Abstract
Half a century after the major histocompatibility complex (MHC) was discovered, its functional roles in health and disease remain poorly understood. Many hallmarks of the MHC, including its unusual evolution, structurefunction properties of its gene products and allele-specific associations with dozens of diseases and health traits cannot be convincingly explained by the tenets of existing paradigms. It is therefore becoming increasingly apparent that in order to better understand MHC-health/disease association—a phenomenon that impacts the health of millions—heterodox ideas are critically needed. Here we propose a testable, novel theory concerning the functional role of MHC molecules in health and disease. At the focus of this theory is an evolutionarily-conserved, tri-dimensional cusp-like prominence (‘kink’), found in the midst of one of the two α helices that form the perimeter of the groove of all MHC molecules. Based on structural, functional and evolutionary considerations, as well as our recent experimental data, it is proposed here that the MHC cusp region is enriched in allele-specific signal transduction ligands that interact with non-MHC cell surface receptors and trigger signaling events. Aberrations in these pathways could lead to disease development, or affect the severity of such diseases.
Key words: HLA, evolution, disease, autoimmunity, function, structure
Introduction
Borrowing a phrase from Sir Winston Churchill, the major histocompatibility complex (MHC) is a riddle wrapped in a mystery inside an enigma. Half a century after the MHC was discovered, its functional roles in health and disease remain poorly understood. Many hallmarks of the MHC, including its unusual evolution, unclear structure-function properties and enigmatic allele-specific associations with dozens of diseases and health traits cannot be convincingly explained by the tenets of existing paradigms. It is therefore becoming increasingly apparent that in order to better understand MHC-health/disease association—a phenomenon that directly impacts the health of millions—heterodox ideas are critically needed. Here we propose a testable, novel theory concerning a previously unrecognized functional role of MHC molecules in health and disease.
Beyond the Groove
The MHC encompasses some of the most polymorphic gene loci in vertebrates. For example, the 2010 WHO HLA Nomenclature1 lists 53 genes that account for over 4400 HLA (human leukocyte antigen) alleles. The best-characterized function of MHC-encoded molecules is the presentation of antigenic peptides to T lymphocytes in a stringent self-MHC context, a process called “MHC restriction”.2 Notwithstanding the critical importance of MHC-restricted antigen presentation in the adaptive immune system, there are strong indications that MHC molecules may also perform a variety of important biologic functions that have little to do with antigen presentation.
The impetus for the new theory presented here stems from the growing realization that the MHC-restricted antigen presentation paradigm, a Nobel Prize-winning discovery that has dominated the field over the past 4 decades, is incongruent with many observations concerning the epidemiology, biology, structure and evolution of MHC molecules. The following are few examples of inconsistency:
Dozens of human diseases and health traits have been shown to associate with particular HLA alleles. The antigen presentation hypothesis postulates that the mechanistic basis of these associations involves immune response to putative self or foreign peptides. However, that explanation is not well supported by scientific evidence. For example: (1) Despite decades of extensive research efforts, the identities of the putative target antigens remain elusive in the vast majority of MHC-associated diseases; (2) There are many alleles that associate each with several conditions that do not share pathogeneses, target tissues or putative antigens; (3) MHC class II alleles known to associate with human rheumatoid arthritis (RA) contribute to inflammatory arthritis in mice as well.3 Likewise, susceptibility to inflammatory arthritis in dogs is associated with class II MHC alleles containing a sequence motif known to increase RA susceptibility in humans, called “shared epitope” (SE).4 Such ‘inter-species susceptibility’ phenomenon is difficult to reconcile with MHC-restricted antigen presentation. (4) The most significant class II MHC-disease association documented to date has been found in narcolepsy,5 a condition that is not known to involve antigen presentation, (5) MHC associations have been shown to exist with certain health traits (e.g., cognition, reviewed in ref. 6) that do not have any immune basis, let alone antigen presentation; (6) The antigen presentation paradigm does not offer a plausible explanation for allele-dose impact on disease susceptibility and severity, nor can it explain allele-dose effects on concordance rates in genetically susceptible monozygotic twins.7
MHC and MHC-like molecules can perform other functions besides antigen presentation, including regulation of iron metabolism,8 olfaction,9 transport of immunoglobulins,10 activation of innate immune signaling (certain HLA-DR and class I MHC molecules, reviewed in ref. 11) and more. Some of these functions display strict allele-specificities, yet are independent of the presence or the identity of groove-bound peptides.
