Abstract
γδ T-cells represent a minor T-cell subset mainly distributed in mucosal surfaces. Two distinct lymphomas derived from these cells have been recognized: hepatosplenic γδ T-cell lymphoma (HSTL) and primary cutaneous γδ T-cell lymphoma (PCGD-TCL). However, whether other anatomic sites may also be involved and whether they represent a spectrum of the same disease is not well studied. The lack of TCRβ expression has been used to infer a γδ origin when other methods are not available.
We studied 35 T-cell tumors suspected to be γδ TCL using monoclonal antibodies reactive with TCR δ or γ in paraffin sections. We were able to confirm γδ chain expression in 22 of 35 cases. We identified 8 PCGD-TCL, 6 HSTL, and 8 γδ TCL without hepatosplenic or cutaneous involvement involving mainly extranodal sites. Two such cases were classified as enteropathy associated T-cell lymphoma, type II. The other γδ TCL presented in the intestine, lung, tongue, orbit and lymph node. In addition we observed 13 cases with mainly extranodal involvement that lacked any TCR expression (“TCR silent”). In all cases an NK origin was excluded.
In conclusion, the lack of TCRβ expression does not always predict a γδ T-cell derivation since TCR silent cases may be found. The recognition of γδ TCL presenting in extranodal sites other than skin and liver/spleen expands the clinical spectrum of these tumors. However, non- HSTL γδ TCL do not appear to represent a single entity. The relationship of these tumors to either HSTL or PCGD-TCL requires further study.
Keywords: γδ T-cell lymphomas, γδ T-cell receptor, αβ T-cell receptor, cutaneous γδ T-cell lymphoma, hepatosplenic T-cell lymphoma
INTRODUCTION
Normal γδ T-cells comprise an immunologically distinct lymphoid population that correspond to less than 1%–5% of peripheral blood lymphocytes and up to 50% of T-cells in mucosal sites, particularly intestine and skin. 6, 7, 17, 22–24 γδ T-cells, along with NK-cells are components of the innate immune system and do not require specialized antigen processing and presentation. 4, 8 Furthermore, γδ T-cells are involved in regulation of immune responses including cell recruitment and activation, and tissue repair. 7, 16, 27
The 2008 WHO classification recognizes two main types of γδ TCL, γδ hepatosplenic T-cell lymphoma (HSTL) 5, 11 and primary cutaneous γδ T-cell lymphoma (PCGD-TCL). 2, 12, 26, 35 Nevertheless, infrequent examples of γδ TCL have been reported to present in mucosal sites. 2, 12, 26 The relationship of such lesions to the more common primary cutaneous γδ T-cell tumors is unknown.
The investigation of γδ TCLs is limited by the rarity of these tumors and by the absence of reliable methodologies to assess γδ T-cell origin in routine diagnostic specimens. In this study, we describe the clinical, immunophenotypic and molecular characteristics of 29 non-hepatosplenic T-cell lymphomas suspected of being of γδ TCL, and explore new diagnostic markers potentially useful for diagnosis in routinely processed paraffin-embedded material.
MATERIAL AND METHODS
Tissue samples
A total of 35 lymphomas were collected from the pathology files of the National Cancer Institute (Bethesda, Maryland), Hospital Clínic (Barcelona, Spain), Chi-Mei Medical Center (Tainan, Taiwan), Hospital Henri Mondor (Créteil, France) and Hospital Verge de la Cinta (Tortosa, Spain). Cases were classified according to the current WHO classification.32 Six of the cases had proven expression of the γδ receptor by flow cytometry or frozen section immunohistochemistry, and were used in part to assist in the validation of the immunohistochemical studies. An additional 29 cases of mature T-cell lymphoma (TCL), suspected of being γδ TCL on the basis of absent TCRβ chain expression and a cytotoxic phenotype were studied. Information regarding sites of disease, therapy, and clinical course were obtained. Five patients were previously reported (cases 15, 17–20). 10 This study was approved by the Intramural Research Boards of the participating institutions.
Immunohistochemistry and hybridization studies
Immunohistochemical analysis was performed using a large panel of monoclonal antibodies detecting CD3, CD2, CD4, CD5, CD7, CD8, CD56, CD30, TIA-1 and granzyme B, performed as previously reported. 3, 19 Detection of the TCR-δ chain was performed using a primary monoclonal antibody raised against the human TCRδ constant region (Human Pan TCRγδ1, clone 5A6.E9, Thermo Scientific, IL) (see supplemental information, online only). Only membranous staining was considered positive, irrespective of the intensity. For TCRβ we used a mouse monoclonal antibody (TCR 1151, clone 8A3, Pierce Endogen, IL). In cases with no detectable expression of either δ or β chain and with additional sections available, TCRγ chain detection was performed using the monoclonal antibody TCR 1153 (clone γ3.20, Thermo Scientific, IL). 31 Epstein-Barr virus (EBV) was detected using in situ hybridization with EBER probes (INFORM EBER, Benchmark XT; Ventana Medical Systems, Tucson, AZ).
To determine whether the 5A6.E9 anti TCRδ1 antibody could recognize γδ T-cells with a wide repertoire of TCRδ chains in formalin-fixed paraffin embedded cells we obtained an enriched population of γδ T-cells from peripheral blood mononuclear cells of healthy donors (see supplemental information, online only). After enrichment, the purity of the γδ T-cells was assessed by flow cytometry. A pellet of these cells was formalin fixed and embedded in paraffin and the sections stained with the 5A6.E9 anti TCRδ1 antibody (Supplemental Figure 1, online only). As a control group we also studied normal tissues (Supplemental Figure 2, online only), 8 cytotoxic TCRβ chain positive TCL, NOS, one subcutaneous panniculitis-like TCL, and 6 EBV-positive NK/T-cell malignancies. In all of them, the tumor cells were negative for TCRδ chain expression although some reactive cells were easily identified in the reactive background.
