Abstract
IL-1 cytokine family plays a key role in the innate immune response against pathogen- and danger-associated molecular patterns. More recently, IL-1 receptor type 1 (IL-R1) signaling has been identified as a critical step in the differentiation and commitment of Th17 cells, which mediate the development of autoimmune diseases. Given its significance in the induction of the adoptive immune response, this complex signaling pathway is tightly regulated. Upon binding of IL-1 to IL-1R1, IL-1R accessory protein (AcP) is recruited to form a high affinity IL-1R1-IL-1RAcP heterodimeric receptor, which initiates the downstream signaling cascade. Multiple negative regulators of this pathway, including inhibitory membrane-bound IL-RII, secreted soluble (s)IL-1RI, sIL-RII and sIL-1RAcP, the regulatory IL-1R1 antagonist (IL-1R1a) and the IL-1R1-signlaing-induced single Ig-IL-1R-related (SIGIRR), provide a negative feedback control of this pathway, and suppress excessive IL-1 signaling and Th17 cell differentiation. IL-1R1 signaling induces human Th17 cell differentiation, leading to the expression of IL-1R-associated protein kinase (IRAK)4 and retinoic acid-related orphan nuclear hormone receptor (ROR), Th17 cell lineage transcription factors, which together with signal transducer and activator of the transcription (STAT)3, activate this cell lineage's specific cytokine expression profile, including IL-17A, IL-17F, IL-21 and IL-22. Given the role of IL-1 signaling and Th17 cells in the development of the autoinflammatory and autoimmune diseases, therapeutic strategies inhibiting IL-1R1 signaling are discussed as a novel approach for the treatment of autoimmune diseases and particularly multiple sclerosis (MS).
Key words: IL-1, IL-1R1, Th17 cells, multiple sclerosis
Introduction
The IL-1 receptor (IL-1R)/toll-like receptor (TLR) superfamily plays an essential role in the regulation of inflammatory and immune responses.1 More recently, IL-1R1 signaling has been recognized as a defining step in the differentiation and commitment of Th17 cells, which play a key role in the development of the autoimmune diseases. After IL-1 binds to IL-1RI, an IL-1RAcP is recruited to form a high affinity IL-1R1-IL-1RAcP heterodimeric receptor, which initiates the downstream signaling cascade. Subsequent to the ligand binding, the IL-1RI complex recruits the myeloid differentiation protein (MyD88), IRAKs, and tumor necrosis factor (TNF) receptor associated factor (TRAF)6 to transmit an intracellular signal through its highly conserved toll/IL-1R (TIR) domain, which results in the activation of the nuclear factor (NF)κB, mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) pathways. Recent studies of the human autoimmune diseases have shown that IL-1 signaling represents a key step in the Th17-mediated autoimmune response in MS, rheumatoid arthritis (RA), psoriasis and inflammatory bowel disease.2
In this mini-review, we will discuss the complex regulation of the IL-1R1 signaling, including regulation of the cell surface expression of IL-1α and secretion of IL-1β. Given the importance of the IL-1Ra signaling, complex negative regulators of this pathway, including inhibitory IL-RII, secreted soluble (s)IL-1RI and sIL-RII, as well as the regulatory IL-1R1 antagonist (IL-1R1a) will be considered in therapeutic approaches targeting this signaling pathway. Since IL-1Ra very effectively suppresses IL-1 signaling, several studies have proposed that the IL-1β/IL-1Ra ratio reflects the activity of this signaling pathway better than the isolated cytokine or cytokine receptor expression levels. Furthermore, IL-1R1 expression is regulated via the ubiquitination and transmembrane proteolysis of IL-1R1, whose expression is a key determinant of human T-cells ability to differentiate into the pathogenic Th17 cells. Further mechanisms for the inhibition of the IL-1-induced signaling cascade include an inhibition of the formation of IL-1 and IL-1RAcP heterodimeric complex, and inhibition of the recruitment of IRAK1, IRAK4 and TRAF6 signaling molecules by SIGIRR, which together with a suppressor of cytokine secretion (SOCS)1, and MyD88s (a splice variant of MyD88) provide the intracellular mechanisms for signaling regulation.3–6
Therapeutic approaches that target IL-1 signaling and its regulatory components will be reviewed in the context of the autoimmune response in patients with MS, which is a focus of the studies in our laboratory. Recent data from our laboratory supporting a critical role of IL-1R1 signaling in the development of the autoimmune response in relapsing remitting (RR) MS will also be discussed.
IL-1 Family
The IL-1 family consists of eleven structurally related molecules, among which are IL-1α and IL-1β (stimulatory ligands for the IL-1R complex), and a specific IL-1 receptor antagonist (IL-1Ra) Table 1. The members of the IL-1 family are coded by closely located genes on the human chromosome 2. IL-1α and IL-1β are synthesized as precursor proteins, which are proteolytically cleaved by caspase 1, the IL-1 converting enzyme, to generate biologically active cytokines.3,7 In contrast, IL-1Ra is secreted extracellularly through the endoplasmic reticulum and its binding to the IL-1R1 prevents its association with IL-1RAcP and signaling, upon ligand binding to the receptor.8 All nucleated cells and particularly mononuclear cells, produce IL-1 upon induction by infection, inflammation and cell injury.
Tabe 1.