One of the most remarkable hallmarks of MHC genes is a phenomenon called “trans species polymorphism” (TSP, reviewed in ref. 12): the occurrence of alleles that are more similar in divergent species than alleles within each species. TSP is generated by the passage of alleles from ancestral to descendant species. Although the raison d'être of TSP in the MHC is unknown, antigen presentation is an unlikely explanation. If antigen-driven mechanism had been the cause, the polymorphism would have more likely been species and habitat-specific.
The MHC Cusp Theory
As discussed above, the groove-centric antigen presentation theory lacks explanatory power in many key questions concerning the role of MHC molecules in health and disease. The alternative theory discussed below could close these explanatory gaps. At the focus of this theory is a α-helical ‘kink’—a tri-dimensional cusp-like structural motif—that is shared by all products of the MHC gene family. It is proposed here that the MHC cusp could hold the key to many unanswered questions about the role of MHC in health and disease.
The MHC Cusp theory states that “the MHC codes for allele-specific ligands in the cusp region, which interact with non-MHC receptors and activate various pathways. Aberrations in those pathways could cause MHC-associated diseases.”
The rationale for the theory is based on structural, functional and evolutionary considerations.
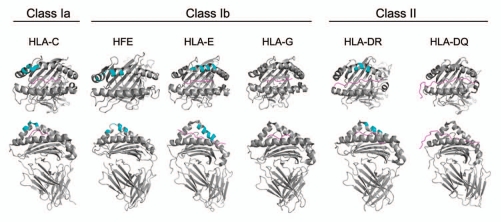
Structure: The first crystal structure of a class II MHC molecule, published in 1993 by Don Wiley's group,13 revealed a remarkable tri-dimensional similarity to a previously reported class I MHC molecule. The degree of the similarity was surprising, given a substantial evolutionary distance between the two molecules and the fact that the peptide-binding groove in class I molecules is encoded by a single gene, while in class II it is formed jointly by the products of two distinct genes. The extent of evolutionary ‘choreography’ required to bring these two disparate MHC molecules to form a near-identical tri-dimensional structure is staggering. One of the notable features of the similarity is a sharp ‘kink’ in the α2 domain of the class I MHC molecule, which can be almost perfectly superimposed on a similar ‘kink’ in the β1 domain of the class II molecule. The ‘kink’ in both molecules involves allele-diversity regions. Subsequent crystal analyses have shown very similar ‘kinks’ in the entire MHC gene product family.14 In all cases, the ‘kink’ forms a pointed protrusion ‘above’ the MHC groove plane that in a ‘side’ view looks very reminiscent of a mathematical cusp—a point where two arcs meet (Fig. 1). The theory discussed here argues that this allelic polymorphism cusp-like region may be responsible for many of the poorly-explained functional attributes of MHC molecules.
Figure 1.
The MHC Cusp. ‘Top’ (upper part) and ‘side’ (lower part) views of representative products of the class I and class II HLA gene family. Groove peptides are shown in pink; receptor-binding AA residues are colored in cyan.
Function: The remarkable conservation of a uniformly-shaped cusp structure in the midst of allele diversity regions in MHC molecules independent of their antigen presentation capabilities strongly suggests that this region may possess important allele-specific, conformationally-dependent non-antigen presentation functions. Indeed, there are some indications that the cusp region performs such functions. For example, in both classical and non-classical (HLA-E) class I MHC molecules, the cusp contains ligands for NK cell receptors;15,16 in HFE (an empty-grooved human class I-like molecule), the cusp region interacts with transferrin receptor.17 In M10 (a mouse class I-like molecule), the cusp region has been proposed as an interaction site with a pheromone receptor.18 Importantly, our group has recently discovered that the allele-specific RA-associated shared epitope (SE), which is located in the HLA-DR cusp region, is acting as a signaling ligand for calreticulin, an established innate immune system receptor.19–26 Thus, there is growing evidence that the MHC cusp performs several allele-dependent, non-antigen presentation functions.