TCR gene rearrangements
Polymerase chain reaction analysis of the TCRγ gene was performed using BIOMED2 protocol as described elsewhere 14, 19, 36 and DNA extracted from formalin-fixed tissues using the QIAamp DNA mini kit (Qiagen, Hilden, Germany ). Cases from the NCI were characterized for TCRγ rearrangement as previously described. 15
RESULTS
The main clinical and pathologic features are summarized in Tables 1 and 2 respectively. Based on review, 8 cases were classified as PCGD-TCL and 6 cases as HSTL. Eight additional cases were proven to be of γδ origin, 2 of which had features of enteropathy-associated T-cell lymphoma (EATL), Type II, following WHO criteria for enteropathy (villous atrophy, crypt hyperplasia and intraepithelial lymphocytosis).32 The remaining 6 γδ TCL involved mainly extranodal sites, including one intestinal case without features of enteropathy. Thirteen cases were classified as TCL, “silent TCR”, and involved mainly extranodal sites. The clinical and pathological features are detailed below.
Table 1.
Clinical features features T-cell lymphoma patients
| Case | Diagnosis | Sex/age | Primary site of diagnosis | Secondary involvement | Therapy | Follow up (mo) |
|---|---|---|---|---|---|---|
| 1 | PCGD-TCL | M/57 | Skin | CT | DoD,14 | |
| 2 | PCGD-TCL | F/61 | Skin | BM, visceral | CT,RT | DoD,13 |
| 3 | PCGD-TCL | F/48 | Skin | Prednisone | DoD,1 | |
| 4 | PCGD-TCL | M/53 | Skin | CT | DoD,16 | |
| 5 | PCGD-TCL | F/63 | Subcutaneous tissue | RT,CT,BMT | AIR,25 | |
| 6 | PCGD-TCL | F/49 | Skin, | Stomach, lymph node, kidney, adrenal gland, ovary | No treatment† | DoD0 |
| 7 | PCGD-TCL | M/24 | Skin, lymph node, BM | Lymph node, liver, PB | CT | AwD,5 |
| 8 | PCGD-TCL | F/16 | Skin | CT | DoD,22 | |
| 9 | HSTL | F/26 | Spleen | Splenectomy | ||
| 10 | HSTL | M/44 | Liver, BM | CT | DoD,16 | |
| 11 | HSTL | F/56 | Spleen, BM | CT,BMT | AIR,35 | |
| 12 | HSTL | M/62 | Spleen, BM | CT, BMT | DoD,15 | |
| 13 | HSTL | F/54 | Spleen | Splenectomy | AIR,32 | |
| 14 | HSTL | F/24 | Liver | Splenectomy | ||
| 15 | EATL type II | M/59 | Small bowel | CT | DoD,9 | |
| 16 | EATL type II | M/72 | Small bowel | Colon | CT | DoD,2 |
| 17 | TCL, intestinal | M/60 | Small bowel | CT | DoD,4 | |
| 18 | TCL, intestinal (TCR silent) | M/49 | Small bowel | CT | DoD,34 | |
| 19 | TCL, intestinal (TCR silent) | M/54 | Small bowel | CT | AIR,22 | |
| 20 | TCL, intestinal (TCR silent) | M/56 | Small bowel | CT | DoD,14 | |
| 21 | Gamma-delta TCL | F/36 | Lung | Skin, liver | CT | DoD,8 |
| 22 | Gamma-delta TCL | F/68 | Orbit | Excision | AIR,48 | |
| 23 | Gamma-delta TCL | F/57 | Lymph node | CT | AIR,10 | |
| 24 | Gamma-delta TCL | M/29 | Tongue | Excision | AIR,15 | |
| 25 | Gamma-delta TCL | M/29 | Lymph node | CT | DoD,5 | |
| 26 | TCL, TCR silent | F/49 | Spleen, PB | CT,BMT | DoD,12 | |
| 27 | TCL, TCR silent | F/23 | Subcutaneous tissue | |||
| 28 | TCL, TCR silent | M/22 | Subcutaneous tissue | |||
| 29 | TCL, TCR silent | M/60 | Skin | Ethmoidal sinus | CT,RT | AwD,15 |
| 30 | TCL, TCR silent | M/84 | Skin | CT | DoD,2 | |
| 31 | TCL, TCR silent | M/57 | Skin | |||
| 32 | TCL, TCR silent | M/54 | Subcutaneous tissue | Submaxillary bone | ||
| 33 | TCL, TCR silent | M/42 | BM, liver and spleen | CT,RT | DoD,11 | |
| 34 | TCL, TCR silent | M/56 | Lymph node | Peripheral blood and skin | CT | DoD,63 |
| 35 | TCL, TCR silent | M/57 | Central nervous system | prednisone | DoD,14 |
CT, chemotherapy; RT, radiotherapy; BMT, bone marrow transplantation; DOD, dead of disease; AIR, alive in remission; AWD, alive with disease.
Diagnosed at autopsy
Table 2.
