Abstract
OBJECTIVE
Although patients who develop scleroderma (SSc) later in life (≥ 65 years) may express the entire clinical spectrum of disease, we hypothesize that patients with late-age onset incur a different risk for specific organ manifestations of disease compared to those with younger-age onset SSc.
METHODS
In total, 2300 SSc patients were evaluated between 1990–2009 and reviewed from a university-based Scleroderma Center cohort. Demographic profile, SSc subtype, autoantibody status, Medsger severity scores, pulmonary function tests, echocardiography, and right heart catheterization parameters were compared between late-age versus younger-age onset patients.
RESULTS
Overall, 2084 (91%) patients developed SSc prior to age 65; whereas 216 (9%) were ≥65 years. Late-age onset patients had a significantly higher proportion of anti-centromere antibodies (42% vs 27%; p=0.001) compared to younger-age onset. Risk of pulmonary hypertension (OR 1.77; 95%CI 1.00, 3.12), muscle weakness (OR 1.85; 95%CI 1.30, 2.64), renal impairment (OR 2.83; 95%CI 1.98, 4.04) and cardiac disease (OR 2.69; 95%CI 1.92, 3.78) was greater among those with late-age onset SSc; although risk of digital ischemia (OR 0.64; 95%CI 0.47, 0.86) was reduced. The cumulative incidence of pulmonary hypertension at 5 years was greater among those with late-age (9%) compared to younger-age (2.5%) onset SSc (log-rank, p<0.001).
CONCLUSION
These findings suggest that older SSc patients are at greater risk for pulmonary hypertension, renal impairment, cardiac disease, and muscle weakness. Awareness of the distinct risk for specific organ manifestations in SSc, in particular pulmonary hypertension, should guide the care of older SSc patients whose disease begins after age 65 years.
Keywords: scleroderma, systemic sclerosis, elderly, aging
Introduction
Scleroderma (SSc) is an uncommon multisystem autoimmune disease characterized by immune abnormalities, fibrosis of the skin and internal organs and an obliterative vasculopathy. It is a heterogeneous disease ranging from mild limited cutaneous features to widespread skin thickening. Internal organ involvement can be minimal with mild gastroesophageal reflux, subtle skin changes and Raynaud phenomenon as the sole manifestations. Alternatively, it can be a fulminant and catastrophic multisystem disease with interstitial lung disease, severe digital ischemia, gastrointestinal (GI) malfunction, myopathy, renal failure and/or fatal pulmonary hypertension (1).
Whereas SSc affects adults across the life spectrum, with peak age of onset between 40 to 50 years, incident cases continue to occur into late adult life (2). In fact, several US epidemiologic studies underscore incident SSc in the sixth, seventh and eighth decades of life (3–6). In one such study, the peak incidence of SSc at diagnosis among Caucasian women occurred in the 65–74 year age group (3).
Phenotypic variation by age has been established for younger SSc patients (i.e. juvenile onset SSc) who have been shown to have an increased prevalence of overlap syndrome with myositis, SSc specific antibodies (anti-PM-Scl, anti-U1RNP), and improved survival compared with adult onset SSc (7). Surprisingly, whether the SSc disease phenotype varies with older age of SSc onset is unclear. This lack of clarity stems in part from inconsistent previous descriptions of SSc features in the elderly. Whereas early case descriptions suggested that late-age onset SSc represented a milder form of disease with limited morbidity and minimal skin and internal organ involvement (8–10); subsequent larger case series, demonstrated the severity and breadth of organ involvement in late-age onset SSc (11–13). Furthermore, whether the limited versus the diffuse cutaneous subset of disease predominates in the elderly is a matter of ongoing debate. For example, both a Hungarian and an American cohort demonstrated that the majority of patients with late-age onset SSc had diffuse disease (12, 13). Other descriptions of SSc in the elderly favor subtle and minimal skin thickening more consistent with the limited subtype (8, 9). It is also unclear whether the cardinal organ systems affected by SSc (e.g. skin, cardiac, pulmonary, musculoskeletal, gastrointestinal, renal) are more or less frequently involved in the older SSc population. In this regard, a prior report from our Center demonstrated a two-fold increase in the risk of pulmonary hypertension among patients with SSc onset after age 60 (14).
Although patients who develop SSc later in life (≥65 years of age) can express the entire clinical spectrum of the disease, we hypothesize that those with late-age onset will have a greater risk for specific organ manifestations compared to those with younger-age onset.
Patients and Methods
Patients and Measurements
Our investigation surveyed a large, well characterized cohort of SSc patients to determine the clinical features of late-age onset SSc. The collection of clinical, serologic, and diagnostic data was approved for this study by the Johns Hopkins Office of Human Subjects Research and Institutional Review Board. Clinical and laboratory data are collected at the first visit to the Johns Hopkins Scleroderma Center (JHSC) and then prospectively ascertained in an ongoing longitudinal database among those with follow-up visits to the Center. Each patient satisfied one of the following three criteria: [A] ACR criteria for scleroderma (15), [B] 3 or more of the 5 features of the CREST syndrome (16, 17), or [C] the combination of definite Raynaud phenomenon, abnormal nailfold capillaries and a SSc-specific autoantibody (18). A total of 2481 patients with SSc were enrolled in the database from 1990 until 2009, among which 2300 had a recorded date of SSc onset and comprehensive assessments, and therefore were included in the present analyses.