IL-1-associated molecules
| Molecules | Abbreviation | Description |
| Interleukin-1 receptor type I | IL-1R I | cytokine receptor which binds interleukin 1 |
| Interleukin-1 receptor type II | IL-1R II | cytokine receptor which binds interleukin 1 |
| Interleukin-1 receptor antagonist | IL-1Ra | antagonist that binds to the cell surface interleukin-1 receptor |
| Interleukin-1 receptor accessory protein | IL-1RAcP | transmembrane protein that interacts with IL-1R, |
| Toll interacting protein | Tollip | inhibitory adaptor protein |
| Interleukin-1 receptor-associated kinase 4 | IRAK4 | protein kinase involved in signaling from Toll-like receptors |
| Myeloid differentiation primary response gene (88) | MYD88 | universal adapter protein recruited after Toll-like receptors ligation |
| Single Ig IL-1-related receptor | SIGIRR | negative regulator of interleukin 1 receptor and Toll-like receptor signaling |
| Interleukin-1 receptor-associated kinase 1 | IRAK1 | protein kinase involved in signaling from Toll-like receptors |
| Interleukin-1 receptor-associated kinase 2 | IRAK2 | protein kinase involved in signaling from Toll-like receptors |
| Tumor necrosis factor receptor-associated factor 6 | TRAF6 | signal transducer in the nuclear factor-kappaB pathway |
| Ubiquitin-conjugating enzyme E2 N | Ubc13 | ubiquitin E2 ligase |
| Ubiquitin-conjugating enzyme E2 variant 1 | Uev1A | ubiquitin E2 ligase |
| Transforming growth factor-β-activated protein kinase 1 | TAK1 | interleukin 1-signaling intermediate |
| Transforming growth factor-β-activated protein kinase-binding protein 1 | TAB1 | interleukin 1-signaling intermediate |
| Nuclear factor-kappaB | NF-κB | protein complex that controls the transcription of DNA |
| Inhibitor of nuclear factor kappaB | IκBα | NF-κB transcription factor inhibitor |
| Nuclear factor-kappaB essential modulator | NEMO | part of the inhibitory complex of nuclear factor kappaB kinase |
| Inhibitor of nuclear factor kappaB kinase subunit alpha | Iκκα | part of the inhibitory complex of nuclear factor kappaB kinase |
| Inhibitor of nuclear factor kappaB kinase subunit beta | Iκκβ | part of the inhibitory complex of nuclear factor kappaB kinase |
| Mitogen-activated protein kinase kinase kinase 3 | MEKK3 | protein kinase that mediates TLR and IL1R signaling-induced gene activation |
| Mitogen-activated protein kinase kinase kinase 6 | MEKK6 | protein kinase that mediates TLR and IL1R signaling-induced gene activation |
| p38 mitogen-activated protein kinases | p38MAPK | mitogen-activated protein kinase responsive to stress stimuli |
| c-Jun N-terminal kinase | JNK | mitogen-activated protein kinase responsive to stress stimuli |
| Extracellular signal-regulated protein kinase | ERK | protein kinase intracellular signalling molecule |
| c-Jun | c-Jun | protein that interacts with specific target DNA sequences to regulate gene expression |
| c-Fos | c-Fos | transcription factor that regulates gene expression |
| Activator protein 1 | AP1 | transcription factor, a heterodimeric protein composed of c-Fos and c-Jun |
IL-1 is a pleiotropic mediator of the response to infection and injury, and it coordinates the activities of other cells and cytokines. It is produced by macrophages, dendritic cells (DCs), B- and T-cells in the peripheral circulation, where it stimulates the secretion of other cytokines, such as granulocyte colony-stimulating factor (G-CSF), TNFα, IL-6, IL-8, IL-11 and IL-17. IL-1 cytokines link cell injury to the adaptive immune response. High mobility group nucleosome-binding (HMGN)1, a nuclear DNA binding protein that is passively released from necrotic cells, induces monocyte secretion of IL-1β,9 which then leads to the augmented Th1 and Th17 cell differentiation and an adaptive immune response. IL-1 upregulates multiple cytokine receptors, inducing IL-1R1, the IL-2Rα chain, and the receptors for interferon (IFN)γ, IL-13 and granulocyte macrophage colony-stimulating factor (GM-CSF), thereby promoting the synergistic action of those cytokines with IL-1. When produced locally, IL-1 acts as an autocrine and paracrine costimulator of the early innate inflammatory and adaptive immune responses, enhancing CD4 T-cells' antigen-driven differentiation and proliferation of the Th1, Th2 and Th17 cell subsets.9,10 In the context of the autoimmune diseases, IL-1 is responsible for the activation of autoreactive T-cells through induction of their expression of CD40L and OX40 11,12 and matrix metalloproteinases,13 which facilitate cell migration to the sites of inflammation.