Evolution: One of the enigmas surrounding MHC genes is TSP,12 an evolutionary pattern that is difficult to reconcile with the antigen presentation paradigm (see discussion above). The MHC Cusp theory, proposing polymorphic regions that interact with non-MHC, inter-speciously-conserved receptors, is much more consistent with the TSP phenomenon. In this context, it is worth commenting that the modern MHC is likely a descendent of archaic self-non-self discrimination systems, which, in turn, likely evolved from basic cell-cell contact systems. The MHC Cusp theory argues that the antigen presentation-independent functions of the cusp region may have been preserved through evolution due to their fundamental biologic properties.
The Rheumatoid Arthritis Shared Epitope: a Prototypic Cusp Ligand
The MHC Cusp theory proposes that the cusp region is enriched in ligands that interact with non-adaptive immune system receptors, as suggested by few anecdotal examples.15–18 Our recent discovery of the ligand function of the SE19–26 provides an important impetus to the MHC Cusp theory. The SE is a five amino acid sequence motif in the cusp region (residues 70–74 of the DRβ chain) coded by HLA-DRB1 alleles that are carried by the vast majority of RA patients. The SE is the single most significant genetic risk factor for RA.27 In addition to increasing susceptibility to RA, SE-coding HLA-DRB1 alleles have been shown to associate with more severe disease28 and to exhibit allele-dose effect, i.e., patients with 2 SE-coding alleles tend to experience more severe disease than patients with 1 allele, who, in turn, have more severe RA than SE-negative patients.
The mechanism underlying the effect of the SE is unclear. The prevailing paradigms postulate that the SE allows presentation of arthritogenic self-peptides,29 molecular mimicry with foreign antigens,30 or aberrant T cell repertoire selection.31 However, despite its theoretical appeal, the antigen presentation hypothesis is difficult to reconcile with the fact that data supporting antigen-specific responses as the primary event in RA are inconclusive. Additionally, several other human diseases that are unrelated to RA32–36 as well as several distinct animal disease models3,4,37,38 have been shown to be associated with SE-encoding DRB1 alleles as well. Thus, the SE associates with a variety of disease processes that do not share target antigen or pathogenic mechanisms. These promiscuities are incongruent with fundamental tenets of MHC-restricted antigen presentation theory.
The inconsistencies of SE-RA association with the antigen presentation-based theory have prompted us to examine an alternative hypothesis concerning the role of the SE in RA.19–26 Based on its topology in the cusp region, where anecdotal cases of MHC-coded ligands have been previously identified,15–18 we postulated that the SE may be acting as a signal transduction ligand that can trigger innate immune signaling. Notably, the cusp in HLA-DR molecules hosts the third allelic hypervariable region of the class II β chain, a domain which has been previously shown to display TSP characteristics.39 Indeed, the SE has been previously shown to possess trans-species functional properties and associate with inflammatory arthritis not only in humans, but also in dogs4 and mice.3
Our studies to date19–26 have substantiated the signaling ligand hypothesis. Whether expressed in its native conformation on the cell surface; as a cell-free HLA-DR tetrameric molecule; engineered into large recombinant proteins; or as a short synthetic peptide, the SE activated in all cases nitric oxide (NO)-mediated signaling and production of reactive oxygen species (ROS) in trans in a strictly allelespecific manner. SE-triggered signaling is transduced via cell surface calreticulin (CRT), a known innate immunity receptor.40 The SE binding site on CRT has been recently mapped to a well-defined region in the P-domain of the molecule.25
Directly relevant to the MHC Cusp theory, our recent data show that the SE ligand activates an important immune regulatory function through its effect on dendritic cells (DCs). DCs have a central role in immune tolerance and T cell regulation. Their immune regulatory effect is partially dependent on the tolerogenic enzyme indoleamine 2,3 dioxygenase (IDO).41 Consistent with the reported inhibitory effect of NO on IDO42 and our previous findings that the SE ligand activates NO generation,19 we have recently found that the SE ligand potently inhibits activation of IDO in DCs. In addition, the SE activates production of important pro-inflammatory cytokines, IL-6 and IL-23.23 IDO inhibition43 and increased IL-6 levels44 are known to reduce the development of regulatory T cells (Treg). Therefore, we asked whether the SE interferes with Treg differentiation in vitro. To this end, naïve CD4+ T cells were co-cultured with SE-stimulated DCs under Treg differentiation condition. The results confirmed that the SE potently inhibits Treg cell differentiation.