Phenotypical and genotypical features T-cell lymphoma patients
| Case # | Diagnosis | γδ-TCR | αβ-TCR | PCR TCR | CD3 | CD4 | CD5 | CD7 | CD8 | CD56 | TIA-1 | Gr-B | EBV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PCGD-TCL | + | − | germline | + | − | − | − | + | + | + | − | |
| 2 | PCGD-TCL | + | − | clonal | + | − | − | − | − | + | − | ||
| 3 | PCGD-TCL | + | − | clonal | + | − | − | − | − | + | + | + | − |
| 4 | PCGD-TCL | + | − | clonal | + | − | − | − | − | + | + | − | |
| 5 | PCGD-TCL | + | − | clonal | + | − | − | + | + | ||||
| 6 | PCGD-TCL | + | − | clonal | + | − | − | − | + | + | + | + | |
| 7 | PCGD-TCL | + | − | nd | + | − | − | + | − | + | + | ||
| 8 | PCGD-TCL | + | − | clonal | + | − | + | + | − | − | + | + | − |
| 9 | HSTL | + | − | clonal | + | − | − | − | − | + | + | − | |
| 10 | HSTL | + | − | clonal | + | − | − | − | + | + | − | ||
| 11 | HSTL | + | − | clonal | + | − | − | + | − | + | + | + | − |
| 12 | HSTL | + | − | clonal | + | − | − | + | − | + | + | − | |
| 13 | HSTL | + | − | clonal | + | − | − | + | − | + | + | − | − |
| 14 | HSTL | + | − | nd | + | − | − | − | + | + | + | − | |
| 15 | EATL type II | + | − | clonal | + | − | − | + | + | + | + | + | − |
| 16 | EATL type II | + | − | clonal | + | − | − | + | + | + | + | − | |
| 17 | TCL, intestinal | + | − | clonal | + | − | − | + | + | + | + | − | − |
| 18 | TCL, intestinal (TCR silent) | − | − | clonal | + | − | − | + | + | + | − | + | − |
| 19 | TCL, intestinal (TCR silent) | − | − | clonal | + | − | − | + | + | + | + | − | − |
| 20 | TCL, intestinal (TCR silent) | − | − | clonal | + | − | − | + | + | + | + | − | − |
| 21 | Gamma-delta TCL | + | − | clonal | + | − | − | − | + | + | − | ||
| 22 | Gamma-delta TCL | + | − | clonal | + | − | − | + | + | − | + | + | − |
| 23 | Gamma-delta TCL | + | − | clonal | + | − | − | − | − | − | + | + | + |
| 24 | Gamma-delta TCL | + | − | clonal | + | − | − | + | + | − | |||
| 25 | Gamma-delta TCL | + | − | clonal | + | − | + | + | − | − | + | − | |
| 26 | TCL, TCR silent | − | − | clonal | + | − | − | − | − | + | + | − | − |
| 27 | TCL, TCR silent | − | − | clonal | + | − | + | − | + | + | |||
| 28 | TCL, TCR silent | − | − | clonal | − | ||||||||
| 29 | TCL, TCR silent | − | − | clonal | + | − | + | + | + | − | |||
| 30 | TCL, TCR silent | − | − | germline | + | − | − | − | + | + | − | ||
| 31 | TCL, TCR silent | − | − | clonal | + | − | − | ||||||
| 32 | TCL, TCR silent | − | − | clonal | + | + | + | − | − | − | + | − | |
| 33 | TCL, TCR silent | − | − | clonal | + | − | − | + | − | − | + | − | − |
| 34 | TCL, TCR silent | − | − | germline | + | + | + | + | − | − | + | + | − |
| 35 | TCL, TCR silent | − | − | clonal | + | − | − | + | + | − | + | − |
Primary cutaneous γδ T-cell lymphoma
Eight cases were classified as PCGD-TCL. This tumor occurred mainly in adults (median age, 46 years; age range, 16–63 years). The male to female ratio was 3:5. A mean follow-up of 14 months was recorded (range, 1–25). Six patients were treated with multiagent systemic chemotherapy and two cases received additional radiotherapy. One patient was treated with corticosteroids alone. Most patients experienced an aggressive clinical course with extranodal and nodal dissemination. Six patients died as a result of the disease between 1 and 22 months after the initial diagnosis. One patient (case 5) underwent allogeneic bone marrow transplantation and had no evidence of disease 25 months after diagnosis. One patient (case 7) experienced persistence of the disease at the last follow-up.
Histologically, six of these tumors were composed of small/medium-sized atypical lymphocytes. In all cases, the tumor involved the subcutis and in six extended into the dermis. All tumors had an activated cytotoxic phenotype. Six cases were double negative for CD4 and CD8, whereas two cases expressed CD8. Expression of CD56 was observed only in 3 of 7 patients. In all cases γδ TCR expression was convincingly demonstrated by positive staining for TCRδ chain. In two cases, γδ TCR expression was further confirmed, by flow cytometry in one case, and by immunohistochemistry on frozen sections in a second case.
A clonal TCRγ gene rearrangement was observed in 6 cases, whereas 1 case showed a germline configuration of the TCRγ gene. Epstein-Barr virus infection was observed in two cases (cases 6 and 7) in which the strong δ chain expression excluded a NK-cell origin (Figure 1). In one EBV-positive case TCRγ gene rearrangement confirmed a T-cell origin, but PCR studies could not be performed in the second case.
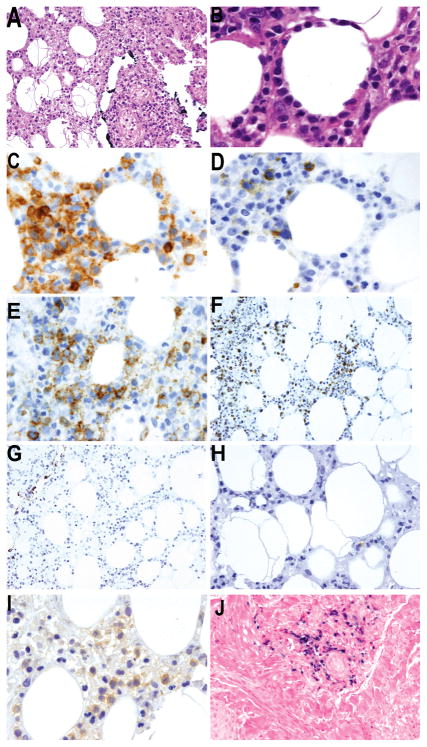
Figure 1. Primary cutaneous γδ T-cell lymphoma.