Systemically, in addition to its pro-inflammatory effects, IL-1 causes hyperthermia, the activation of the hypothalamic-pituitary adrenal axis,14,15 and contributes to the systemic hypotension induced by bacterial infections.16
Studies of patients with deletion of individual or multiple genes of the IL-1 family, provide an invaluable insight into the systemic and local effects of IL-1β and multiple components of this signaling pathway in humans.17–19 Autoinflammatory diseases include neonatal-onset multi-inflammatory disease, Muckle-Wells syndrome, familial cold autoinflammatory syndrome, hyper IgD syndrome, familial Mediterranean fever, urate crystal arthritis (gout) and type 2 diabetes mellitus.2
As our laboratory focuses on the role of IL-1β in the development of the autoimmune response in RR MS, it is important to emphasize that polymorphisms encoded within the IL-1 gene cluster have been associated with susceptibility to RRMS.16 IL-1 is detected in active MS lesion in the microglia, astrocytes and in brain endothelial cells,20,21 where it regulates through upregulation of intracellular adhesion molecule (ICAM) and the vascular cell adhesion molecule (VCAM)-1, leukocyte transmigration into the central nervous system (CNS) inflammatory sites.11 It has been reported that IL-1 induces astrocyte activation and is in turn secreted from the activated astrocytes. IL-1 produced within the CNS lesions induces permeability of the blood brain barrier via induction of the vascular endothelial growth factor (VEGF)-A. In addition, IL-1β promotes apoptosis of neurons and oligodendrocytes, thereby contributing to the inflammation-mediated damage of the CNS parenchyma.20 Interestingly, IL-1 is also produced by neurons, which at a later time point also produce IL-1Ra. The relative levels of IL-1 and IL-1Ra determine the extent of tissue injury in the CNS; these levels are tightly regulated by cell type-specific regulatory pathways.22
IL-1R1 Signaling
IL-1R is related to TLRs and its structure is characterized by the presence of extracellular immunoglobulin (Ig)-like domains and an intracellular TIR domain. The three Ig domains are responsible for ligand binding, while the conserved TIR domain—characterized by the presence of three homologous regions (boxes 1, 2 and 3), is critical for signaling.23 IL-1RI activation is induced by IL-1α and IL-1β binding, followed by the association of IL-1RAcP with the receptor complex.23 IL-1R1 is expressed on the surface of a variety of cells, including monocytes, DCs, fibroblasts, epithelial cells and T-cells.24 IL-1α and IL-1β also bind to the surface IL-1RII (expressed predominantly on B-cells and neutrophils),25 which is dispensable for signaling and acts as a decoy receptor.26 In addition, IL-1RII inhibits IL1R1's association with IL1RAcP by sequestering it and preventing effective IL-1RI signaling.27 Soluble IL-1RI and IL-1RII bind to IL-1β with higher affinity than do IL-1α and IL-1Ra, effectively preventing IL-1R1 signaling.28 The secreted receptor antagonist IL-1Ra binds to IL-1RI with a similar affinity as IL-1 and inhibits the binding of both IL-1α and IL-1β and their induction of signaling.29 In addition to decoy and antagonistic IL-1 receptors, TRAF6 can induce ubiquitination and regulates intramembrane proteolysis of IL-1RI,30–32 which further contributes to the regulation of this important signaling pathway.
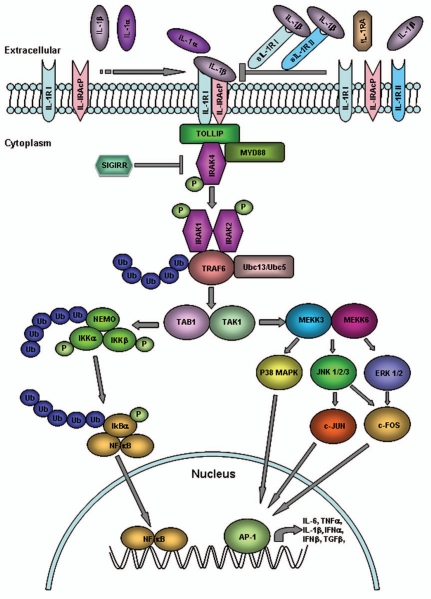
IL-1β binding to IL-1R1 induces its formation of a heterodimer with the IL-1RAcP, followed by the recruitment of the MyD88 adapter and its activation and association with IRAK4, which phosphorylates IRAK1 and IRAK2 and bind TRAF6. The formation of this complex leads to the induction of the MAPK p38 cascade and the NF-κB pathway (Fig. 1).1,2,33
Figure 1.
IL-1RI signaling. Upon binding of IL-1 to IL-1RI, an IL-1RAcP is recruited to form a high affinity IL-1R1-IL-1RAcP heterodimeric receptor, which initiates the downstream signaling cascade. The trimeric complex rapidly assembles MyD88 and IRAK4 and forms a stable IL-1-induced first signaling module, which subsequently phosphorylates IRAK1 and IRAK2, and recruits TRAF6. Complexes of IRAK1, IRAK2 and TRAF6 dissociate from the initial receptor complex and promote TGFβ-activated protein kinase (TAK)1/TAK-binding protein (TAB)1 association, which is followed by the activation of NFκB, JNK and p38 MAPK pathways. Activation of the IKK complex by IL-1 promotes IκBα ubiquitination. The nuclear translocation of NFκB with the nuclear translocation of c-JUN, induced by the activation of JNK and p38 MAPK, modulates the gene expression of IL-6, TNFα, IL-1β, IFNα, IFNβ and TGFβ. Multiple negative regulators of this pathway, including inhibitory IL-RII, secreted soluble (s)IL-1RI and sIL-RII, regulatory IL-1R1a and SIGIRR provide a negative feedback control of this pathway and suppress excessive IL-1 signaling.
