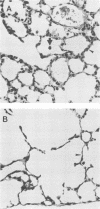
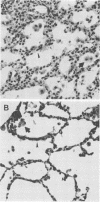
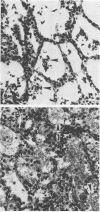
Abstract
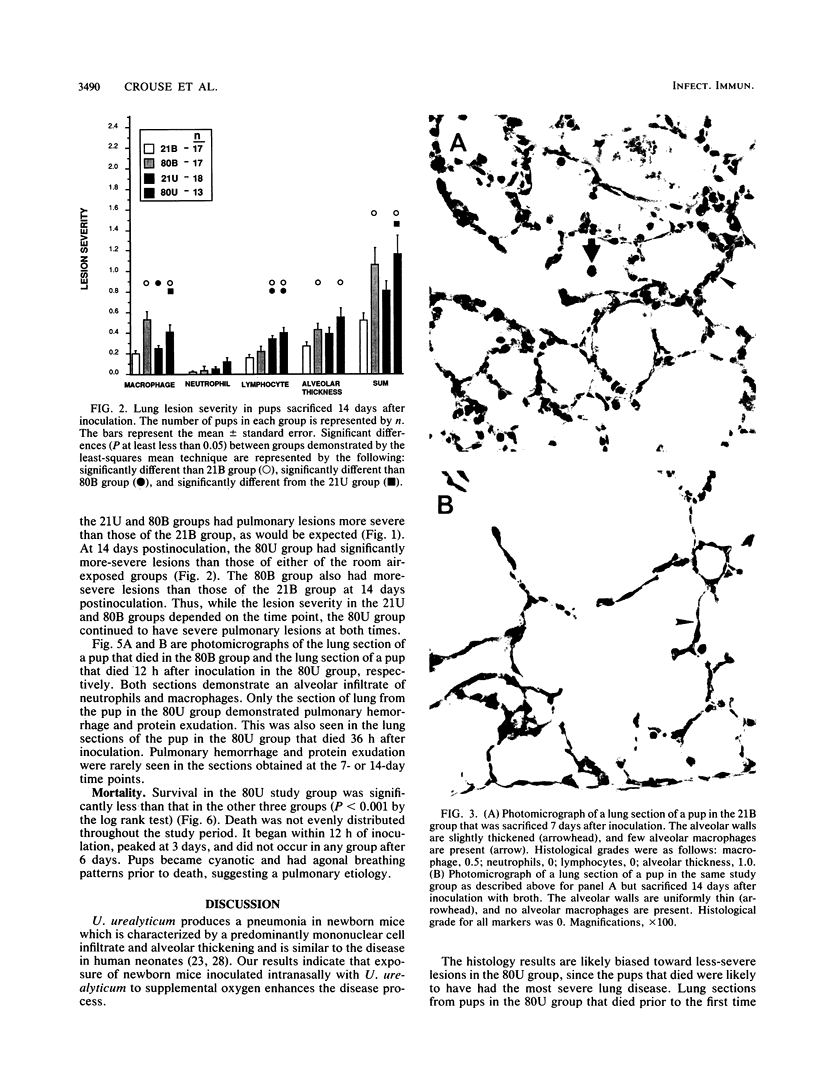
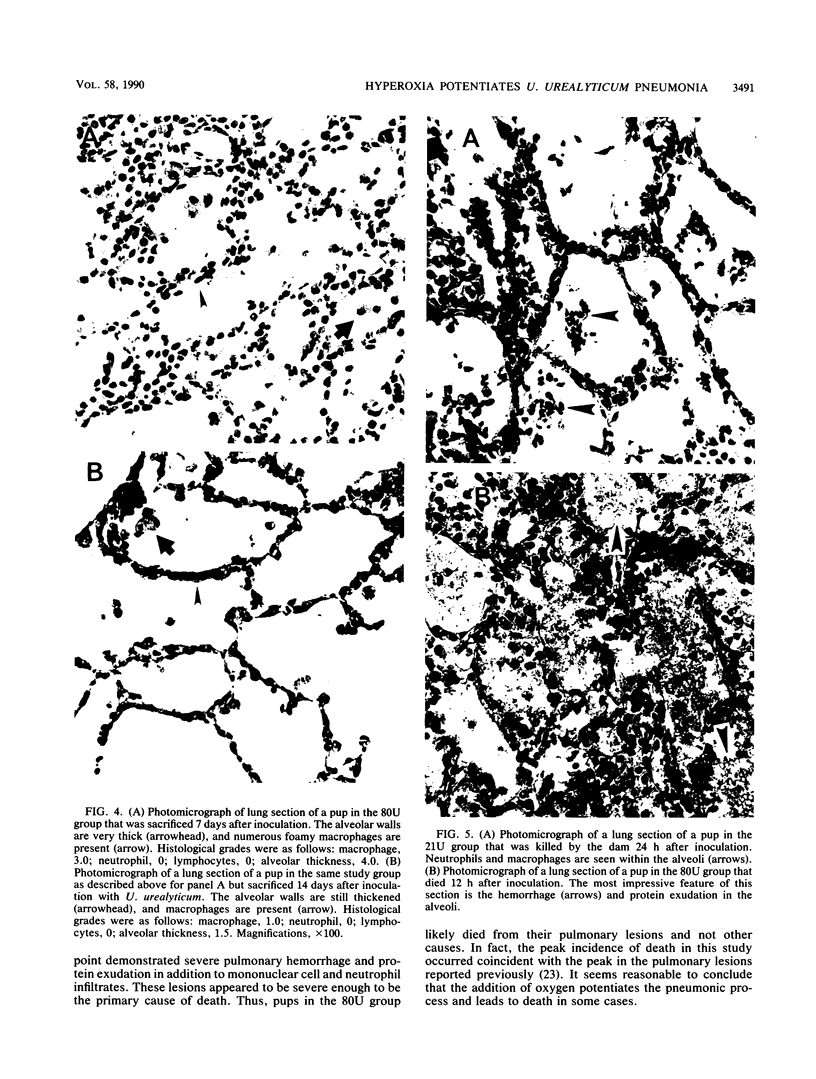
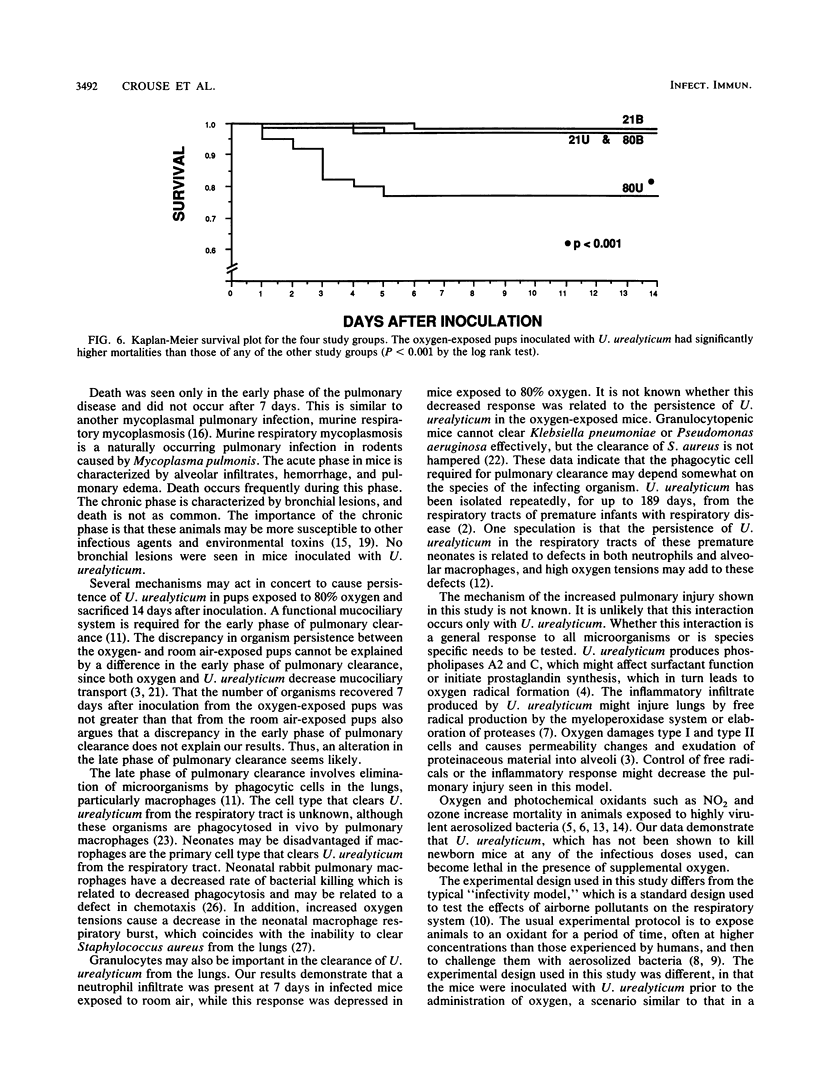
The effect of continuous exposure to 80% oxygen on newborn mice with Ureaplasma urealyticum pneumonia was determined. Mice were inoculated intranasally with either U. urealyticum or sterile broth and then housed in either 80% oxygen or room air (21% oxygen). The mice were sacrificed at either 7 or 14 days after inoculation. Significantly more mice in the U. urealyticum group housed in 80% O2 than in the room air-exposed group were culture positive 14 days after inoculation (P = 0.042), but no difference was found at 7 days. The presence of alveolar macrophages, neutrophils, and lymphocytes and alveolar wall thickness were determined. Overall, the group housed in 80% O2 and inoculated with U. urealyticum had severe pulmonary lesions at both time points, while the lesion severity in the room air-exposed group inoculated with U. urealyticum and the group housed in 80% O2 and inoculated with sterile broth was dependent on the time point. Mortality was significantly higher in the group housed in 80% O2 and inoculated with U. urealyticum than it was in all other groups (P less than 0.001). Our results indicate that hyperoxia causes the persistence of U. urealyticum in the lungs of newborn mice, acutely potentiates the inflammatory response, and turns an otherwise self-limited pneumonia into a lethal disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broderson J. R., Lindsey J. R., Crawford J. E. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol. 1976 Oct;85(1):115–130. [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Waites K. B., Crouse D. T., Rudd P. T., Canupp K. C., Stagno S., Cutter G. R. Association of Ureaplasma urealyticum infection of the lower respiratory tract with chronic lung disease and death in very-low-birth-weight infants. Lancet. 1988 Jul 30;2(8605):240–245. doi: 10.1016/s0140-6736(88)92536-6. [DOI] [PubMed] [Google Scholar]