A) Subcutaneous involvement with panniculitic like features (H&E X20) B) by medium sized atypical lymphocytes with adipocyte rimming (H&E X60) C) The atypical lymphocytes are CD3 positive (X60) and D) negative for CD5 (X60) E) Some tumor cells express CD8 (X60) and F) exhibit an activated cytotoxic phenotype with granzyme-B expression (X20) G) Neoplastic lymphocytes are negative for CD56 (X20) H) Very few reactive lymphocytes express TCRβ, which is absent in tumor cells (X40) I) Homogenous TCRδ expression is observed in the tumor (X60) J) Some perivascular tumor cells are infected by Epstein-Barr virus in the dermis (EBER, X20)
Hepatosplenic T-cell lymphoma
Six cases were classified as HSTL following the criteria of the WHO classification. No male predominance was observed; two patients were men while four were women. The median age was 44 years (range 24–62). Follow up was available in four patients. Two patients achieved a complete remission (CR) following chemotherapy, which included allogeneic stem-cell transplantation in one patient (case 11) and autologous stem-cell transplantation in other case (case 12). Median overall survival was 25 months (range 15 to 35 months), and one patient was still alive 35 months from the initial diagnosis. Case 13 did not receive any adjuvant therapy and was alive and in remission 32 months following splenectomy. The remaining two patients, after initial response to therapy, died as result of progression of lymphoma.
In all cases the tumor cells exhibited a CD3+, CD56+, CD4- and CD8- phenotype with expression of TIA-1; two cases were also positive for granzyme B (Figure 2). Staining for TCRδ chain was positive in all cases and expression was confirmed in two by flow cytometry. In situ hybridization for EBV (EBER) was performed in five cases and was negative in all cases. Five cases had TCRγ gene rearrangement.
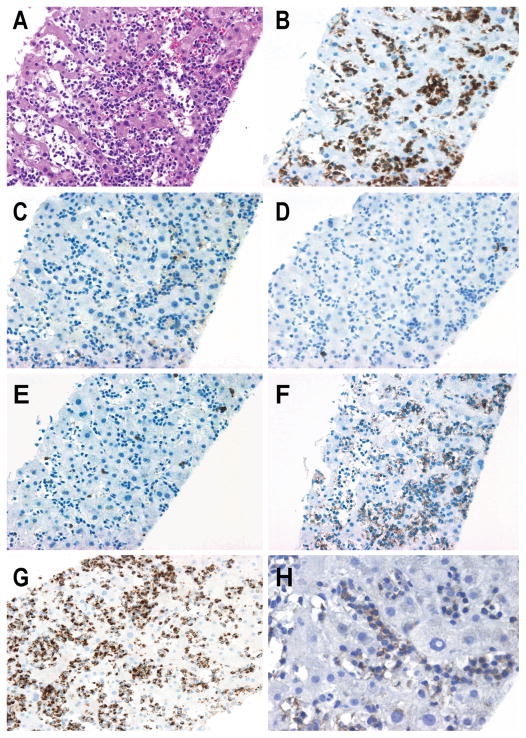
Figure 2. Hepatosplenic T-cell lymphoma.
A) Diffuse intrasinusoidal liver involvement by monotonous medium sized atypical lymphocytes (H&E X20) that strongly express B) CD3 (X20) being negative for C,D,E) CD4, CD8 and CD5 (X20). F) The atypical cell expresses CD56 (X20) and G) TIA-1 (X20). H) TCRδ chain is strongly expressed in the tumor cells (X40) expression by immunohistochemistry (X40).
T-cell lymphomas with intestinal involvement
There were six male patients with a median age of 58 years old (range: 49 to 72). None of them had prior history of celiac disease and five patients were Asian and one Hispanic. All patients underwent surgical resection and adjuvant chemotherapy. The median follow-up duration was 14 months. Five patients died of disease within 2 to 34 months.
Tumor cells were small to medium in all cases, and extended through the submucosa, with perforation in five cases. Histologically, all tumors showed a diffuse monotonous lymphoid infiltrate, as described in the monomorphic or Type II variant of EATL. However, features of enteropathy with increased intraepithelial lymphocytes in the mucosa adjacent to the tumor were only clearly seen in two cases (cases 15, 16), one Asian and one Hispanic, both males. All tumors expressed CD3, CD7, CD8, CD56 and cytotoxic granule associated proteins (Figure 3), while stains for CD4 (6/6), CD5 (6/6) and CD30 (2/3) were negative. Three cases showed unequivocal TCRγδ derivation with strong TCRδ chain expression (Figure 3H), including the two cases of EATL, Type II. Three cases were negative for both TCRδ and TCRβ chain. Admixed reactive TCRγδ and TCRαβ tumor infiltrating T-cells were present confirming the validity of the techniques. In addition, immunohistochemistry for the TCRγ chain was performed and the tumor cells also lacked TCRγ chain expression. All cases had a clonal TCRγ gene rearrangement and were EBV negative by in situ hybridization.
