Abstract
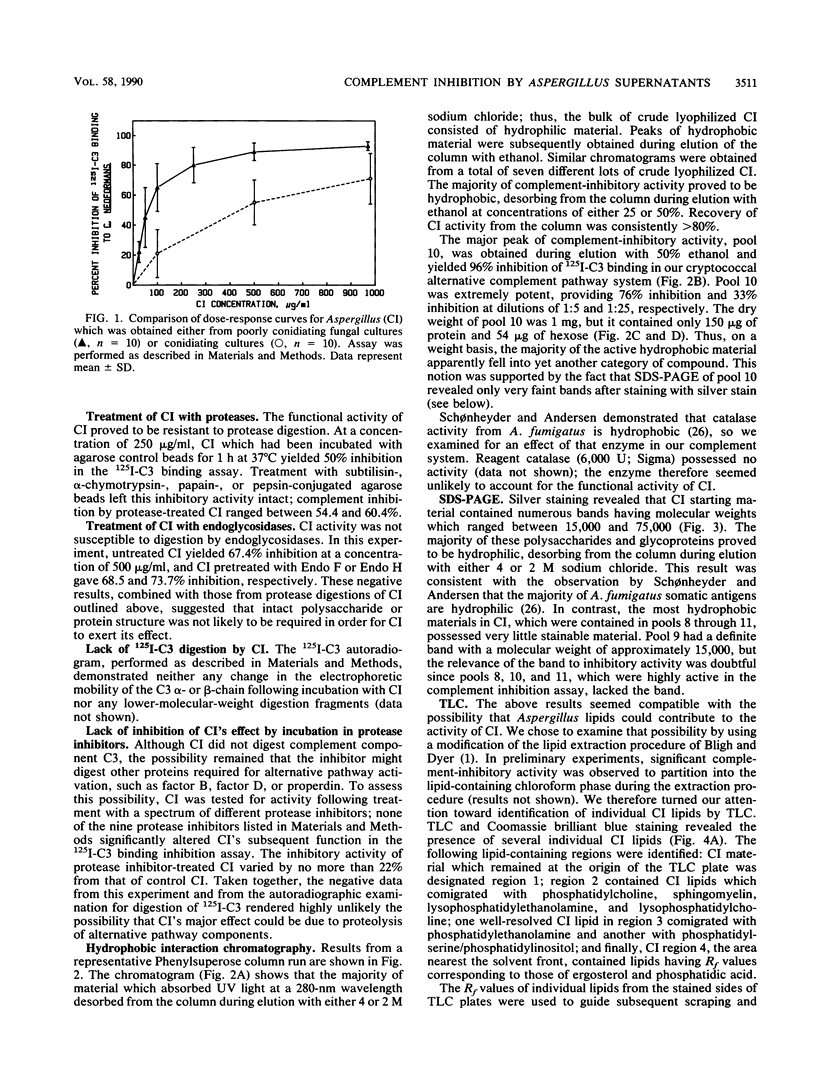
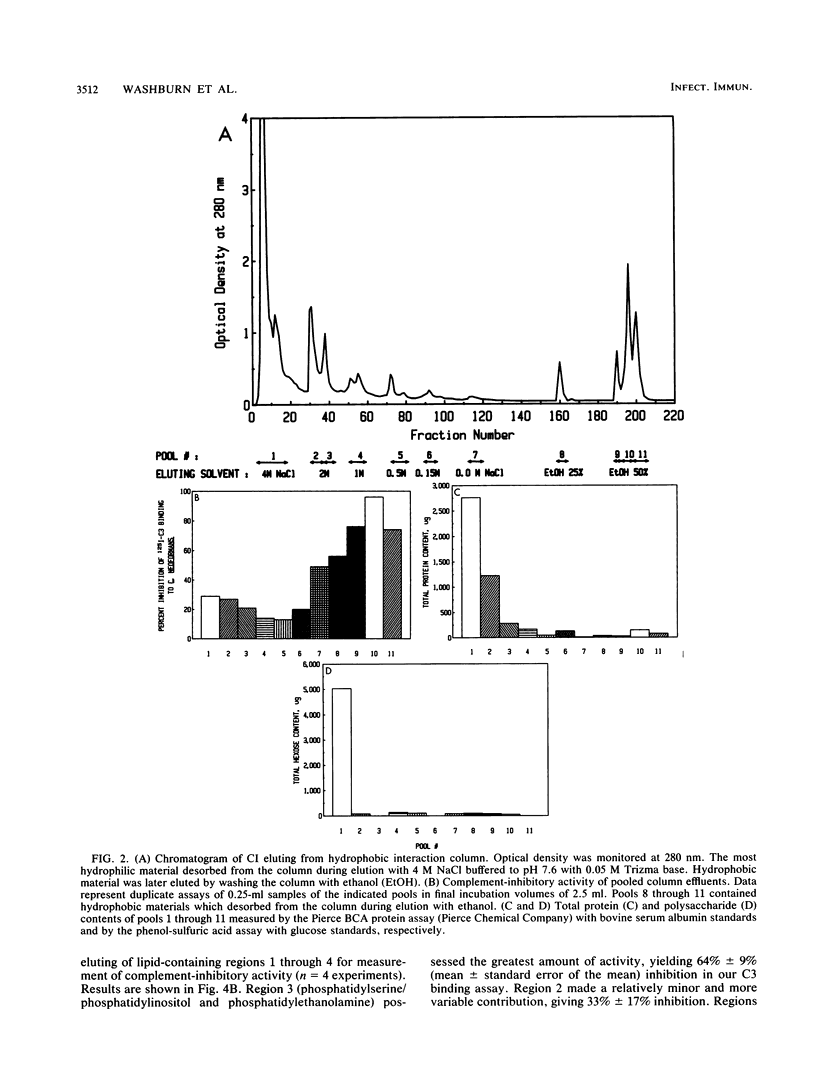
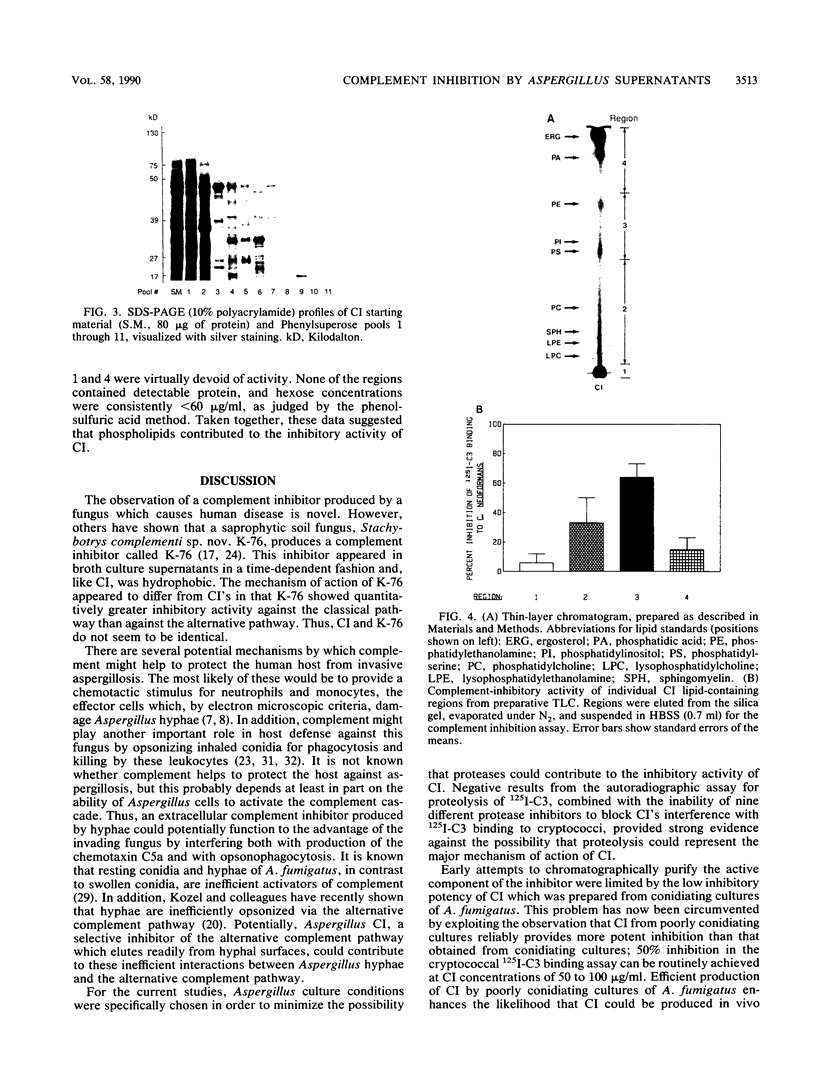
Aspergillus fumigatus has previously been shown to produce a soluble extracellular inhibitor of the alternative complement pathway, called Aspergillus complement inhibitor, or CI. We now report an efficient method for production of CI which relies on the fact that poorly conidiating cultures yielded CI activity with approximately sevenfold-higher potency than CI produced by conidiating cultures. CI from poorly conidiating cultures provided 50% inhibition of alternative pathway-mediated binding of 125I-labeled complement component C3 to cryptococcal blastoconidia at a mean concentration of 60 micrograms/ml. The ability of crude CI to inhibit the alternative complement pathway seemed to be independent of intact protein or polysaccharide structure, as evidenced by resistance of inhibitory activity to digestion by proteases, including subtilisin, alpha-chymotrypsin, papain, and pepsin as well as endoglycosidases F and H. Separation of the active inhibitory component of CI from contaminating materials contained in crude CI preparations was achieved by using Phenylsuperose hydrophobic interaction chromatography in a fast protein liquid chromatography system. The active material proved to be extremely hydrophobic, desorbing from the column only during elution with ethanol; it contained only 15% protein and 5% polysaccharide. Furthermore, results from preparative thin-layer chromatography indicated that lipids which comigrated with phosphatidylserine/phosphatidylinositol and phosphatidylethanolamine possessed significant complement-inhibitory activity. Taken together, these data suggested that phospholipids from A. fumigatus contributed to the functional activity of CI.