In addition to inhibiting Treg differentiation, the SE has a reciprocal effect on IL-17 producing T helper cells (Th17). SE-treated DCs when co-cultured with naïve CD4+ T cells in the presence of Th17-polarizing condition, induces a significant increase in the differentiation of Th17 cells. Moreover, supernatants of SE-treated DCs co-cultured with CD4+ T cells show higher production of IL-17. Experiments using CFSE-labeled cells revealed that the SE decreases Treg and increases Th17 expansion. Thus the SE polarization effect on naïve CD4+ cells is compounded by its enhancement of their expansion. Importantly, the SE affects Th17 differentiation and IL17 production in vivo as well, as evidenced by expansion of Th17 and increased IL-17 production in mice immunized with collagen type 2 in the presence of a SE ligand.
Thus, the SE, a cusp region allele diversity sequence acts as a signal transduction ligand that activates non-antigen presentation functions through interaction with a cell surface receptor. Beyond illuminating an important new role of the SE in RA pathogenesis, these findings lend support to the MHC Cusp theory.
Proposed Role of Cusp Ligands in Health and Disease
The MHC Cusp theory states that in physiologic conditions, cusp region ligands activate beneficial biologic functions (e.g., Th17 polarization by the SE could improve immune response to pathogens). Under certain circumstances, however, these ligands could trigger excessive or aberrant signaling events that could lead to pathology. We propose the following model (Fig. 2):
Figure 2.
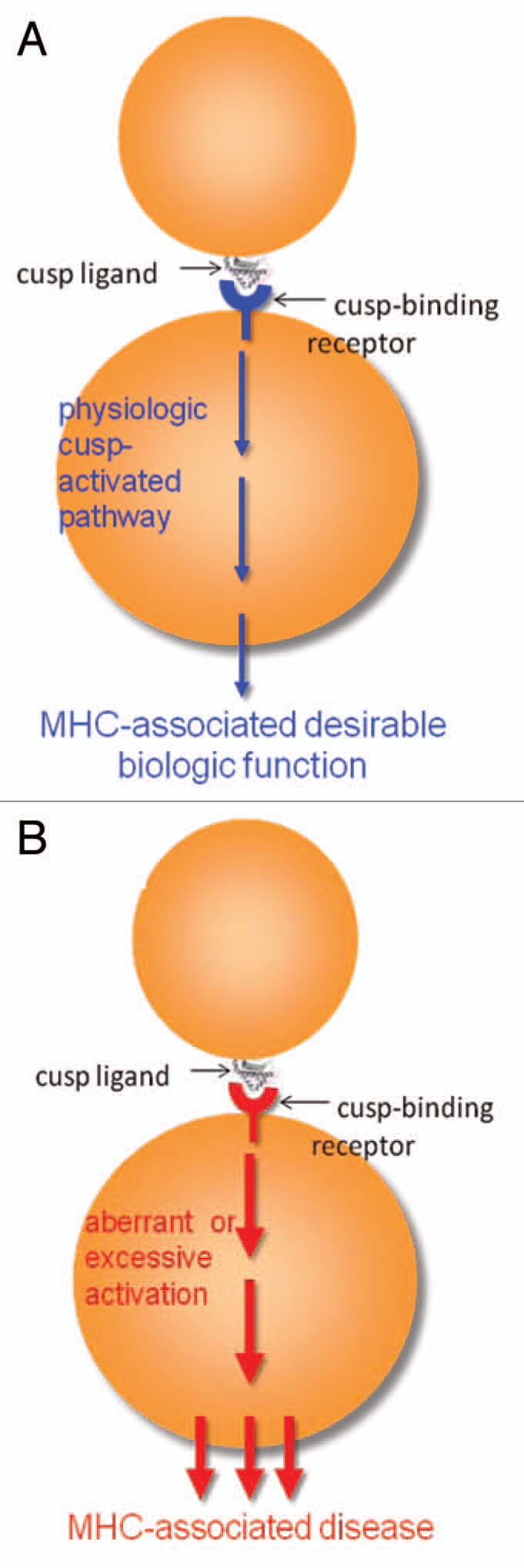
The role of the MHC cusp in health and disease. (A) Under physiologic conditions, the cusp region is acting as signal transduction ligand that interacts with cell surface receptors. This interaction allows activation of important physiologic functions. (B) Over time, due to environmentally-triggered or other stochastic events, the affinity of cusp ligands interaction with their receptors could increase with resultant aberrant activation of the pathway and development of MHC-associated disease.
