narrow, the distance between clusters decreases to the point where speciation is hindered by the inability to achieve complete reproductive isolation. In consequence, narrow phenotypic competition kernels (corresponding to small values of c) impede speciation in the sexual case (Fig. 2c, d), but not in the asexual case (Fig. 2a, b), if isolation by distance is the only isolating mechanism considered and gradients are not steep enough to limit dispersal. The same trend was also observed by Heinz et al. (2009).
Previous studies of conditional dispersal in metapopulation structures have observed a reduction in dispersal thresholds in the absence of dispersal costs (Travis and Dytham, 1999; Metz and Gyllenberg, 2001). In the presence of explicit costs, such as increased mortality, the dispersal threshold is often found to equilibrate at the patch carrying capacity (Metz and Gyllenberg, 2001; Travis et al., 2009). Here, we have found similar results in populations structured in continuous space and subject to the implicit costs of an environmental gradient; dispersal thresholds typically evolve toward values near the population-level average death rate. Whether the dispersal threshold evolves to a value less than or greater than this population-level average is directly related to the severity of the cost imposed by the environmental gradient and the mode of reproduction, with sexual populations generally evolving higher dispersal thresholds.
The environmental gradient considered in this study influenced the carrying capacity experienced by an individual, as a function of the individual’s spatial position and ecological character. In a recent model-based study of range expansions in metapopulations, Kubisch et al. (2010) demonstrated that the elasticity of range limits varied between different kinds of environmental gradients. For environmental gradients that affected either dispersal mortality or per capita growth rate, range contractions were observed after an initial period of expansion. In contrast, when the environmental gradient impacted patch capacity or patch extinction rate, range contractions were never observed. These results highlight the potential sensitivity of ecological and evolutionary processes to different types of environmental gradients. How alternative environmental gradients influence dispersal evolution and speciation in the model investigated herein is an exciting challenge for future work.
We considered impermeable boundary conditions for the spatial dimension in which the environmental gradient varied. To test the sensitivity of our results to this assumption, we performed additional experiments in which competitive interactions are mirrored about the x-boundary. Such reflective boundary conditions led to an overall reduction in population size, relative to the impermeable case, resulting from the now increased competition experienced at the boundaries. The evolved dispersal thresholds, however, were indistinguishable between the two boundary conditions. The evolved dispersal distances were also indistinguishable for sufficiently steep environmental gradients, but for shallow gradients, they were lower for reflective boundary conditions than for impermeable boundary conditions. This is because large dispersal steps toward the boundaries are favored for the case of impermeable boundary conditions, provided the inherent risk of dispersal is low, as it is on shallow gradients. It is only in this case that the evolved dispersal distances differ between the two boundary conditions. We also note that Heinz et al. (2009) found dispersal evolution to be robust to various forms of boundary conditions.
Empirical evidence is still needed to determine the true shape of conditional dispersal strategies in natural populations (Travis et al., 2009). A variety of functional forms have been considered in theoretical studies of conditional dispersal (Bach et al., 2007; Kun and Scheuring, 2006; Metz and Gyllenberg, 2001; Travis and Dytham, 1999), and while these model details certainly matter (Ronce, 2007), most studies have observed the evolution of some form of threshold-based strategy. The question addressed here was not focused on the functional form of conditional dispersal per se, but instead on how conditional dispersal, of any form, affects parapatric speciation driven by frequency-dependent competition along environmental gradients. Preliminary experimentation with the conditional dispersal functions considered by Travis and Dytham (1999) and Kun and Scheuring (2006) produced qualitatively similar results to those reported herein.
As discussed by van Baalen and Hochberg (2001), “the ecological and evolutionary implications of how dispersal may create or destroy biological patterns are only starting to be appreciated.” Our results contribute to our understanding of these implications, by demonstrating how both spatial and phenotypic pattern formation can be generated and subsequently annihilated by the evolution of conditional dispersal, and how this relates to speciation processes. These results indicate that the stability of current spatial and phenotypic distributions should be considered in the context of dispersal plasticity, as shifts in environmental cues, e.g., such as those expected from climate change events, may severely disrupt population stability and threaten biodiversity.
Acknowledgments
J.L.P. was partially supported by Vermont EPSCoR (NSF Grant no. EPS 0701410) and a summer research grant awarded by the IEEE Computational Intelligence Society. Part of this research was conducted during J.L.P.’s participation in the Young Scientists Summer Program at the International Institute for Applied Systems Analysis, with support from the National Academy of Sciences. R.M. and U.D. gratefully acknowledge support from the Vienna Science and Technology Fund (WWTF). U.D. also gratefully acknowledges financial support from the European Commission, through the Marie Curie Research Training Network FishACE and the Specific Targeted Research Project FinE, funded under the European Community’s Sixth Framework Program. U.D. received additional support by the European Science Foundation, the Austrian Science Fund, and the Austrian Ministry of Science and Research. The authors would like to thank the two anonymous reviewers for their helpful comments and Leithen M’Gonigle, Jan Ohlberger, and Davnah Urbach for valuable discussions and comments on earlier versions of this work.