- De Silva N. S., Quinn P. A. Endogenous activity of phospholipases A and C in Ureaplasma urealyticum. J Clin Microbiol. 1986 Feb;23(2):354–359. doi: 10.1128/jcm.23.2.354-359.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke S. M., Fanburg B. L. Normobaric oxygen toxicity of the lung. N Engl J Med. 1980 Jul 10;303(2):76–86. doi: 10.1056/NEJM198007103030204. [DOI] [PubMed] [Google Scholar]
- Ehrlich R., Findlay J. C., Gardner D. E. Effects of repeated exposures to peak concentrations of nitrogen dioxide and ozone on resistance to streptococcal pneumonia. J Toxicol Environ Health. 1979 Jul;5(4):631–642. doi: 10.1080/15287397909529775. [DOI] [PubMed] [Google Scholar]
- Ehrlich R. Interaction between environmental pollutants and respiratory infections. Environ Health Perspect. 1980 Apr;35:89–99. doi: 10.1289/ehp.803589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Feltner D. E., Brieland J. K., Ward P. A. Phagocytic cell-derived inflammatory mediators and lung disease. Chest. 1987 Mar;91(3):428–435. doi: 10.1378/chest.91.3.428. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. E. Oxidant-induced enhanced sensitivity to infection in animal models and their extrapolations to man. J Toxicol Environ Health. 1984;13(2-3):423–439. doi: 10.1080/15287398409530508. [DOI] [PubMed] [Google Scholar]
- Goldstein E. An assessment of animal models for testing the effect of photochemical oxidants on pulmonary susceptibility to bacterial infection. J Toxicol Environ Health. 1984;13(2-3):415–421. [PubMed] [Google Scholar]
- Green G. M. Similarities of host defense mechanisms against pulmonary infectious diseases in animals and man. J Toxicol Environ Health. 1984;13(2-3):471–478. doi: 10.1080/15287398409530510. [DOI] [PubMed] [Google Scholar]