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Butnick N. Z., Yager L. N., Hermann T. E., Kurtz M. B., Champe S. P. Mutants of Aspergillus nidulans blocked at an early stage of sporulation secrete an unusual metabolite. J Bacteriol. 1984 Nov;160(2):533–540. doi: 10.1128/jb.160.2.533-540.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Linehan L., Wadsworth E., Sandberg A. L. Identification of C3d receptors on Candida albicans. Infect Immun. 1988 Jan;56(1):252–258. doi: 10.1128/iai.56.1.252-258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay P., Banerjee S. K., Sen K., Chakrabarti P. Lipid profiles of Aspergillus niger and its unsaturated fatty acid auxotroph, UFA2. Can J Microbiol. 1985 Apr;31(4):352–355. doi: 10.1139/m85-067. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P., Banerjee S. K., Sen K., Chakrabarti P. Lipid profiles of conidia of Aspergillus niger and a fatty acid auxotroph. Can J Microbiol. 1987 Dec;33(12):1116–1120. doi: 10.1139/m87-195. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Isturiz R. E., Malech H. L., Root R. K., Wilfert C. M., Gutman L., Buckley R. H. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med. 1981 Jul;71(1):59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Huber E., Haudenschild C. C. Mechanisms of destruction of Aspergillus fumigatus hyphae mediated by human monocytes. J Infect Dis. 1983 Mar;147(3):474–483. doi: 10.1093/infdis/147.3.474. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M. A., Frank M. M., Bennett J. E. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974 Jun;112(6):2260–2270. [PubMed] [Google Scholar]
- Edwards J. E., Jr, Gaither T. A., O'Shea J. J., Rotrosen D., Lawley T. J., Wright S. A., Frank M. M., Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986 Dec 1;137(11):3577–3583. [PubMed] [Google Scholar]