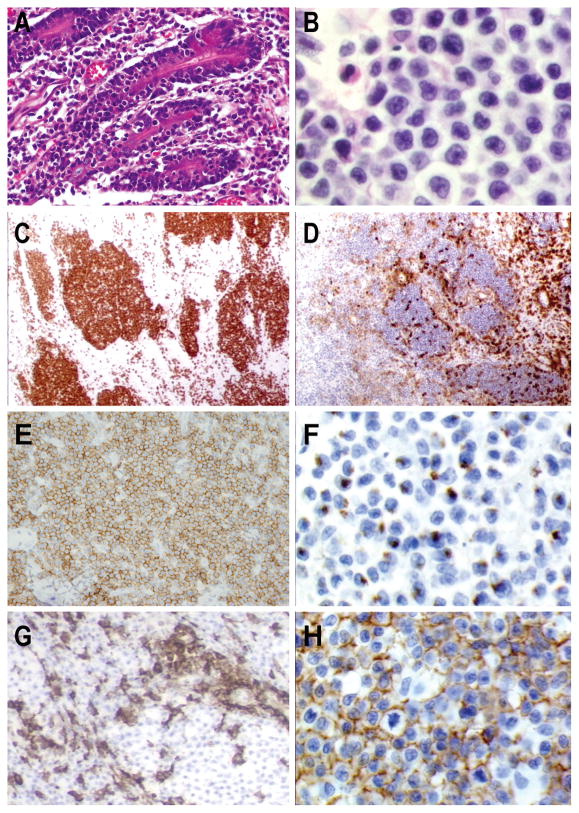
Figure 3. T-cell lymphoma of γδ origin with intestinal involvement. Type II variant of enteropathy-associated T-cell lymphoma.
A) The tumor surround mucosal crypts with intraepithelial involvement (H&EX20) B) Monotonous lymphoid infiltrate of small medium sized tumor cells (H&E X60) The atypical lymphocytes are C) CD8 positive (X10), D) CD5 negative (X10) and E) CD56 positive (X40) F) Neoplastic lymphocytes have an activated cytotoxic phenotype with granzyme-B expression (X60) G) Reactive lymphocytes are positive for TCRβ that is absent in tumor cells (X40) H) Homogenous TCRδ expression is observed in the tumor (X60)
Other γδ T-cell lymphomas
Five patients presented with γδ T-cell tumors exhibiting strong δ chain expression that did not correspond to any of the WHO categories; these were designated as γδ TCL, not otherwise specified. There were two males and three females with a median age of 44 years (range, 29 to 68). None of them had cutaneous or hepatosplenic involvement at diagnosis. Two cases had primary nodal involvement (Figure 4). The remaining three patients showed extranodal disease involving lung (case 21), orbit (case 22) and tongue (case 24). The patients were treated with chemotherapy (cases 21, 23 and 25) or only underwent surgical excision (cases 22 and 24). During follow-up one patient developed skin lesions and liver involvement (case 21). At the time of last follow-up (median follow-up 17 months, range, 5 to 48 months), 2 patients died of disease, while 3 patients were in complete remission.
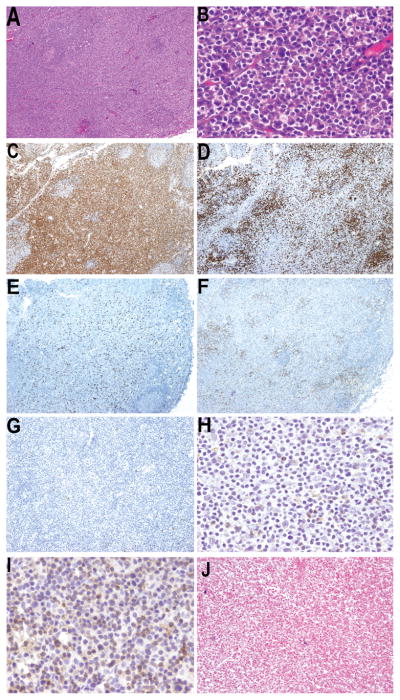
Figure 4. Other γδ T-cell lymphomas.
A) Diffuse lymph node involvement (H&E X4) by B) small atypical lymphocytes. C) CD3 positive neoplastic cells (X4) that are D) CD5 negative (X4). The atypical lymphocytes are double negative for E) CD4 (X4) and F) CD8 (X4) lacks G) CD56 (X4) H) TCRβ is expressed by scattered reactive T-cells (X40) I) TCRδ is expressed in the tumor cells (X40) that are negative for J) Epstein-Barr virus (EBER, X10)
All cases exhibited an activated cytotoxic phenotype, with expression of TIA-1 and granzyme B in all tested cases. Three cases were double negative for CD4 and CD8, whereas 2 cases were CD8 positive. CD5 was lost in 5/7 tested cases and all cases were CD56-positive. TCRδ expression was confirmed in two cases by flow cytometry. A clonal peak for TCRγ was observed in all cases. Epstein-Barr virus infection was detected in one case (case 23), which presented in lymph node.
Peripheral T-cell lymphoma, TCR silent
Lack of any form of TCR expression was observed in 13 cases: three were classified as TCL with intestinal involvement, and are discussed with the other intestinal neoplasms. The remaining 10 cases were grouped as TCL, TCR silent. There was a marked male predominance (8/10 were males). The median age of the patients in this category was 50 years, range 22–84. At initial presentation, six cases had cutaneous lesions (cases 27–32), two cases had features of HSTL (cases 26 and 33) and the remaining cases involved lymph node and central nervous system (cases 34 and 35, respectively). All but one patient was treated with chemotherapy, and in 2 of the 5 patients with available clinical records radiotherapy was added. Mean follow-up was 20 months. Most patients experienced an aggressive clinical course and 5 patients died with progression of the disease.