NF-κB is a critical transcription factor for the immune and inflammatory responses since it regulates the transcription of multiple proinflammatory cytokines. The key event in the NF-κB activation is the IL-1-induced phosphorylation and degradation of the inhibitory cytoplasmic protein IκB, which leads to the release of NF-κB, its translocation to the nucleus and binding and transcriptional regulation of its target genes (Fig. 1).34
IL-1R1 Signaling in Antigen Presenting Cells (APCs)
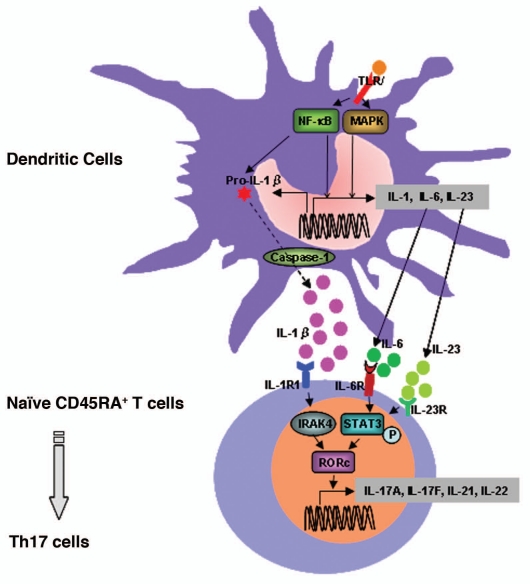
During activation of the innate immune response by infection, APCs, such as macrophages and DCs, can recognize the pathogen's structural components using TLR/IL-1R and produce pro-inflammatory cytokines to induce T-cell differentiation and the activation of the adaptive immune response.32 DCs are professional antigen-presenting cells, able to capture and process antigens, migrate into lymphoid organs and present antigenic peptides to naive T-cells.35 The APCs' capacity to induce Th17 responses is defined by their cytokine secretion profile. Acosta-Rodriguez et al. have reported that activated monocytes and circulating conventional DCs produce large amounts of IL-1β and IL-6, representing the most efficient APCs for Th17 differentiation (Fig. 2).35 Monocyte-induced Th17 differentiation was inhibited by anti IL-1β and anti IL-6 neutralizing antibodies and by inhibitors of Caspase 1, required for the IL-1β release. Importantly, the proportion of cells producing IL-17 was higher after priming naive T-cells by APCs than by anti-CD3 and anti-CD28 mAbs.27
Figure 2.
IL-1-induced Th17 cell differentiation. IL-1R1 signaling is critical for the differentiation and commitment of Th17 cells. IL-1β is induced via activation of the TLR signaling pathways and is processed by caspase-1. Activation of TLR pathways also induces the production of proinflammatory cytokines including IL-6 and IL-23. Naïve T-cells primed by APCs such as DCs can differentiate into Th17 cells depending upon the cytokine environment. Priming in the presence of IL-1/IL-6/IL-23 promotes the differentiation of Th17. In naïve CD45RA+ T-cells, Th17 cell differentiation induced by IL-1 signaling may be mediated through the induction of IRF4 and RORc, key transcription factors for Th17 cell differentiation. IL-1R1 signaling also induces IL-23 secretion and the subsequent activation of STAT3, which induces RORc expression and the transcription of Th17 cytokines, including IL-17A, IL-17F, IL-21 and IL-22.
DCs can also migrate into the CNS, where together with microglia and astrocytes, they participate in the myelin antigen presentation to T-cells. During chronic inflammation, these APCs can perpetuate the self-destructive inflammatory response by secretion of inflammatory cytokines, which further enhance their antigen presentation to the autoreactive T-cells.
It has been shown that TRAF6 is involved in the signaling cascade downstream of the IL-1R, coupling IL-1RI to the NF-κB activation. TRAF6 is also a critical factor for DCs' activation and maturation. DCs from MyD88-deficient mice failed to produce inflammatory cytokines (IL-1β, IL-6, TNFα, TGFβ, IL-12) in response to IL-1, suggesting that MyD88 is indispensable for inflammatory cytokine production in IL-1R signaling.29 Further evidence that TRAF6 plays a critical role in DCs' function is provided by the observation that lipopolysaccharide (LPS) treatment of TRAF6-deficient DCs fails to augment their T-cell stimulatory capacity.30
The major cell types responsible for an inflammatory response in the brain are microglia and astrocytes, which secrete inflammatory cytokines upon activation.31 IL-1 is rapidly expressed by microglia and astrocytes in response to neuronal injury and elevated levels of IL-1 further contribute to the CNS injury and astrogliosis.32
IL-1R Signaling in T-cells
IL-1 plays an important role in the regulation of the T-cells', B-cells' and natural killer cells' activation. IL-1R1 expression in T cells, which is induced by IL-6, is necessary for the early T helper (Th) 17 cell differentiation, as it induces IFN regulatory factor (IRF)4 and RORγτ transcription factor expression.8,15 Since IL-1R1−/− T-cells have lower IL-23R expression, it is conceivable that IL-1 may enhance IL-23R expression in Th17 cells.8 Furthermore, a disinhibited IL-1 signaling in IL-1Ra-deficeint mice induces a strong IL-23 production, which enhances Th17 cell differentiation.33 IL-1RI−/− mice have a reduced incidence of experimental autoimmune encephalomyelitis (EAE), associated with failure to induce autoantigen-specific Th17 cells.27,34 IL-1R1 expression is increased in Th17 cells in comparison to the Th1 or Th2 cell subsets,8 and IL-1 signaling maintains Th17 cell expansion even in the absence of TCR stimulation.8