References
- Bach L, Ripa J, Lundberg P. On the evolution of conditional dispersal under environmental and demographic stochasticity. Evolutionary Ecology Research. 2007;9:663–673. [Google Scholar]
- Barton N. The evolutionary consequences of gene flow and local adaptation: future approaches. In: Clobert J, Danchin E, Dhondt A, Nichols J, editors. Dispersal. Oxford University Press; 2001. pp. 329–340. [Google Scholar]
- Dieckmann U, Doebeli M. On the origin of species by sympatric speciation. Nature. 1999;400:354–357. doi: 10.1038/22521. [DOI] [PubMed] [Google Scholar]
- Doebeli M, Blok HJ, Leimar O, Dieckmann U. Multimodal pattern formation in phenotype distributions of sexual populations. Proceedings of the Royal Society of London B. 2006;274:347–357. doi: 10.1098/rspb.2006.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebeli M, Dieckmann U. Speciation along environmental gradients. Nature. 2003;421:259–264. doi: 10.1038/nature01274. [DOI] [PubMed] [Google Scholar]
- Doligez B, Danchin E, Clobert J. Public information and breeding habitat selection in a wild bird population. Science. 2002;297:1168–1170. doi: 10.1126/science.1072838. [DOI] [PubMed] [Google Scholar]
- Eppstein M, Payne J, Goodnight C. Underdominance, multiscale interactions, and self-organizing barriers to gene flow. Artificial Evolution and Applications. 2009 725049. [Google Scholar]
- Friedenberg N. Experimental evolution of dispersal in spatiotemporally variable microcosms. Ecology Letters. 2003;6:953–959. [Google Scholar]
- Gandon S, Capowiez Y, Duboid Y, Michalakis Y, Olivieri I. Local adaptation and gene-for-gene coevolution in a metapopulation model. Proceedings of the Royal Society of London B. 1996;263:1003–1009. [Google Scholar]
- Gillespie DT. A general method for numerically simulating the stochastic time evolution of coupled chemical equations. Journal of Computational Physics. 1976;22:403–434. [Google Scholar]
- Gyllenberg M, Kisdi E, Utz M. Evolution of condition dependent dispersal under kin competition. Journal of Mathematical Biology. 2008;57:285–307. doi: 10.1007/s00285-008-0158-2. [DOI] [PubMed] [Google Scholar]
- Heinz S, Mazzucco R, Dieckmann U. Speciation and the evolution of dispersal along environmental gradients. Evolutionary Ecology. 2009;23:53–70. [Google Scholar]
- Hodgson D. An experimental manipulation of the growth and dispersal strategy of a parasitic infection using monoclonal aphid colonies. Evolutionary Ecology Research. 2002;4:133–145. [Google Scholar]
- Ims RA, Andreassen HP. Spatial synchronization of vole population dynamics by predatory birds. Nature. 2000;408:194–196. doi: 10.1038/35041562. [DOI] [PubMed] [Google Scholar]
- Ims RA, Hjermann DØ. Condition dependent dispersal. In: Clobert J, Danchin E, Dhondt A, Nichols J, editors. Dispersal. Oxford University Press; 2001. pp. 203–216. [Google Scholar]
- Kendall B, Bjørnstad O, Bascompte U, Keitt T, Fagan W. Dispersal, environmental correlation, and spatial synchrony in population dynamics. American Naturalist. 2000;155:628–636. doi: 10.1086/303350. [DOI] [PubMed] [Google Scholar]
- Kubisch A, Hovestadt T, Poethke H. On the elasticity of range limits during periods of expansion. Ecology. 2010;91:3094–3099. doi: 10.1890/09-2022.1. [DOI] [PubMed] [Google Scholar]
- Kun Á, Scheuring I. The evolution of density-dependent dispersal in a noisy spatial population model. OIKOS. 2006;115:308–320. [Google Scholar]
- Kunert G, Otto S, Röse U, Gershenzon J, Weisser WW. Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecology Letters. 2005;8:596–603. [Google Scholar]
- Leimar O, Doebeli M, Dieckmann U. Evolution of phenotypic clusters through competition and local adaptation along an environmental gradient. Evolution. 2008;62:807–822. doi: 10.1111/j.1558-5646.2008.00334.x. [DOI] [PubMed] [Google Scholar]
- Lenormand T. Gene flow and the limits of natural selection. Trends in Ecology and Evolution. 2002;17:183–189. [Google Scholar]
- Metz J, Gyllenberg M. How should we define fitness in structured metapopulation models? Including an application to the calculation of evolutionary stable dispersal strategies. Proceedings of the Royal Society of London B. 2001;268:499–508. doi: 10.1098/rspb.2000.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinen K, Dieckmann U, Gyllenberg M, Metz J. Evolution of dispersal in metapopulations with local density dependence and demographic stochasticity. Journal of Evolutionary Biology. 2003;16:143–153. doi: 10.1046/j.1420-9101.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- Ronce O. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annual Review of Ecology, Evolution, and Systematics. 2007;38:231–253. [Google Scholar]
- Roughgarden J. Evolution of niche width. American Naturalist. 1972;106:683–718. [Google Scholar]
- Roughgarden J. Species packing and the competition function with illustration from coral reef fish. Theoretical Population Biology. 1974;5:163–186. doi: 10.1016/0040-5809(74)90039-2. [DOI] [PubMed] [Google Scholar]
- Travis J, Dytham C. The evolution of density-dependent dispersal. Proceedings of the Royal Society of London B. 1999;266:1837–1842. [Google Scholar]
- Travis J, Mustin K, Benton T, Dytham C. Accelerating invasion rates result from the evolution of density-dependent dispersal. Journal of Theoretical Biology. 2009;259:151–158. doi: 10.1016/j.jtbi.2009.03.008. [DOI] [PubMed] [Google Scholar]
- van Baalen M, Hochberg ME. Dispersal in antagonistic interactions. In: Clobert J, Danchin E, Dhondt A, Nichols J, editors. Dispersal. Oxford University Press; 2001. pp. 299–310. [Google Scholar]
- Wright S. Isolation by distance. Genetics. 1943;28:114–138. doi: 10.1093/genetics/28.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. The Theory of Gene Frequencies. University of Chicago Press; 1969. [Google Scholar]