We defined SSc onset at age of first non-Raynaud symptom. Patients were categorized as having late-age onset SSc if this occurred at age 65 or older. Demographic data including sex, race, diabetes, smoking status (dichotomized as current or former versus never), and SSc subtype, based on classification criteria established by LeRoy et al (16, 18), were recorded on initial presentation to the JHSC and updated if new information became available. Patients with skin thickening proximal to the elbows or knees (at any time during their illness) were categorized as having the diffuse cutaneous scleroderma subtype; all other patients were categorized as having limited cutaneous disease. Serologic status was recorded on initial presentation to the JHSC and updated if new information became available. Clinical data regarding patients' SSc included all available pulmonary function testing, echocardiography, Medsger severity scores (MSS) (19, 20) and modified Rodnan skin scores. For patients with return visits to JHSC, these data were updated every 6 months or as it became available. Functional status was evaluated using the Health Assessment Questionnaire Disability Index (HAQ-DI) and the Scleroderma Health Assessment Questionnaire (SHAQ) on the first visit to the JHSC and on follow-up if applicable. Measurement of lung volumes (FVC, FEV1, TLC) and diffusing capacity (DLCO) by pulmonary function testing were standardized (21, 22) and reported as percent predicted.
Given that disease features may vary with time and in keeping with our hypothesis that patients with late-age onset SSc have a greater risk for organ impairment compared to younger-age onset SSc patients, we characterized the involvement of each major organ system by its most abnormal value. We abstracted the maximum recorded HAQ-DI, SHAQ, MSS, and Rodnan skin scores to define organ impairment any time during initial or follow-up visits to the JHSC. Similarly, an individual's minimum value for each lung function parameter and ejection fraction was used to define peak pulmonary and cardiac disease, respectively.
Definitions for organ involvement
Pulmonary hypertension was defined by right heart catheterization with a resting mean pulmonary artery pressure ≥ 25 mmHg in the setting of a pulmonary capillary wedge pressure <15 mmHg. We also examined the outcome of pulmonary hypertension defined by echocardiographic evidence of elevated pulmonary vascular pressures (RVSP ≥40mmHg) or right heart cardiac catheterization parameters.
Restrictive lung disease (RLD) was defined as FVC less than 70% in an individual with a non-obstructive (FEV1/FVC ≥70%) pattern on pulmonary function testing.
Cardiac disease was defined as cardiac MSS ≥1 which includes clinically significant conduction or structural cardiac abnormalities or overt heart failure.
Digital ischemia was defined by peripheral vascular / Raynaud phenomenon MSS ≥2 which includes digital pitting, ulcers or gangrene and indicates the presence of tissue damage.
Severe GI involvement was defined as GI MSS ≥ 2 which includes any GI involvement beyond gastroesophageal reflux requiring routine treatment.
Renal involvement was defined as renal MSS ≥1, which is based exclusively on renal indices, and includes a creatinine ≥1.3 mg/dL or ≥2+ protein on urine dipstick.
Muscle weakness was defined by muscle MSS ≥1 which equates to motor strength <5/5 in upper or lower extremity proximal muscle groups.
Statistical Analysis
Continuous, normally distributed variables were summarized as mean value ± standard deviation. Discrete variables were summarized as proportions. The MSS is an ordinal measurement; hence central tendency was reported as a median with nonparametric testing (Wilcoxon rank sum test) to determine equality of the distributions. To account for missing data, denominators in proportions only included individuals with data available for that particular variable. Differences between means and proportions were examined using Student t-test for continuous and Chi-square test for discrete, dichotomous variables.
Logistic regression was used to calculate odds ratios and determine the risk of SSc organ involvement as a function of age at SSc onset. Age was modeled as a dichotomous variable (cut-off ≥65 years) to specifically evaluate the impact of late-age compared to younger-age SSc onset. Age was also modeled as a continuous variable in 10-year periods to determine the effect of age across the entire life spectrum. Multivariate logistic regression was performed for each outcome adjusting, as appropriate, for race, gender, SSc subtype, smoking status, disease duration, and FVC (continuous variable).
We undertook stratified analyses based on potential effect modifiers. Specifically, obstructive lung disease (FEV1/FVC <70%) is common in the older population (23–25) and could be an effect modifier when evaluating for the effect of age on risk of RLD. In addition, diabetes may be a potential effect modifier of digital ulcer severity, and we therefore stratified on this variable as well.
Kaplan-Meier cumulative incidence plots were generated to evaluate time to development of pulmonary hypertension among all cohort participants with 2 or more visits to the Scleroderma Center (26). Pulmonary hypertension was selected as the outcome for this analysis because previous literature has described its association with age of SSc onset (14, 27–29). Kaplan-Meier cumulative incidence curves using both definitions of pulmonary hypertension were generated. These analyses were conducted with the origin defined as SSc onset (first non-Raynaud SSc symptom) and the patients dichotomized by age of SSc onset. Pulmonary hypertension is a prominent vascular manifestation of SSc, and analyses were repeated using `onset of Raynaud phenomenon' as the origin and then dichotomized by age of Raynaud onset. Log-rank testing was used to compare the incidence rates in each analysis between those with SSc onset before and after age 65 (30). Statistical analyses were performed using Stata IC 10.0. All reported p-values are two sided with α=0.05.
Results
Overall Characteristics of the Population
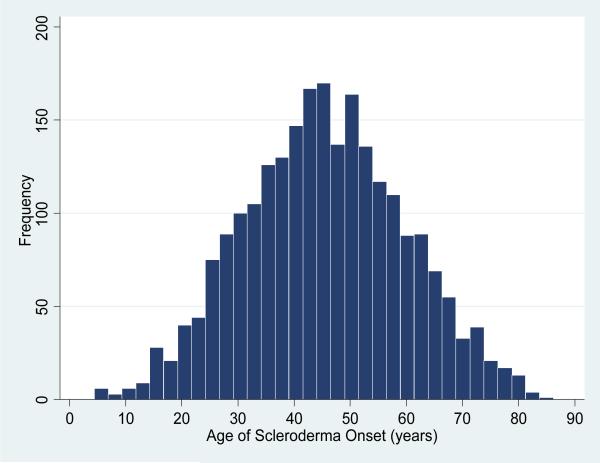
Among the study population of 2300 patients with SSc, 1909 (83%) were women and 403 (18%) were African-American. In addition, 1900 (83%) met ACR criteria for SSc, 384 (17%) satisfied 3 or more of the 5 features of the CREST syndrome, and 16 (0.7%) met criteria by the combination of definite Raynaud phenomenon, abnormal nailfold capillaries and a SSc-specific autoantibody. The mean age of SSc onset among the entire cohort was 45.5 years (Figure 1). In the cohort, 2084 (91%) had disease onset before age 65 and 216 (9%) at 65 years or older. Of those with late-age onset SSc, 105 (49%) had onset between 65–70 years, 68 (31%) between 70–75 years, 36 (17%) between 75–80 years, and 7 (3%) had onset at greater than 80 years of age. For the overall cohort, the mean age of Raynaud onset was 42 ± 15 years, mean age of first non-Raynaud symptom was 46 ± 14.3 years; 1449 (63%) had the limited SSc subtype.