In humans, IL-1β has been implicated as an essential cytokine for the Th17 cells differentiation,36,37 since IL-1β in naïve CD4 cells induced RORc expression and Th17 differentiation, which was enhanced by IL-6 and IL-23 (Fig. 2). In human peripheral blood, CD4+ T-cells that express IL-1R1 have the capacity to produce IL-17 in naive and memory T-cells. IL-R1+ memory cells had increased gene expression of IL-17, RORc and IRF4, even before TCR triggering, suggesting that IL-1R1 expression renders cells committed to Th17 differentiation.27 IL-1RI+ memory CD4+ T-cells produce higher levels of IL-17 in response to TCR triggering, as well as increased gene expression of IRF4, RORc, RORα, IL-17A, IL-17F, IL-26 and IL-23R, in comparison to IL-1RI− memory CD4+ T-cells. IL-1RI+ naive CD4+ T-cells produce higher levels of IL-17 in response to a combination of IL-1β and TCR triggering compared to IL-1RI− naive CD4+ T-cells, which are capable of producing IL-17 only upon TCR stimulation-induced IL-1R1 expression. The decoy IL-1RII expression was expressed at higher levels in IL-1R1+ than in IL-1R1− cells, but both cells had similar levels of IL-1RAcP expression, indicating that inducible IL-1RII has a role in limiting the response to IL-1β.38
Recent data from our laboratory have demonstrated that IL-1R1 expression is significantly higher in both the naïve and memory CD4+ T-cells derived from RRMS patients in comparison to those from healthy controls (HCs). Moreover, in-vitro differentiated Th17 cells expressed higher levels of IL-R1 than did Th1 or Th2 differentiated cells. Finally, the siRNA silencing of IL-1R1 in naïve CD4+ cells inhibited in-vitro Th17 cell differentiation, by inhibiting IRF4 and RORc, as well as IL-17A, IL-17F, IL-21, IL-22 and IL-23R gene expression. Cytokine secretion measurements detected a significantly decreased IL-17A and IL-21 secretion by the siRNA IL-1R1-transfected naïve in-vitro polarized Th17 cells (Sha et al. manuscript in preparation).
Regulatory T cells (Tregs) are critical in the active suppression of the autoimmune responses. The expression of IL-1R1 is higher on resting mature Tregs compared to naive or memory T-cells. However, IL-1R1 expression does not affect Tregs' ability to suppress T-cell proliferation, but more likely represents a specialized Tregs subset that is better equipped to respond to IL-1 at the site of inflammation. It is proposed that IL-1R1 could be used as an activated Tregs-specific marker, allowing separation of purified activated Tregs, which could be utilized as a therapeutic strategy in autoimmune diseases.39
IL-1R1 Signaling in the Development of Autoimmune Diseases
A pivotal role of IL-1 signaling in the induction of autoimmune diseases is demonstrated by the induction of EAE exacerbations following IL-1α or IL-1β administration.11 The paralysis of the IL-1-treated mice with EAE lasted longer, was more severe and animals exhibited more severe weight loss.40 Furthermore, mice deficient for IL-1R1 or IRAK1, which did not develop Th17 cells, were resistant to EAE.11,15
Elevated IL-1β cerebrospinal fluid (CSF) and serum levels have been reported in patients with RRMS in comparison to healthy controls.20 Increased IL-1β levels have also been detected in MS lesions, where it is produced predominantly by microglia and astrocytes.41 Families with a high IL1-β to IL-1Ra production ratio are at greater risk of having a relative with RRMS than are those with a low ratio,21,42 suggesting that the balance between IL-1β and IL-1Ra production may be more relevant for predicting the risk for RRMS than the cytokine levels.
In the arthritis model, the injection of IL-1β into normal rabbit joints caused severe arthritis, while anti-IL-1-blocking antibodies ameliorated the disease.14 In contrast, mice deficient for IL-1Ra spontaneously develop arthritis, due to the disinhibited IL-1β-induced IL-23 production, which induced Th17 cell differentiation.14 However, mice deficient in IL-1Ra did not develop spontaneous arthritis when also deficient for IL-17.43
A number of human diseases associated with the deletion of the gene for IL-1 regulatory molecule (IL-1Ra) exhibit unopposed IL-1 signaling, while patients with the mutation of NOD-like receptor family 3 and inhibition of IL-1 converting enzyme do not produce secreted IL-1β. These rare diseases with identified mutation of the IL-1 family genes have been successfully treated with therapeutic administration of the recombinant IL-1Ra, which was more recently used also in the treatment of the IL-1-mediated autoimmune disease, RA.2
Inhibition of IL-1R1 Signaling as a Therapeutic Approach in Autoimmune Diseases
Currently available strategies to counter pathological IL-1 signaling rely on a recombinant IL-1Ra, which directly competes with IL-1 for its binding site.16 Badinovac et al.44 have reported that recombinant IL-1Ra delayed the EAE onset and suppressed the severity and duration of the clinical disease activity, by influencing the activation and proliferation of encephalitogenic cells. Both IL-1Ra and sIL-1R1 have been proposed to suppress IL-1-mediated inflammation in RA.45 Anakinra, a recombinant IL-1Ra has been reported to be safe and effective in suppressing disease activity in patients with RA46 and juvenile idiopathic arthritis.47 Furthermore, more selective reversible noncompetitive small peptide antagonist (RYTVELA) of IL-1R1 may only diminish the binding activity of IL-1, in contrast to recombinant IL-1Ra which disables all functions triggered by the IL-1R1 receptor.16
Based on evidence that IL-1RAcP interacts with the IL-1R subunit of the IL-1R1 receptor complex and that the recombinant extracellular portion of IL-1RAcP can interfere with IL-1R1's actions, Smeets et al. have designed a peptide that is rationally derived from an extracellular loop of the IL-1RAcP regions and interferes with IL-1R1 signaling.48 Anti-IL-1RAcP mAb has also been used to inhibit IL-1α, IL-1β and the related IL-33 signal transduction.