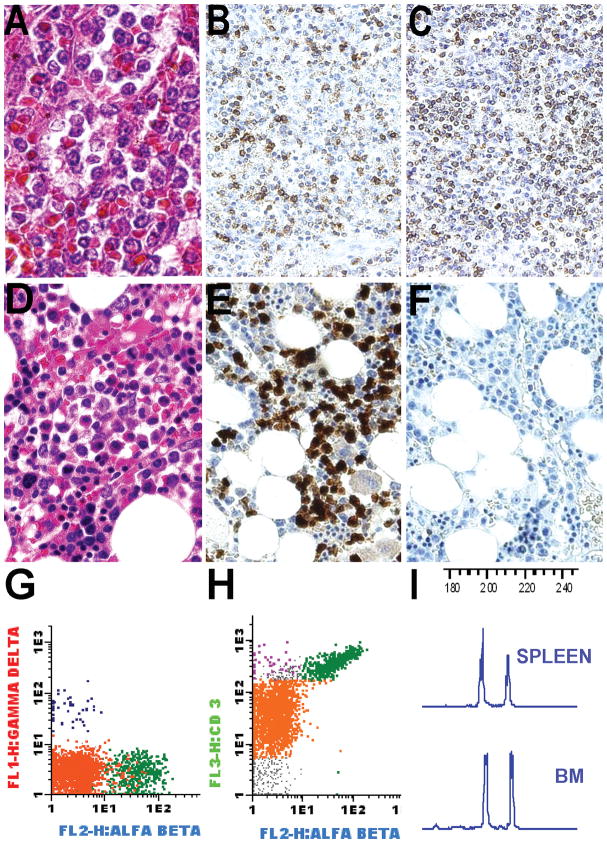
The tumor cells were negative for TCRδ and TCRβ chain expression by immunohistochemistry, although some reactive lymphocytes expressing both TCR receptors were easily identified in the reactive background. In four cases with additional available sections, immunohistochemistry for the TCRγ chain was performed and the tumor cells also lacked TCRγ chain expression. The tumor cells expressed CD3 but not CD4 or CD5. Three cases showed CD8 expression. In all cases a diagnosis of NK/T-cell lymphoma, nasal type was excluded, either by a monoclonal T-cell gene rearrangement (8 cases) or absence of EBV infection (8 cases). Interestingly, case 26 was originally diagnosed as an αβ variant of HSTL (Figure 5). This case was negative for TCRδ chain and positive for TCRβ chain by both immunohistochemistry and flow cytometry of the splenectomy specimen. In subsequent studies following splenectomy, an abnormal T-cell population with an identical neoplastic phenotype but lacking TCRβ chain expression was demonstrated in the bone marrow and peripheral blood compartments by flow cytometry. A molecular study for TCRγ chain gene rearrangement demonstrated a clone of identical size in the spleen, bone marrow and peripheral blood examined both before and after surgery (Figure 5).
Figure 5. T-cell lymphoma, TCR silent.
A) Diffuse red pulp involvement by monotonous medium sized atypical lymphocytes (H&E X60) that strongly express B) CD3 (X10) and C) TCRβ (X10) D) Interstitial bone marrow involvement (H&E X60) E) CD3 staining highlights neoplastic cells (X40) F) Neoplastic lymphocytes lack TCRβ expression by immunohistochemistry (X40) G,H) Flow cytometry on bone marrow aspirate confirms a silent phenotype with lack of TCRβ and TCRδ expression (green: residual αβ T-cells, purple: residual γδ T-cells, orange: abnormal T-cells) I) TCR gene rearrangement studies demonstrated the same clonal peak pattern in the spleen and bone marrow.
DISCUSSION
T-cell lymphomas of γδ cell origin represent a rare form of lymphoma with aggressive behavior and poor outcome. 5,38 HSTL and PCGD-TCL are recognized in the 2008 WHO classification of lymphoid neoplasms as the two main types of TCL that express a γδ TCR. 32, 32 In the absence of flow cytometry studies or frozen material these cases are diagnosed based on clinicopathological features and the expression of a typical CD3+, CD56+ phenotype with absence of the TCRβ chain and CD5, and usually a double negative (CD4/CD8) phenotype. Some cases with uncommon clinical presentations or not fulfilling this phenotype are more difficult to categorize, and may not be appreciated as being of γδ T-cell origin.
Several studies have described the presence and the distribution of γδ TCR’s in frozen tissue sections 17, 20, 22 but their characterization in routinely processed tissues has been less successful. 29,31 The detection of a γδ TCR in formalin-fixed paraffin-embedded tissues is currently a technological challenge that hampers the diagnosis and understanding of γδ T-cell lymphoproliferative disorders. In this study, we have demonstrated the mutually exclusive expression of the TCRβ and TCRδ chains in several types of TCL in routinely processed tissues.
TCRδ chain expression was demonstrated in 8 of 14 tumors initially diagnosed as PCGD-TCL and 6 of 8 HSTL. Of note, TCRδ chain expression was observed in 8 cases of TCRβ negative TCL without hepatosplenic or cutaneous involvement at diagnosis. Three of these patients presented with intestinal involvement, two of which had features of EATL, Type II. The remaining cases had the characteristic phenotype of PCGD-TCL but presented with pulmonary, orbital, nodal, and tongue involvement. In contrast to most γδ TCL, three of the non-intestinal cases had an indolent clinical course. This heterogeneity in histological and clinical features suggests that non-hepatosplenic γδ TCL is not a homogeneous clinical entity 2.