Figure 1.
Age of scleroderma onset as defined by age of first non-Raynaud's scleroderma symptom among 2300 patients evaluated at the Johns Hopkins Scleroderma Center from 1990–2009
Overall, 1521 (66%) patients had more than one recorded visit to the JHSC; however, the median number of follow-up visits was significantly higher among the younger compared to the older SSc patients (Table 1). The mean duration of follow-up at the JHSC among this group was also significantly greater for those with younger compared to late-age onset SSc (5±4 years vs 4±3 years; p<0.001). On average, patients with late-age onset SSc were diagnosed by a physician faster than those with younger age of SSc onset (0.5±2.4 years vs 2±5 years; p<0.001), and similarly had a shorter disease duration at initial presentation to the JHSC (2±3 years vs 6±8 years; p<0.001).
Table 1.
Demographics, disease duration, and serologic profile among 2300 SSc patients evaluated from 1990–2009.
| Age of onset < 65 years (n = 2084) | Age of onset > 65 years (n = 216) | p-value | |
|---|---|---|---|
| Female | 1725 (83%) | 184 (85%) | 0.369 |
| African-American | 379 (18%) | 24 (11%) | 0.040 |
| Limited subtype | 1302 (62%) | 147 (68%) | 0.108 |
| Smoking status current or former§ | 992 (48%) | 105 (50%) | 0.601 |
| Median number of visits to Scleroderma Center, range | 3 (1,28) | 2 (1,19) | <0.001 |
| Mean number of years of follow up from first visit to Scleroderma Center among patients with >1 visit§§ (± SD*) | 5 ± 4 | 4 ± 3 | <0.001 |
| Scleroderma Duration, mean ± SD | |||
| Disease duration (years) at time of 1st visit to Scleroderma Center | 6 ± 8 | 2 ± 3 | <0.001 |
| Duration (years) of RP** at time of 1st visit to Scleroderma Center† | 9 ± 10 | 8 ± 13 | 0.4113 |
| Age of first non-RP symptom in years | 43 ± 12 | 71 ± 5 | <0.001 |
| Age of RP onset in years† | 40 ± 13 | 65 ± 13 | <0.001 |
| Years from RP onset to first non-RP SSc symptom† | 3 ± 8 | 6 ± 13 | <0.001 |
| Years from first non-RP SSc symptom to diagnosis of SSc by physician†† | 2 ± 5 | 0.5 ± 2.4 | <0.001 |
| Serology | |||
| ANA | 1259/1306 (96%) | 120/124 (97%) | 0.831 |
| Anti-topoisomerase I | 277/1175 (23%) | 18/107 (17%) | 0.112 |
| Anti-centromere | 348/1288 (27%) | 50/119 (42%) | 0.001 |
| Anti-U1RNP | 102/1084 (9%) | 3/99 (3%) | 0.033 |
Data analyzed from 2272 individuals with data recorded regarding smoking status
Data analyzed from 1521 individuals with more than one visit to the Scleroderma Center
SD = standard deviation
RP = Raynaud phenomenon
Data analyzed from 2227 individuals with a reported date of onset for Raynaud phenomenon
Data analyzed from 2291 individuals with a reported date of diagnosis by a physician
Patient demographics, SSc duration, and serology are summarized in Table 1 by age of SSc onset. Individuals with late-age onset SSc had a mean age of Raynaud onset of 65 ± 13 years, mean age of first non-Raynaud symptom of 71 ± 5 years, and 147 (68%) had the limited SSc subtype. Among individuals with SSc onset after age 75 (n=43), 34 (79%) had the limited subtype. Late-age onset patients had more anti-centromere antibodies (50 of 119 [42%] vs 348 of 1288 [27%]; p=0.001) but less anti-U1RNP antibodies (3 of 99 [3%] vs 102 of 1084 [9%]; p=0.033) than those with younger-age onset disease.
Prevalence of Organ Involvement
Late-age onset SSc patients had the same median maximum MSS as their younger counterparts in the domains of renal, cardiac, GI, and muscle reflecting the highly skewed distribution of these measures. However, by non-parametric testing the MSS distributions between older and younger patients were significantly different (p<0.05). Older SSc patients had a lower median MSS in the Raynaud domain compared to younger-age onset SSc patients (1 vs 2; p<0.001). SSc organ involvement by dichotomized MSS is summarized in Table 2. In addition, there was no significant difference between prevalence of renal crisis between the older compared to younger-age onset SSc patients (11 of 216 [5%] vs 63 of 2084 [3%]; p=0.101).
Table 2.