- Hill H. R. Biochemical, structural, and functional abnormalities of polymorphonuclear leukocytes in the neonate. Pediatr Res. 1987 Oct;22(4):375–382. doi: 10.1203/00006450-198710000-00001. [DOI] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Higuchi J. H., Woods D. E., Gomez P., Coalson J. J. Dissemination of Pseudomonas aeruginosa during lung infection in hamsters. Role of oxygen-induced lung injury. Am Rev Respir Dis. 1985 Aug;132(2):358–361. doi: 10.1164/arrd.1985.132.2.358. [DOI] [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Lindsey J. R., Cassell H. Experimental Mycoplasma pulmonis infection in pathogen-free mice. Models for studying mycoplasmosis of the respiratory tract. Am J Pathol. 1973 Jul;72(1):63–90. [PMC free article] [PubMed] [Google Scholar]
- Northway W. H., Jr, Petriceks R., Shahinian L. Quantitative aspects of oxygen toxicity in the newborn: inhibition of lung DNA synthesis in the mouse. Pediatrics. 1972 Jul;50(1):67–72. [PubMed] [Google Scholar]
- Northway W. H., Jr, Rosan R. C., Porter D. Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967 Feb 16;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- Parker R. F., Davis J. K., Cassell G. H., White H., Dziedzic D., Blalock D. K., Thorp R. B., Simecka J. W. Short-term exposure to nitrogen dioxide enhances susceptibility to murine respiratory mycoplasmosis and decreases intrapulmonary killing of Mycoplasma pulmonis. Am Rev Respir Dis. 1989 Aug;140(2):502–512. doi: 10.1164/ajrccm/140.2.502. [DOI] [PubMed] [Google Scholar]