Soluble IL-1R1 (sIL-1R1) is a naturally occurring regulatory protein that inhibits the biological activities of IL-1. sIL-1R1 has been reported to improve the survival of heart allografts, protect from experimental autoimmune diabetes mellitus and decrease the severity of arthritis.30 In studies of mouse peritoneal macrophages incubated with LPS, addition of sIL-1R1 significantly inhibited the bioactivity of IL-1, suggesting that sIL-1R1 is a potential therapeutic agent for targeting IL-1-mediated pathways.49 Most recently, novel strategies have been utilized for the development of anti-inflammatory therapeutics based on interference with the function of the TIR domain of members of the IL-1R superfamily (Fig. 2).50
Recently described negative regulator of IL-1R and TLR signaling SIGIRR is induced during Th17 cell differentiation upon IL-1's binding to the IL-1R1. This IL-1-induced regulator of excessive IL-1 signaling and Th17 cell differentiation represents a unique target for therapeutic intervention in the Th17 cell-mediated autoimmune diseases (Fig. 2).15 SIGIRR is expressed in T-cells, B-cells and DCs and mediates the fine tuning of the inflammatory response, since its deficiency is associated with hypersusceptibility to autoimmune diseases, including systemic lupus, EAE and DSS-induced colitis.51 Consistent with its IL-1R1 signaling-dependent function, SIGIRR is expressed at higher levels in Th17 cells compared to Th1 or naïve CD4 cells. Even though SIGIRR does not bind to the IL-1β, it negatively regulates IL-1 signaling through its interaction with the IL-1R1 complex by inhibiting heterodimerization between IL-1R1 and IL-RAcP,52 and by attenuating the recruitment of proximal signaling components (MyD88, IRAK4/IRAK1 and TRAF6) to the receptor complex (Fig. 2).15,52,53 Therefore, SIGIRR functions as a negative feedback switch that prevents detrimental autoimmune and chronic inflammatory responses. In SIGIRR-deficient cells, Th17 differentiation is associated with an enhanced response to IL-1 and the induction of IRF4 and RORc.54 Thus, the biological outcome of the IL-1R1 activation is the result of a balance between its activation and dampening of the signaling by SIGRR, which represents a novel and not yet therapeutically manipulated target.55 Interestingly, SIGIRR is expressed in the CNS by neurons, microglia and astrocytes, where it negatively regulates microglia activation.56
In addition to the above-proposed direct manipulation of IL-1R1 signaling by its inhibitors, multiple naturally occurring anti-inflammatory and immunomodulatory therapies were reported to provide therapeutic effect by targeting IL-1R1 signaling. Corticosteroids, frequently used as anti-inflammatory agents in patients with acute MS relapses, were reported to induce the elevation of IL-1Ra and IL-1RII in the sera of treated patients, suggesting that their therapeutic mechanisms may in part be mediated by the activation of IL-1 antagonistic mechanisms.20
Treatment of MS patients with IFNβ-1b has been reported to induce IL-1Ra in treated patients,57,58 while study of glatiramer acetate, another approved immunomodulatory therapy for MS, reportedly elevated sIL-1Ra blood levels.59
Summary
In conclusion, the IL-1R1 signaling pathway has received significant attention due to its recently described role in Th17 cell differentiation and the generation of autoimmune responses. Its complex regulation by multiple naturally occurring inhibitors opens new possibilities for the treatment of autoinflammatory and autoimmune diseases. Since clinical trials targeting specific genetic defects in the IL-1/IL-1R signaling pathway showed remarkable efficacy in systemic autoinflammatory diseases, we propose that multiple available compounds, including recombinant IL-1Ra, IL-1R and IL-1RAcp (IL-1 Trap), soluble IL-1RII, anti-IL-RAcP and it soluble peptide antagonists, as well as anti-IL-1 mAbs may provide new opportunities for the treatment of autoimmune diseases in the future.