Organ involvement and functional measures among 2300 SSc patients evaluated from 1990–2009.
| Frequency of Dichotmized Medsger Severity Scores (%) | |||||
|---|---|---|---|---|---|
| Age of onset < 65 years | Age of onset ≥ 65 years | ||||
| No | Yes | No | Yes | p-value | |
| Cardiac Disease* | 1386 (77) | 425 (23) | 113 (63) | 67 (37) | <0.001 |
| Digital Ischemia§ | 893 (43) | 1180 (57) | 133 (62) | 80 (37) | <0.001 |
| GI Involvement† | 206 (19) | 857 (81) | 31 (34) | 59 (66) | 0.001 |
| Renal Involvement** | 1536 (84) | 302 (16) | 127 (69) | 57 (31) | <0.001 |
| Muscle Weakness†† | 1384 (77) | 418 (23) | 118 (68) | 56 (32) | 0.008 |
| Maximum Scleroderma Health Assessment Questionnaire Score, mean ± SD (N) | |||
|---|---|---|---|
| Age of onset < 65 years | Age of onset ≥ 65 years | p-value | |
| General disease | 1.58 ± 2.70 (1478) | 1.50 ± 0.91 (138) | 0.742 |
| Intestine | 0.95 ± 0.97 (1475) | 0.79 ± 0.90 (136) | 0.059 |
| Breathing | 1.00 ± 0.98 (1482) | 1.07 ± 1.01 (135) | 0.435 |
| Raynaud phenomenon | 1.28 ± 1.04 (1461) | 0.94 ± 0.92 (131) | <0.001 |
| Digital ulcers | 0.97 ± 1.22 (1463) | 0.66 ± 0.97 (129) | 0.005 |
| Maximum General Health Assessment Questionnaire Disability Index, mean ± SD (N) | |||
|---|---|---|---|
| Score | 1.11 ± 0.8 (1847) | 1.23 ± 0.8 (185) | 0.055 |
Cardiac MSS ≥1 which includes clinically significant conduction or structural cardiac abnormalities or overt heart failure
Raynaud MSS ≥2 which includes digital pitting, ulcers or gangrene and indicates the presence of tissue damage
GI MSS ≥ 2 which includes any GI involvement beyond gastroesophageal reflux requiring routine treatment
Renal MSS ≥1 which includes a creatinine ≥1.3 mg/dL or ≥2+ protein on urine dipstick
Muscle MSS ≥1 which equates to motor strength <5/5 in upper or lower extremity proximal muscle groups
The mean maximum HAQ-DI was higher for older versus younger SSc patients, but this difference was not statistically significant (mean maximum score 1.23 ± 0.8 vs 1.11 ± 0.8; p=0.055). The mean maximum HAQ-DI increased with age of SSc onset: 65–69 years (1.13±0.82), 70–74 years (1.28±0.78), 75–79 years (1.34±0.80), ≥80 years (1.68±0.66).
SSc lung involvement, as assessed by pulmonary function testing (Table 3), demonstrated a greater proportion of late-age onset patients with an obstructive pattern (FEV1/FVC <70%) compared to younger-age onset patients (56 of 179 [31%] vs 363 of 1808 [20%]; p=0.001). Among those with a non-obstructive pattern, FVC (76 ± 22% vs 69 ± 21%; p=0.001) and TLC (85 ± 26% vs 78 ± 21%; p=0.001) were significantly higher in the older compared to younger SSc patients. In contrast, percent predicted DLCO was similar between the two groups (65 ± 29% vs 62 ± 26%; p=0.176).
Table 3.
Cardiopulmonary diagnostic testing among 2300 SSc patients evaluated from 1990–2009.
| Age of onset < 65 years | Age of onset > 65 years | p-value | |
|---|---|---|---|
| Pulmonary Function Tests | |||
| No obstruction: FEV1/FVC ≥ 70% | 1445/1808 (80%) | 123/179 (69%) | 0.001 |
| Mean ± SD* FEV1/FVC | 0.79 ± 0.06 | 0.79 ± 0.06 | 0.109 |
| Mean ± SD minimum percent predicted FVC | 69 ± 21 | 76 ± 22 | 0.001 |
| Mean ± SD minimum percent predicted TLC | 78 ± 21 | 85 ± 26 | 0.001 |
| Mean ± SD minimum percent predicted DLCO | 62 ± 26 | 65 ± 29 | 0.176 |
| Obstruction: FEV1/FVC < 70% | 363/1808 (20%) | 56/179 (31%) | 0.001 |
| Mean ± SD FEV1/FVC | 0.62 ± 0.12 | 0.61 ± 0.09 | 0.654 |
| Mean ± SD minimum percent predicted FVC | 70 ± 21 | 74 ± 17 | 0.129 |
| Mean ± SD minimum percent predicted TLC | 83 ± 21 | 93 ± 26 | 0.002 |
| Mean ± SD minimum percent predicted DLCO | 58 ± 23 | 54 ± 25 | 0.211 |
| Echocardiography, mean ± SD | |||
| Minimum ejection fraction (n=1736) | 56 ± 9 | 55 ± 10 | 0.133 |
| Maximum RVSP, mmHg (n=1428) | 44 ± 21 | 49 ± 20 | 0.007 |
| Right heart cardiac catheterization measures | |||
| PA pressure ≥25mmHg and PCWP ≤15 mmHg | 151 (7%)* | 19 (9%)* | 0.413 |
| PA pressure ≥25mmHg and PCWP >15 mmHg | 35 (2%)* | 6 (3%)* | 0.248 |
260 right heart catheterizations were completed among patients with younger SSc onset (< 65 years); and 35 right heart catheterizations were completed among patients with late-age SSc onset (≥ 65 years). The percentages represent number of catheterizations meeting criteria out of total number of younger SSc onset patients (n=2084) or late-age SSc onset patients (n=216).
By echocardiography (Table 3) there was no difference in minimum ejection fraction (55 ± 10% vs 56 ± 9%; p=0.133) between the two groups. However, the older patients did have a higher mean maximum RVSP compared to younger patients (49 ± 20mmHg vs 44 ± 21mmHg; p=0.007). Thirty-five (16%) individuals with late-age onset SSc and 260 (12%) with younger-age SSc onset underwent right heart catheterization. There was an approximately equal proportion of late-age onset patients with pulmonary hypertension by right heart catheterization as among younger-age onset patients (19 of 216 [9%] vs 151 of 2084 [7%]; p=0.413). Using the broader definition of pulmonary hypertension consisting of both right heart catheterization parameters and elevated RVSP by echocardiography, there was a significantly higher proportion of patients with pulmonary hypertension among older compared to younger SSc patients (97 of 216 [45%] vs 627 of 2084 [30%]; p<0.001).