Six cases of TCL in our study primarily involved the small bowel and showed a monomorphous small-medium lymphoid infiltrate with CD3+, CD8+, CD56+, CD7+ and CD5-, βF1-immunophenotype. In three cases a γδ T-cell origin was proven. However, only two of them showed intraepithelial lymphocytosis fulfilling the criteria for type II EATL. EATL is defined as an intestinal tumor of intraepithelial lymphocytes (IEL) with villous atrophy and crypt hyperplasia in the adjacent, non-neoplastic small intestinal mucosa. The monomorphic variant (type II EATL) may occur sporadically, without risk factors for celiac disease. Our two EATL type II cases were from patients of Asian and Hispanic ethnicities, in whom celiac disease and enteropathy-associated TCL have rarely been reported. 10 EATL Type II has been suggested to be derived from IELs with a γδ phenotype. 13, 21, 28 The fact that the two cases of EATL Type II in our series had a γδ phenotype may support this theory. 1, 9, 37 Notably three additional TCL with intestinal involvement lacking diagnostic criteria for EATL had similar clinical, cytological and immunophenotypic features but lacked TCRβ, TCRδ and TCRγ chain expression and were defined as TCR silent. Recently a benign gastrointestinal NK-cell enteropathy has been reported that may closely mimic EATL.30, 33 The cells of NK cell enteropathy are CD8-negative, lack TCR expression and evidence of TCR rearrangement. In contrast, the intestinal TCR silent cases were TCRγ clonal and CD8 positive.
We identified a total of 13 TCL that were negative for both TCRδ and TCRβ chains. In 7 of these cases, we performed immunostaining for TCRγ chain which was negative as well. Rare TCL with a lack of TCR expression had been previously recognized in cases studied by frozen section immunohistochemistry and had been designated as TCR silent TCL. 20 Six of our TCR silent TCL cases had clinical and pathological features resembling PCGD-TCL while two mimicked a HSTL. As noted above, three presented with intestinal disease. We believe it is most likely that the TCR silent phenotype represents a common phenomenon of “TCR instability” in different tumor types, rather than representing a distinct clinicopathological entity. In favor of this hypothesis was the observation of a TCR αβ HSTL that evolved to a TCR silent phenotype with an identical TCR gene rearrangement pattern after splenectomy. A similar phenomenon had been reported in γδ HSTL associated with histological progression and in enteropathy-associated T-cell lymphomas evolving from refractory celiac disease. 18, 34 Thus, the lack of TCR expression may represent a phenotypic aberration in TCL, usually encountered at the time of progression. This finding indicates further that the lack of TCRβ chain expression cannot be used to infer a γδ T-cell origin with certainty.
Although most of our cases were unrelated to EBV infection, EBV-encoded RNA was detected in the neoplastic cells of three cases of γδ TCL. This phenomenon has been observed previously in rare examples of HSTL and non-hepatosplenic γδ TCL. 2, 5 Whether such cases should be classified as extranodal NK/T-cell lymphomas of true T-cell derivation, or γδ TCL, EBV-positive is difficult to resolve in the absence of other data, since even gene expression profiles may be similar.25 The absence of angiocentricity, the strong TCRδ chain expression and the evidence of a clonal TCR gene rearrangement favored a γδ T-cell origin. Moreover, EBV positivity is more common in individuals of Asian origin and Native American descent in Central and South America, and one of our cases was diagnosed in a patient from Mexico.
In summary, our observations indicate that the spectrum of γδ derived TCL is broader than previously appreciated. Although HSTL and PC-GDTCL are recognized in the 2008 WHO classification, we identified a number of other γδ TCL presenting in other mainly extranodal or more rarely nodal sites. The heterogeneity of these cases suggests that non-hepatosplenic γδ TCL is not a single entity. It also appears that a high proportion of type II EATL is derived from γδ T-cells. However, there is still a subset of T-cell lymphomas with intestinal involvement that exhibits either a γδ-TCR or TCR silent phenotype and lacks features of enteropathy. Finally, the recognition of a group of silent TCL’s indicates that a lack of TCRβ expression cannot be used to predict a γδ T-cell origin. The addition of genetic or genomic data in the future may help to resolve the apparent diversity in these heterogeneous and aggressive neoplasms.
Supplementary Material
Acknowledgments
Supported by Instituto de Salud Carlos III, Fondo de Investigación Sanitaria) PI080095 (AM) and PI050458 (TE). The Spanish Comisión Interministerial de Ciencia y Tecnología (CICYT) SAF08-3630 (EC), Red Temática de Investigación Cooperativa del Cáncer (RTICC) RD06/0020/0039 (EC), AGH is a fellow supported by the Instituto de Salud Carlos III. This work was also supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute.
Footnotes
Disclosure/Conflict of interest: The authors declare no conflict of interest.