Abbreviations
- IL-1R1
IL-1 receptor type 1
- IL-1RAcP
IL-1R accessory protein
- s
soluble
- IL-1Ra
IL-1R antagonist
- SIGIRR
single Ig-IL-1R-related receptor
- IRAK
IL-1R-associated kinase
- RORc
retinoic acid-related orphan nuclear hormone receptor c
- STAT
signal transducer and activator
- MS
multiple sclerosis
- TLR
toll-like receptors
- MyD88
myeloid differentiation primary response protein 88
- TRAF
tumor necrosis factor (TNF) associated factor
- TIR
Toll/IL-1R
- NF-κB
nuclear factor κB
- MAPK
mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
- RA
rheumatoid arthritis
- SOCS
suppressor of cytokine secretion
- RR
relapsing remitting
- DCs
dendritic cells
- G-CSF
granulocyte colony-stimulating factor
- TNF
tumor necrosis factor
- HMGN
high mobility group nucleosome-binding
- IFN
interferon
- GM-CSF
granulocyte macrophage CSF
- ICAM
intracellular adhesion molecule
- VCAM
vascular cell adhesion molecule
- CNS
central nervous system
- VEGF
vascular endothelial growth factor
- APCs
antigen presenting cells
- LPS
lipopolysaccharide
- IRF
IFN regulatory factor
- EAE
experimental autoimmune encephalomyelitis
- HCs
healthy controls
- Tregs
regulatory T-cells
- EAE
experimental autoimmune encephalomyelitis
- CSF
cerebrospinal fluid
- TAK
TGFβ-activated protein kinase
- TAB
TAK-binding protein
- TGF
tumor growth factor
References
- 1.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3:1–6. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 3.Weber A, Wasiliew P, Kracht M. Interleukin-1beta (IL-1beta) processing pathway. Sci Signal. 2010;3:1–2. doi: 10.1126/scisignal.3105cm2. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello CA. The many worlds of reducing interleukin-1. Arthritis Rheum. 2005;52:1960–1967. doi: 10.1002/art.21107. [DOI] [PubMed] [Google Scholar]
- 6.Sriskantharajah S, Ley SC. Cell biology. Turning off inflammation signaling. Science. 2010;327:1093–1094. doi: 10.1126/science.1187271. [DOI] [PubMed] [Google Scholar]
- 7.Mills KH, Dunne A. Immune modulation: IL-1, master mediator or initiator of inflammation. Nat Med. 2009;15:1363–1364. doi: 10.1038/nm1209-1363. [DOI] [PubMed] [Google Scholar]
- 8.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–577. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao DA, Tracey KJ, Pober JS. IL-1alpha and IL-1beta are endogenous mediators linking cell injury to the adaptive alloimmune response. J Immunol. 2007;179:6536–6546. doi: 10.4049/jimmunol.179.10.6536. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 12.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson U, Kurrer MO, Sonderegger I, Iezzi G, Tafuri A, Hunziker L, et al. Activation of dendritic cells through the interleukin 1 receptor 1 is critical for the induction of autoimmune myocarditis. J Exp Med. 2003;197:323–331. doi: 10.1084/jem.20021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quiniou C, Sapieha P, Lahaie I, Hou X, Brault S, Beauchamp M, et al. Development of a novel noncompetitive antagonist of IL-1 receptor. J Immunol. 2008;180:6977–6987. doi: 10.4049/jimmunol.180.10.6977. [DOI] [PubMed] [Google Scholar]
- 17.Reddy S, Jia S, Geoffrey R, Lorier R, Suchi M, Broeckel U, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, et al. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206:1029–1036. doi: 10.1084/jem.20082481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dujmovic I, Mangano K, Pekmezovic T, Quattrocchi C, Mesaros S, Stojsavljevic N, et al. The analysis of IL-1beta and its naturally occurring inhibitors in multiple sclerosis: The elevation of IL-1 receptor antagonist and IL-1 receptor type II after steroid therapy. J Neuroimmunol. 2009;207:101–106. doi: 10.1016/j.jneuroim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 21.de Jong BA, Huizinga TW, Bollen EL, Uitdehaag BM, Bosma GP, van Buchem MA, et al. Production of IL-1beta and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis. J Neuroimmunol. 2002;126:172–179. doi: 10.1016/s0165-5728(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 22.Liu JS, Amaral TD, Brosnan CF, Lee SC. IFNs are critical regulators of IL-1 receptor antagonist and IL-1 expression in human microglia. J Immunol. 1998;161:1989–1996. [PubMed] [Google Scholar]
- 23.Slack JL, Schooley K, Bonnert TP, Mitcham JL, Qwarnstrom EE, Sims JE, et al. Identification of two major sites in the type I interleukin-1 receptor cytoplasmic region responsible for coupling to pro-inflammatory signaling pathways. J Biol Chem. 2000;275:4670–4678. doi: 10.1074/jbc.275.7.4670. [DOI] [PubMed] [Google Scholar]
- 24.Sims JE, March CJ, Cosman D, Widmer MB, MacDonald HR, McMahan CJ, et al. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988;241:585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- 25.McMahan CJ, Slack JL, Mosley B, Cosman D, Lupton SD, Brunton LL, et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colotta F, Re F, Muzio M, Bertini R, Polantarutti N, Sironi M, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 27.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 28.Wang D, Zhang S, Li L, Liu X, Mei K, Wang X. Structural insights into the assembly and activation of IL-1beta with its receptors. Nat Immunol. 2010;11:905–911. doi: 10.1038/ni.1925. [DOI] [PubMed] [Google Scholar]