Multivariate Analysis of Organ Risk
Table 4 summarizes the risk of organ involvement by age of SSc onset. When dichotomizing age of SSc onset at 65 years, the relationship between those with late-age onset and pulmonary hypertension defined by cardiac catheterization was of borderline significance (p=0.05) after adjustment for race, gender, SSc subtype, FVC, and disease duration (OR 1.76; 95%CI 1.00, 3.12). Furthermore, when age was modeled as a continuous variable, there was a 9% increase in the unadjusted odds of having pulmonary hypertension (OR 1.09; 95%CI 0.98, 1.22) as measured by right heart catheterization for each 10-year increase in age of SSc onset. This relationship was strengthened and was statistically significant after adjustment for race, gender, SSc subtype, FVC, and disease duration (OR 1.34; 95%CI 1.17, 1.54). The relationship between late-age onset SSc and odds of pulmonary hypertension was also strengthened when the definition of pulmonary hypertension was broadened to include both right heart catheterization and elevated RVSP by echocardiography; this association was maintained when age was modeled as a continuous (OR 1.66; 95%CI 1.52,1.81) or dichotomous variable (OR 3.28; 95%CI 2.34, 4.60) demonstrating more than three times the risk of developing pulmonary hypertension among those with late-age onset SSc compared to younger-age onset SSc in the adjusted analysis.
Table 4.
Risk of organ involvement in scleroderma◇
| Outcome | Age-onset† modeled as dichotomous variable OR (95% CI) | Age-onset† modeled as continuous variable†† OR (95% CI) |
|---|---|---|
| Pulmonary hypertension (right heart catheterization) n=2300 | ||
| Unadjusted | 1.23 (0.75, 2.03) | 1.09 (0.98, 1.22) |
| Adjusted* | 1.76 (1.00, 3.12) | 1.34 (1.17, 1.54) |
| Pulmonary hypertension (echocardiogram or right heart catheterization) n=2300 | ||
| Unadjusted | 1.89 (1.42, 2.52) | 1.25 (1.17, 1.33) |
| Adjusted* | 3.28 (2.34, 4.60) | 1.66 (1.52, 1.81) |
| Restrictive lung disease (FVC <70%): among non-obstructed n=1568 | ||
| Unadjusted | 0.69 (0.47, 1.00) | 0.95 (0.88, 1.02) |
| Adjusted** | 0.80 (0.54, 1.19) | 1.01 (0.93, 1.09) |
| Cardiac disease n=1991 | ||
| Unadjusted | 1.93 (1.40, 2.66) | 1.30 (1.21, 1.40) |
| Adjusted** | 2.69 (1.92, 3.78) | 1.60 (1.46, 1.75) |
| Muscle weakness n=1976 | ||
| Unadjusted | 1.57 (1.12, 2.20) | 1.09 (1.01, 1.17) |
| Adjusted§ | 1.85 (1.30, 2.64) | 1.14 (1.05, 1.23) |
| Digital ischemia n=2286 | ||
| Unadjusted | 0.45 (0.34, 0.61) | 0.75 (0.71, 0.80) |
| Adjusted§§ | 0.64 (0.47, 0.86) | 0.82 (0.76, 0.87) |
| Severe gastrointestinal involvement n=1153 | ||
| Unadjusted | 0.46 (0.29, 0.72) | 0.92 (0.83, 1.02) |
| Adjusted§ | 0.80 (0.49, 1.30) | 1.11 (0.99, 1.25) |
| Renal impairment n=2022 | ||
| Unadjusted | 2.28 (1.63, 3.19) | 1.27 (1.17, 1.38) |
| Adjusted§ | 2.83 (1.98, 4.04) | 1.41 (1.28, 1.55) |
Age modeled as both dichotomous and continuous variables in logistic regression
Late-onset defined as first non-Raynaud's scleroderma symptom ≥ 65 years of age
Age-onset per ten years
Adjusted for race, sex, subtype, disease duration, FVC
Adjusted for race, sex, subtype, disease duration, smoking status
Adjusted for race, sex, subtype, disease duration
Adjusted for race, sex, subtype, disease duration, smoking status
Among those with non-obstructive pulmonary function tests (n=1568, FEV1/FVC ≥70%), age of SSc onset did not significantly increase the risk of RLD (FVC ≤70%) (Table 4) when modeled as a continuous (OR 1.01; 95%CI 0.93, 1.09) or dichotomized (OR 0.80; 95%CI 0.54, 1.19) variable. The adjusted risk of cardiac disease, as assessed by MSS, was significantly greater among those with late-age onset SSc (OR 2.69; 95%CI 1.92, 3.78).
Muscle weakness was associated with age (OR 1.09; 95%CI 1.01, 1.17), particularly among those with SSc onset after age 65 (OR 1.57; 95%CI 1.12, 2.20). After adjusting for race, gender, SSc subtype, and disease duration, those with SSc onset after age 65 had an 85% increase in the odds of weakness compared to those with younger-age onset (OR 1.85; 95%CI 1.30, 2.64).
Late-age onset SSc patients had a 36% reduction in risk for digital ischemia (OR 0.64; 95%CI 0.47, 0.86) compared to those with younger-age onset disease after adjustment for race, gender, SSc subtype, disease duration and smoking status. This finding was congruent with less impairment on the digital ulcer domain of the SHAQ (Table 2). Among diabetics (n=95), there was not an increase in risk of digital ischemia among those with late-age compared younger-age onset SSc (OR 2.99; 95%CI 0.58, 15.39). Risk of severe GI involvement yielded conflicting results but did not reach statistical significance (Table 4). Late-age onset SSc patients had an increased risk of renal impairment (OR 2.83; 95%CI 1.98, 4.04).