References
- 1.Akiyama T, Okino T, Konishi H, et al. CD8+, CD56+ (natural killer-like) T-cell lymphoma involving the small intestine with no evidence of enteropathy: clinicopathology and molecular study of five Japanese patients. Pathol Int. 2008;58:626–34. doi: 10.1111/j.1440-1827.2008.02281.x. [DOI] [PubMed] [Google Scholar]
- 2.Arnulf B, Copie-Bergman C, Delfau-Larue MH, et al. Nonhepatosplenic gammadelta T-cell lymphoma: a subset of cytotoxic lymphomas with mucosal or skin localization. Blood. 1998;91:1723–31. [PubMed] [Google Scholar]
- 3.Balague O, Martinez A, Colomo L, et al. Epstein-Barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T-cell lymphomas: a phenomenon with distinctive clinicopathologic features. Am J Surg Pathol. 2007;31:1310–22. doi: 10.1097/PAS.0b013e3180339f18. [DOI] [PubMed] [Google Scholar]
- 4.Bartl S, Baltimore D, Weissman IL. Molecular evolution of the vertebrate immune system. Proc Natl Acad Sci U S A. 1994;91:10769–70. doi: 10.1073/pnas.91.23.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102:4261–9. doi: 10.1182/blood-2003-05-1675. [DOI] [PubMed] [Google Scholar]
- 6.Borst J, van Dongen JJ, Bolhuis RL, et al. Distinct molecular forms of human T cell receptor gamma/delta detected on viable T cells by a monoclonal antibody. J Exp Med. 1988;167:1625–44. doi: 10.1084/jem.167.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–45. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 8.Chien YH, Jores R, Crowley MP. Recognition by gamma/delta T cells. Annu Rev Immunol. 1996;14:511–32. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 9.Chott A, Vesely M, Simonitsch I, et al. Classification of intestinal T-cell neoplasms and their differential diagnosis. Am J Clin Pathol. 1999;111:S68–S74. [PubMed] [Google Scholar]
- 10.Chuang SS, Chang ST, Chuang WY, et al. NK-cell lineage predicts poor survival in primary intestinal NK-cell and T-cell lymphomas. Am J Surg Pathol. 2009;33:1230–40. doi: 10.1097/PAS.0b013e3181a95c63. [DOI] [PubMed] [Google Scholar]
- 11.Cooke CB, Krenacs L, Stetler-Stevenson M, et al. Hepatosplenic T-cell lymphoma: a distinct clinicopathologic entity of cytotoxic gamma delta T-cell origin. Blood. 1996;88:4265–74. [PubMed] [Google Scholar]
- 12.de Wolf-Peeters C, Achten R. gammadelta T-cell lymphomas: a homogeneous entity? Histopathology. 2000;36:294–305. doi: 10.1046/j.1365-2559.2000.00893.x. [DOI] [PubMed] [Google Scholar]
- 13.Deusch K, Luling F, Reich K, et al. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur J Immunol. 1991;21:1053–9. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- 14.Dippel E, Assaf C, Hummel M, et al. Clonal T-cell receptor gamma-chain gene rearrangement by PCR-based GeneScan analysis in advanced cutaneous T-cell lymphoma: a critical evaluation. J Pathol. 1999;188:146–54. doi: 10.1002/(SICI)1096-9896(199906)188:2<146::AID-PATH334>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Dojcinov SD, Venkataraman G, Raffeld M, et al. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405–17. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–6. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 17.Falini B, Flenghi L, Pileri S, et al. Distribution of T cells bearing different forms of the T cell receptor gamma/delta in normal and pathological human tissues. J Immunol. 1989;143:2480–8. [PubMed] [Google Scholar]
- 18.Farcet JP, Gaulard P, Marolleau JP, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood. 1990;75:2213–9. [PubMed] [Google Scholar]
- 19.Garcia-Herrera A, Colomo L, Camos M, et al. Primary cutaneous small/medium CD4+ T-cell lymphomas: a heterogeneous group of tumors with different clinicopathologic features and outcome. J Clin Oncol. 2008;26:3364–71. doi: 10.1200/JCO.2008.16.1307. [DOI] [PubMed] [Google Scholar]
- 20.Gaulard P, Bourquelot P, Kanavaros P, et al. Expression of the alpha/beta and gamma/delta T-cell receptors in 57 cases of peripheral T-cell lymphomas. Identification of a subset of gamma/delta T-cell lymphomas. Am J Pathol. 1990;137:617–28. [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman T, Lefrancois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–8. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- 22.Groh V, Porcelli S, Fabbi M, et al. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–94. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 24.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, de RA, de LL, et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood. 2010;115:1226–37. doi: 10.1182/blood-2009-05-221275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaffe ES, Krenacs L, Raffeld M. Classification of cytotoxic T-cell and natural killer cell lymphomas. Semin Hematol. 2003;40:175–84. doi: 10.1016/s0037-1963(03)00132-x. [DOI] [PubMed] [Google Scholar]
- 27.Jameson J, Ugarte K, Chen N, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–9. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 28.Janeway CA, Jr, Jones B, Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988;9:73–6. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 29.Macon WR, Salhany KE. T-cell subset analysis of peripheral T-cell lymphomas by paraffin section immunohistology and correlation of CD4/CD8 results with flow cytometry. Am J Clin Pathol. 1998;109:610–7. doi: 10.1093/ajcp/109.5.610. [DOI] [PubMed] [Google Scholar]
- 30.Mansoor A, Pittaluga S, Beck PL, et al. NK-cell enteropathy: a benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: clinicopathologic features and follow-up in a unique case series. Blood. 2011;117:1447–52. doi: 10.1182/blood-2010-08-302737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roullet M, Gheith SM, Mauger J, et al. Percentage of {gamma}{delta} T cells in panniculitis by paraffin immunohistochemical analysis. Am J Clin Pathol. 2009;131:820–6. doi: 10.1309/AJCPMG37MXKYPUBE. [DOI] [PubMed] [Google Scholar]
- 32.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 33.Takeuchi K, Yokoyama M, Ishizawa S, et al. Lymphomatoid gastropathy: a distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood. 2010;116:5631–7. doi: 10.1182/blood-2010-06-290650. [DOI] [PubMed] [Google Scholar]
- 34.Tjon JM, Verbeek WH, Kooy-Winkelaar YM, et al. Defective synthesis or association of T-cell receptor chains underlies loss of surface T-cell receptor-CD3 expression in enteropathy-associated T-cell lymphoma. Blood. 2008;112:5103–10. doi: 10.1182/blood-2008-04-150748. [DOI] [PubMed] [Google Scholar]
- 35.Toro JR, Beaty M, Sorbara L, et al. gamma delta T-cell lymphoma of the skin: a clinical, microscopic, and molecular study. Arch Dermatol. 2000;136:1024–32. doi: 10.1001/archderm.136.8.1024. [DOI] [PubMed] [Google Scholar]
- 36.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 37.Vega F, Medeiros LJ, Gaulard P. Hepatosplenic and other gammadelta T-cell lymphomas. Am J Clin Pathol. 2007;127:869–80. doi: 10.1309/LRKX8CE7GVPCR1FT. [DOI] [PubMed] [Google Scholar]
- 38.Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:838–45. doi: 10.1182/blood-2007-04-087288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.