- 29.Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, et al. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- 30.Elzinga BM, Twomey C, Powell JC, Harte F, McCarthy JV. Interleukin-1 receptor type 1 is a substrate for gamma-secretase-dependent regulated intramembrane proteolysis. J Biol Chem. 2009;284:1394–1409. doi: 10.1074/jbc.M803108200. [DOI] [PubMed] [Google Scholar]
- 31.Twomey C, Qian S, McCarthy JV. TRAF6 promotes ubiquitination and regulated intramembrane proteolysis of IL-1R1. Biochem Biophys Res Commun. 2009;381:418–423. doi: 10.1016/j.bbrc.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes Infect. 2004;6:1333–1338. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Shephard F, Kim HB, Palmer IR, McHarg S, Fowler GJ, et al. TILRR, a novel IL-1RI co-receptor, potentiates MyD88 recruitment to control Ras-dependent amplification of NFkappaB. J Biol Chem. 2010;285:7222–7232. doi: 10.1074/jbc.M109.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NFkappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 35.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 36.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 37.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 38.Lee WW, Kang SW, Choi J, Lee SH, SHa K, Eynon EE, et al. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs CA, Baker PE, Roux ER, Picha KS, Toivolla B, Waugh S, et al. Experimental autoimmune encephalomyelitis is exacerbated by IL-1alpha and suppressed by soluble IL-1 receptor. J Immunol. 1991;146:2983–2989. [PubMed] [Google Scholar]
- 41.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 42.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle RC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, et al. STAT3 and NFkappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 44.Badovinac V, Mostarica-Stojkovic M, Dinarello CA, Stosic-Grujicic S. Interleukin-1 receptor antagonist suppresses experimental autoimmune encephalomyelitis (EAE) in rats by influencing the activation and proliferation of encephalitogenic cells. J Neuroimmunol. 1998;85:87–95. doi: 10.1016/s0165-5728(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 45.Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149:217–225. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleischmann RM, Schechtman J, Bennett R, Handel ML, Burmster GR, Tesser J, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trial. Arthritis Rheum. 2003;48:927–934. doi: 10.1002/art.10870. [DOI] [PubMed] [Google Scholar]
- 47.Allantaz F, Chaussabel D, Stichweh D, Bennett L, Allman W, Mejias A, et al. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. J Exp Med. 2007;204:2131–2144. doi: 10.1084/jem.20070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smeets RL, Joosten LA, Arntz OJ, Bennink MB, Takashi N, Carlsen H, et al. Soluble interleukin-1 receptor accessory protein ameliorates collagen-induced arthritis by a different mode of action from that of interleukin-1 receptor antagonist. Arthritis Rheum. 2005;52:2202–2211. doi: 10.1002/art.21108. [DOI] [PubMed] [Google Scholar]
- 49.Netea MG, Kullberg BJ, Boerman OC, Verschueren I, DInarello CA, Van der Meer JW. Soluble murine IL-1 receptor type I induces release of constitutive IL-1 alpha. J Immunol. 1999;162:4876–4881. [PubMed] [Google Scholar]
- 50.Loiarro M, Ruggiero V, Sette C. Targeting TLR/IL-1R signaling in human diseases. Mediators Inflamm. 2010;2010:674363. doi: 10.1155/2010/674363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci USA. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin J, Qian Y, Yao J, Grace C, Li X. SIGIRR inhibits interleukin-1 receptor- and toll-like receptor 4-mediated signaling through different mechanisms. J Biol Chem. 2005;280:25233–25241. doi: 10.1074/jbc.M501363200. [DOI] [PubMed] [Google Scholar]
- 53.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 54.Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D'Angelo C, et al. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J Immunol. 2008;180:4022–4031. doi: 10.4049/jimmunol.180.6.4022. [DOI] [PubMed] [Google Scholar]
- 55.O'Neill LA. SIGIRR puts the brakes on Toll-like receptors. Nat Immunol. 2003;4:823–824. doi: 10.1038/ni0903-823. [DOI] [PubMed] [Google Scholar]
- 56.Watson MB, Costello DA, Carney DG, McQuillan K, Lnch MA. SIGIRR modulates the inflammatory response in the brain. Brain Behav Immun. 2010;24:985–995. doi: 10.1016/j.bbi.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Comabella M, Julia E, Tintore M, Brieva L, Tellez N, Rio J, et al. Induction of serum soluble tumor necrosis factor receptor II (sTNF-RII) and interleukin-1 receptor antagonist (IL-1ra) by interferonbeta-1b in patients with progressive multiple sclerosis. J Neurol. 2008;255:1136–1141. doi: 10.1007/s00415-008-0855-1. [DOI] [PubMed] [Google Scholar]
- 58.Heesen C, Sieverding F, Buhmann C, Gbadamosi J. IL-1ra serum levels in disease stages of MS—a marker for progression? Acta Neurol Scand. 2000;101:95–97. doi: 10.1034/j.1600-0404.2000.101002095.x. [DOI] [PubMed] [Google Scholar]
- 59.Burger D, Molnarfi N, Weber MS, Brandt KJ, Benkhoucha M, Gruaz L, et al. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1beta in human monocytes and multiple sclerosis. Proc Natl Acad Sci USA. 2009;106:4355–4359. doi: 10.1073/pnas.0812183106. [DOI] [PMC free article] [PubMed] [Google Scholar]