Cumulative Incidence of Pulmonary Hypertension
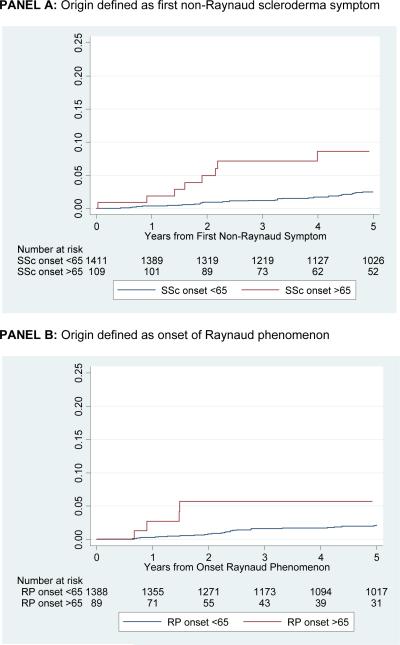
Figure 2a shows time to development of pulmonary hypertension by right heart catheterization from the onset of SSc. Regardless if the origin is defined at time of first non-Raynaud SSc symptom (Figure 2a, Panel A), or at the onset of Raynaud phenomenon (Figure 2a, Panel B) individuals with late-age onset disease develop pulmonary hypertension at a greater rate than those with younger age onset disease (p <0.001). The cumulative incidence of pulmonary hypertension at 5 years from SSc onset was 9% (95% CI 4, 17%) among the older compared to 2% (95% CI 2, 3%) among the younger SSc patients (log-rank p-value <0.001).
Figure 2a.
Cumulative incidence of pulmonary hypertension, defined by right heart catheterization, by age of scleroderma onset among patients with two or more visits to the Johns Hopkins Scleroderma Center. P<0.001
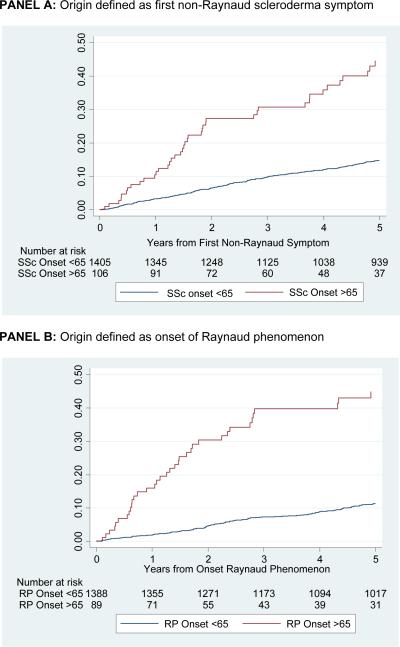
Figure 2b shows time to development of pulmonary hypertension as measured by right heart catheterization or RVSP ≥40mmgHg by echocardiography. Similar to the findings for Figure 2a, regardless if the origin is defined at time of first non-Raynaud SSc symptom (Figure 2b, Panel A), or at the onset of Raynaud phenomenon (Figure 2a, Panel B) patients with late-age onset SSc have a higher cumulative incidence of pulmonary hypertension (log-rank p-value <0.001).
Figure 2b.
Cumulative incidence of pulmonary hypertension, defined by right heart catheterization or echocardiogram, by age of scleroderma onset among patients with two or more visits to the Johns Hopkins Scleroderma Center. P<0.001
Discussion
While the full spectrum of SSc is seen among those with late-age onset SSc, our study suggests that these patients are at greater risk for pulmonary hypertension, cardiac disease, muscle weakness, and renal impairment compared to those with younger-age onset disease. However, older patients surprisingly have less severe Raynaud with reduced risk for digital ischemic ulcers compared to those with younger-age SSc onset. In addition, older patients have a higher prevalence of anti-centromere antibodies but not the limited cutaneous subtype, compared to their younger counterparts. The prevalence of anti-U1RNP antibodies was significantly less frequent among patients with late-age onset SSc. These data confirm that late-age onset SSc (age ≥65 years) occurs frequently, representing almost 10% of this large university-based cohort. Our study in fact demonstrated that the new onset of SSc occurs even in the very old with 43 individuals over the age of 75, including seven octogenarians, in our cohort.
We confirmed that pulmonary hypertension is a significant health concern among older SSc patients. Using the gold standard for pulmonary hypertension measurement, cardiac catheterization, individuals with SSc onset after age 65 had almost twice the risk of developing pulmonary hypertension compared to their younger counterparts. Our study, therefore, is similar to the findings of other investigators regarding the strong association between age of SSc onset and pulmonary hypertension (14, 29, 31). However, our study is unique in that we examined this relationship by cardiac catheterization which allowed for a more precise measurement of right sided pressures, particularly in an older population where there may be considerable left-sided heart disease (27). This may explain some of the increase in risk when an echocardiographic definition was included as diastolic dysfunction is prevalent among the older population (32).
By pulmonary function testing, late-age onset SSc patients had more obstructive lung disease (FEV1/FVC <70%). We speculate this is predominately an effect of age, an established risk factor for chronic obstructive pulmonary disease (25). Among non-obstructed patients, there was suggestion of less RLD risk as measured by FVC among older SSc patients which may be expected in a group of patients with higher rates of anti-centromere positivity. The influence of age-related changes in lung and SSc is not known nor has it been described.
Late-age onset SSc was surprisingly protective against digital ischemia in our cohort. This was consistent from the patient perspective (Raynaud severity and digital ulcer severity domains of SHAQ), provider assessment (MSS), and in multivariate logistic regression. Young age of Raynaud onset has previously been described as a risk factor for digital ulcers in SSc (33, 34). Walker et al, examined a cohort of 3064 SSc patients and categorized them into `early' or `late-onset' SSc by age of Raynaud onset in relation to the cohort mean (42.9 years) (33). With this dichotomization, they found that individuals with early-onset Raynaud had more digital ulcers. One can speculate that Raynaud phenomenon in an older host with SSc may be influenced by a number of factors, such as: the impact of an aging immune system, aging related changes of the skin's microcirculation response to cold (35, 36), generalized decreases in vascular compliance (37), and differences in cold exposure between younger and older individuals (i.e. more cold avoidance among the elderly). At this time, the reasons for these differences between those with younger-age versus older-age onset SSc are unknown and warrant further study with longer prospective follow up among older SSc patients to determine if this SSc feature develops over time.