- Quinn P. A., Gillan J. E., Markestad T., St John M. A., Daneman A., Lie K. I., Li H. C., Czegledy-Nagy E., Klein A. Intrauterine infection with Ureaplasma urealyticum as a cause of fatal neonatal pneumonia. Pediatr Infect Dis. 1985 Sep-Oct;4(5):538–543. doi: 10.1097/00006454-198509000-00020. [DOI] [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Pierce A. K. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980 Aug;66(2):194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd P. T., Cassell G. H., Waites K. B., Davis J. K., Duffy L. B. Ureaplasma urealyticum pneumonia: experimental production and demonstration of age-related susceptibility. Infect Immun. 1989 Mar;57(3):918–925. doi: 10.1128/iai.57.3.918-925.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer M. H., Edwards D. K., Spector S. A. Cytomegalovirus infection and bronchopulmonary dysplasia in premature infants. Am J Dis Child. 1987 Mar;141(3):303–305. doi: 10.1001/archpedi.1987.04460030081030. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Condiotti R. Hyperoxia damages phagocytic defenses of neonatal rabbit lung. J Appl Physiol (1985) 1987 Feb;62(2):684–690. doi: 10.1152/jappl.1987.62.2.684. [DOI] [PubMed] [Google Scholar]
- Sherman M., Goldstein E., Lippert W., Wennberg R. Neonatal lung defense mechanisms: a study of the alveolar macrophage system in neonatal rabbits. Am Rev Respir Dis. 1977 Sep;116(3):433–440. doi: 10.1164/arrd.1977.116.3.433. [DOI] [PubMed] [Google Scholar]
- Sánchez P. J., Regan J. A. Ureaplasma urealyticum colonization and chronic lung disease in low birth weight infants. Pediatr Infect Dis J. 1988 Aug;7(8):542–546. [PubMed] [Google Scholar]
- Waites K. B., Crouse D. T., Philips J. B., 3rd, Canupp K. C., Cassell G. H. Ureaplasmal pneumonia and sepsis associated with persistent pulmonary hypertension of the newborn. Pediatrics. 1989 Jan;83(1):79–85. [PubMed] [Google Scholar]
- Wang E. E., Frayha H., Watts J., Hammerberg O., Chernesky M. A., Mahony J. B., Cassell G. H. Role of Ureaplasma urealyticum and other pathogens in the development of chronic lung disease of prematurity. Pediatr Infect Dis J. 1988 Aug;7(8):547–551. [PubMed] [Google Scholar]