- Gilmore B. J., Retsinas E. M., Lorenz J. S., Hostetter M. K. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988 Jan;157(1):38–46. doi: 10.1093/infdis/157.1.38. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Viswanathan L., Venkitasubramanian T. A. A comparative study of the lipids of a toxigenic & a non-toxigenic strain of Aspergillus flavus. Indian J Biochem. 1970 Jun;7(2):108–111. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. The physiological breakdown of the third component of human complement. Mol Immunol. 1980 Jan;17(1):9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Miyazaki W., Izawa T., Inoue K. An anticomplementary agent, K-76 monocarboxylic acid: its site and mechanism of inhibition of the complement activation cascade. J Immunol. 1979 Jun;122(6):2418–2423. [PubMed] [Google Scholar]
- Kaneko I., Fearon D. T., Austen K. F. Inhibition of the alternative pathway of human complement in vitro by a natural microbial product, complestatin. J Immunol. 1980 Mar;124(3):1194–1198. [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun. 1986 Apr;52(1):1–5. doi: 10.1128/iai.52.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Farrell T. P., Levitz S. M. Activation of C3 and binding to Aspergillus fumigatus conidia and hyphae. Infect Immun. 1989 Nov;57(11):3412–3417. doi: 10.1128/iai.57.11.3412-3417.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Pfrommer G. S., Schlageter A. M. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989 Jul;57(7):1922–1927. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. Mechanisms of resistance of Aspergillus fumigatus Conidia to killing by neutrophils in vitro. J Infect Dis. 1985 Jul;152(1):33–42. doi: 10.1093/infdis/152.1.33. [DOI] [PubMed] [Google Scholar]