The risk for SSc organ involvement, as assessed by MSS, was greater for those with late-age onset SSc in renal and cardiac domains. We speculate these findings may be due to age-related changes and comorbid conditions. Particularly for these two domains, the MSS is not specific for SSc-related disease. When examining other measures of kidney and cardiac disease, we found no difference in the prevalence of renal crisis or ejection fraction (measured by echocardiography) based on age of SSc onset. The risk for severe GI involvement, as assessed by MSS, yielded conflicting results and objective testing was not available for comparison. The impact of age on these SSc-specific domains has not previously been described.
Finally, in our cohort we identified significant functional limitations among individuals with late-age onset SSc. Individuals with late-age onset disease were more disabled overall as measured by HAQ-DI scores compared to their younger counterparts. They also had more muscle weakness. These findings persisted in multiple logistic regression with a 57–85% increase in the odds of muscle weakness among those with SSc onset after age 65. To our knowledge, the association we describe between muscle weakness and age of SSc onset has not been described in other epidemiologic SSc cohorts. The reason for this difference in strength is unclear. Muscle mass and strength have been demonstrated to decline with age (38). Loss of muscle density and weakness in the general older population has been associated with increased risk of hospitalization (39), and muscle weakness has been associated with pathologic states of aging such as frailty (40). HAQ-DI scores also increase with age in the general population (41). This is most notable after age 75 when the mean HAQ-DI increases sharply from 0.34 ± 0.64 (women age 70–74 years) to 0.77 ± 0.72 (women age 75–59 years) with even greater increases after age 80 (1.49 ± 1.08) (41). It is notable that older SSc patients in addition to having higher HAQ-DI scores compared to their younger counterparts, also have higher mean HAQ-DI scores compared to age-matched non-SSc counterparts (41).
Although we demonstrated that age was associated with functional decline, as measured by HAQ-DI score, the impact of age-related changes in muscle superimposed on SSc is not known. Objective muscle data (EMG, CK, biopsy) in this cohort was limited to a very small number of patients overall, particularly among those with late-age onset SSc, and therefore was not included in these analyses. Therefore, we are not able to make conclusions if differences in strength are due to structural muscle changes (myopathy, myositis, atrophy) or simply differences in rates of testing.
The reasons for the differences between those with younger-age versus older-age onset SSc are unknown and need further study. These changes which are part of normal aging may lead to a physiology that is exaggerated in the mileu of SSc. The strengths of our study include the large cohort size with a large proportion of elderly and comprehensive clinical assessments. We used two definitions for pulmonary hypertension which support the validity of these findings.
There are limitations of this study. We did not have a control group of elderly age-matched individuals without SSc with whom to make comparisons on non-SSc measures. There may be data acquisition bias in that older individuals may be less likely to be referred for invasive procedures (i.e. cardiac catheterizations, muscle biopsies). Next, approximately 35% of the cohort had a single visit, among whom the older SSc patients were disproportionately represented. In this group, significant future events and organ involvement may be missed. Hence, these data may under-represent the organ severity and involvement in this group. Finally, although the MSS can be a useful standardized tool by which to measure SSc organ involvement broadly, it lacks specificity in certain domains (renal and cardiac domains not specific to SSc) and may be differentially abnormal based on age-related factors. However, our use of additional objective data, such as echocardiography, pulmonary function tests, and right heart catheterization values provided additional corroborative data on which to draw meaningful conclusions.
In conclusion, we have demonstrated in a large cohort of elderly SSc patients that the full spectrum of SSc features can manifest, and patients with late-age onset are at increased risk for specific organ involvement, particularly pulmonary hypertension. Differences in disease characteristics between older and younger-age onset SSc patients should be considered when caring for older SSc patients and in the future may allow for more focused care of multimorbid elderly.
Acknowledgments
Funding Source: The Scleroderma Research Foundation
References
- 1.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007 Jul;66(7):940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steen VD, Medsger TA., Jr. Epidemiology and natural history of systemic sclerosis. Rheum Dis Clin North Am. 1990 Feb;16(1):1–10. [PubMed] [Google Scholar]
- 3.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003 Aug;48(8):2246–55. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 4.Steen VD, Oddis CV, Conte CG, Janoski J, Casterline GZ, Medsger TA., Jr. Incidence of systemic sclerosis in Allegheny County, Pennsylvania. A twenty-year study of hospital-diagnosed cases, 1963–1982. Arthritis Rheum. 1997 Mar;40(3):441–5. doi: 10.1002/art.1780400309. [DOI] [PubMed] [Google Scholar]
- 5.Medsger TA, Jr, Masi AT. Epidemiology of systemic sclerosis (scleroderma) Ann Intern Med. 1971 May;74(5):714–21. doi: 10.7326/0003-4819-74-5-714. [DOI] [PubMed] [Google Scholar]
- 6.Laing TJ, Gillespie BW, Toth MB, Mayes MD, Gallavan RH, Jr, Burns CJ, et al. Racial differences in scleroderma among women in Michigan. Arthritis Rheum. 1997 Apr;40(4):734–42. doi: 10.1002/art.1780400421. [DOI] [PubMed] [Google Scholar]
- 7.Scalapino K, Arkachaisri T, Lucas M, Fertig N, Helfrich DJ, Londino AV, Jr, et al. Childhood onset systemic sclerosis: classification, clinical and serologic features, and survival in comparison with adult onset disease. J Rheumatol. 2006 May;33(5):1004–13. [PubMed] [Google Scholar]
- 8.Systemic sclerosis in old age. Br Med J. 1979 Nov 24;2(6201):1313–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Dalziel JA, Wilcock GK. Progressive systemic sclerosis in the elderly. Postgrad Med J. 1979 Mar;55(641):192–3. doi: 10.1136/pgmj.55.641.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodkinson HM. Scleroderma in the elderly, with special reference to the CRST syndrome. J Am Geriatr Soc. 1971 Mar;19(3):224–8. doi: 10.1111/j.1532-5415.1971.tb02625.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams PL, Gumpel JM. Scleroderma in the elderly. Br Med J (Clin Res Ed) 1981 Mar 21;282(6268):948. doi: 10.1136/bmj.282.6268.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derk CT, Artlett CM, Jimenez SA. Morbidity and mortality of patients diagnosed with systemic sclerosis after the age of 75: a nested case-control study. Clin Rheumatol. 2006 Nov;25(6):831–4. doi: 10.1007/s10067-005-0177-y. [DOI] [PubMed] [Google Scholar]
- 13.Czirjak L, Nagy Z, Szegedi G. Systemic sclerosis in the elderly. Clin Rheumatol. 1992 Dec;11(4):483–5. doi: 10.1007/BF02283102. [DOI] [PubMed] [Google Scholar]
- 14.Schachna L, Wigley FM, Chang B, White B, Wise RA, Gelber AC. Age and risk of pulmonary arterial hypertension in scleroderma. Chest. 2003 Dec;124(6):2098–104. doi: 10.1378/chest.124.6.2098. [DOI] [PubMed] [Google Scholar]
- 15.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980 May;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 16.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988 Feb;15(2):202–5. [PubMed] [Google Scholar]
- 17.Velayos EE, Masi AT, Stevens MB, Shulman LE. The `CREST' syndrome. Comparison with systemic sclerosis (scleroderma) Arch Intern Med. 1979 Nov;139(11):1240–4. doi: 10.1001/archinte.139.11.1240. [DOI] [PubMed] [Google Scholar]
- 18.LeRoy EC, Medsger TA., Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001 Jul;28(7):1573–6. [PubMed] [Google Scholar]
- 19.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999 Oct;26(10):2159–67. [PubMed] [Google Scholar]
- 20.Medsger TA, Jr, Bombardieri S, Czirjak L, Scorza R, Della Rossa A, Bencivelli W. Assessment of disease severity and prognosis. Clin Exp Rheumatol. 2003;21(3 Suppl 29):S42–6. [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Knudson RJ, Kaltenborn WT, Knudson DE, Burrows B. The single-breath carbon monoxide diffusing capacity. Reference equations derived from a healthy nonsmoking population and effects of hematocrit. Am Rev Respir Dis. 1987 Apr;135(4):805–11. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- 23.Lundback B, Gulsvik A, Albers M, Bakke P, Ronmark E, van den Boom G, et al. Epidemiological aspects and early detection of chronic obstructive airway diseases in the elderly. Eur Respir J Suppl. 2003 May;40:3s–9s. doi: 10.1183/09031936.03.00403103. [DOI] [PubMed] [Google Scholar]
- 24.Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc. 2009 Dec 1;6(7):570–2. doi: 10.1513/pats.200909-099RM. [DOI] [PubMed] [Google Scholar]
- 25.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007 Sep 1;370(9589):741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Hsu VM, Moreyra AE, Wilson AC, Shinnar M, Shindler DM, Wilson JE, et al. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol. 2008 Mar;35(3):458–65. [PubMed] [Google Scholar]
- 28.Hachulla E, Launay D, Mouthon L, Sitbon O, Berezne A, Guillevin L, et al. Is pulmonary arterial hypertension really a late complication of systemic sclerosis? Chest. 2009 Nov;136(5):1211–9. doi: 10.1378/chest.08-3042. [DOI] [PubMed] [Google Scholar]
- 29.Chang B, Schachna L, White B, Wigley FM, Wise RA. Natural history of mild-moderate pulmonary hypertension and the risk factors for severe pulmonary hypertension in scleroderma. J Rheumatol. 2006 Feb;33(2):269–74. [PubMed] [Google Scholar]
- 30.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc (A) 1972;135:185–206. [Google Scholar]
- 31.Hachulla E, de Groote P, Gressin V, Sibilia J, Diot E, Carpentier P, et al. The three-year incidence of pulmonary arterial hypertension associated with systemic sclerosis in a multicenter nationwide longitudinal study in France. Arthritis Rheum. 2009 Jun;60(6):1831–9. doi: 10.1002/art.24525. [DOI] [PubMed] [Google Scholar]
- 32.Kardys I, Deckers JW, Stricker BH, Vletter WB, Hofman A, Witteman J. Distribution of echocardiographic parameters and their associations with cardiovascular risk factors in the Rotterdam Study. Eur J Epidemiol. 2010 May 22; doi: 10.1007/s10654-010-9453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker UA, Tyndall A, Czirjak L, Denton C, Farge-Bancel D, Kowal-Bielecka O, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis. 2007 Jun;66(6):754–63. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunderkotter C, Herrgott I, Bruckner C, Moinzadeh P, Pfeiffer C, Gerss J, et al. Comparison of patients with and without digital ulcers in systemic sclerosis: detection of possible risk factors. Br J Dermatol. 2009 Apr;160(4):835–43. doi: 10.1111/j.1365-2133.2008.09004.x. [DOI] [PubMed] [Google Scholar]
- 35.Degroot DW, Kenney WL. Impaired defense of core temperature in aged humans during mild cold stress. Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292(1):R103–8. doi: 10.1152/ajpregu.00074.2006. [DOI] [PubMed] [Google Scholar]
- 36.Smolander J. Effect of cold exposure on older humans. Int J Sports Med. 2002 Feb;23(2):86–92. doi: 10.1055/s-2002-20137. [DOI] [PubMed] [Google Scholar]
- 37.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003 Jan 7;107(1):139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 38.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009 Dec;90(6):1579–85. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009 Aug;57(8):1411–9. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan E, Sokka T, Hakkinen A, Hubert H, Hannonen P. Normative values for the Health Assessment Questionnaire disability index: benchmarking disability in the general population. Arthritis Rheum. 2004 Mar;50(3):953–60. doi: 10.1002/art.20048. [DOI] [PubMed] [Google Scholar]