- Miyazaki W., Tamaoka H., Shinohara M., Kaise H., Izawa T., Nakano Y., Kinoshita T., Hong K., Inoue K. A complement inhibitor produced by Stachybotrys complementi, nov. sp. K-76, a new species of fungi imperfecti. Microbiol Immunol. 1980;24(11):1091–1108. doi: 10.1111/j.1348-0421.1980.tb02914.x. [DOI] [PubMed] [Google Scholar]
- Mold C. Effect of membrane phospholipids on activation of the alternative complement pathway. J Immunol. 1989 Sep 1;143(5):1663–1668. [PubMed] [Google Scholar]
- Schønheyder H., Andersen P. Fractionation of Aspergillus fumigatus antigens by hydrophobic interaction chromatography and gel filtration. Int Arch Allergy Appl Immunol. 1984;73(3):231–236. doi: 10.1159/000233473. [DOI] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Waldorf A. R., Diamond R. D. Neutrophil chemotactic responses induced by fresh and swollen Rhizopus oryzae spores and Aspergillus fumigatus conidia. Infect Immun. 1985 May;48(2):458–463. doi: 10.1128/iai.48.2.458-463.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. C., Packter N. M. Relationship between fatty-acid and phenol synthesis in Aspergillus fumigatus. Eur J Biochem. 1974 Jul 15;46(2):323–333. doi: 10.1111/j.1432-1033.1974.tb03624.x. [DOI] [PubMed] [Google Scholar]
- Washburn R. G., Gallin J. I., Bennett J. E. Oxidative killing of Aspergillus fumigatus proceeds by parallel myeloperoxidase-dependent and -independent pathways. Infect Immun. 1987 Sep;55(9):2088–2092. doi: 10.1128/iai.55.9.2088-2092.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn R. G., Hammer C. H., Bennett J. E. Inhibition of complement by culture supernatants of Aspergillus fumigatus. J Infect Dis. 1986 Dec;154(6):944–951. doi: 10.1093/infdis/154.6.944. [DOI] [PubMed] [Google Scholar]
- Young R. C., Bennett J. E., Vogel C. L., Carbone P. P., DeVita V. T. Aspergillosis. The spectrum of the disease in 98 patients. Medicine (Baltimore) 1970 Mar;49(2):147–173. doi: 10.1097/00005792-197003000-00002. [DOI] [PubMed] [Google Scholar]