In healthy individuals carrying disease-associated HLA alleles (e.g., SE-coding HLA-DRB1 alleles) the cusp ligand interacts at low affinity with non-adaptive immunity cell surface receptors (e.g., CRT in the case of the SE). This interaction allows physiologic activation of health-promoting functional effects (in the case of the SE-Th17 polarization). We further propose that over time, due to environmentally-triggered or other stochastic events, the affinity of cusp ligands interaction with their receptors could increase with resultant aberrant activation of the pathway and development of HLA-associated disease. In the case of the SE, recent data by our group suggest that aberrant pathway activation may be related to posttranslational CRT modifications that allows enhanced interaction with the ligand and amplified signal transduction (unpublished). Given the known role of Th17 in RA, we are presently examining the disease-accelerating effects of the SE in arthritis (de Almeida DE, et al. unpublished).
Consistency of the MHC Cusp Theory with Salient Concepts in the Field
Although the theory discussed here is seemingly antithetic to the prevailing concepts in the MHC disease association field, we submit that it is fully consistent with them. The following paragraphs address the compatibility between the two theories in the form of frequently asked questions:
Are the antigen presentation and the MHC Cusp theories mutually exclusive? No. They complement each other. Taking autoimmune diseases as an example: the existent paradigm may explain antigenic/target tissue specificity, while the MHC Cusp theory pertains to antigen-non-specific features of the response (e.g., T cell polarization, as this group's recent findings demonstrate), which determine the outcome of the response (autoimmunity versus tolerance). As an example, the new SE ligand theory is non-exclusive with other hypotheses in the field. It is conceivable that while SE-expressing HLA-DR molecules are uniquely capable of presenting joint-specific antigens, thereby determining the tissue-specificity of the immune response and its anatomical distribution, it is the SE ligand function that determines the outcome of this response by facilitating Th17 polarization.
Does the cusp region overlap with TCR binding-sites? In class I MHC molecules, the cusp ligand for NK receptors has been previously shown to have distinct topology from that of TCR-binding sites.15 As to class II: (1) TCR binding affinity is rather low and conceivably could be overcome by higher-affinity receptors. For example, in our hands, the affinity of the SE to CRT is ∼100-fold higher than TCR-MHC reported affinities; (2) According to some reports,46 in autoimmune states, the TCR binding site on class II MHC molecules tends to ‘slide’ away from the cusp region, toward the C-terminus of the β chain; (3) Cusp signaling effect could conceivably be more effective in tissue compartments where T cells are scarce.
How does the Cusp theory explain the role of MHC haplotypes in disease-associations? This could be explained in two ways: (1) Cusp ligand coded by two or more MHC genes work synergistically, antagonistically or in an additive fashion by activating complementing pathways that together better carry out the desired physiologic function. When pathway aberrations develop, these haplotypic cusp combinations exert greater impact on disease-causing pathology; (2) Interaction between a cusp-dependent signaling effect, contributed by one MHC gene product and antigenic specificity, contributed by another product. As discussed in point 1 above, such interactions are consistent with the non-exclusivity of the cusp theory with the prevailing antigen presentation-based paradigms.
How does the cusp theory explain low penetrance of MHC-associated diseases? In the same way as it is explained by the antigen presentation paradigm: Disease-associated MHC alleles only determine susceptibility. Disease onset requires a stochastic, possibly environmentally-induced, event.
Why were disease-associated cusps preserved? Because they perform important biologic functions, and because most MHC-associated diseases develop late in life and therefore do not affect genetic fitness.
Summary
Significant progress has been achieved over the past 4 decades in our understanding of the role of the MHC in health and disease. However, many observations concerning the MHC and its associations with health traits and diseases cannot be explained by the tenets of the prevailing paradigms. Based on functional, structural and evolutionary considerations, here we propose a new, MHC Cusp theory focusing on an evolutionarily-conserved, tri-dimensional cusp-like prominence, found in the midst of one of the two α helices that form the perimeter of the groove of all MHC molecules. The MHC Cusp theory asserts that these regions host signal transduction ligands that activate MHC-associated biologically important pathways. Aberrations in these pathways could lead to MHC-associated diseases. As a prototypic example illustrating the proposed mechanism, we discuss our recent findings with the SE, a cusp region motif that has been recently found to function as an innate immune system ligand that help polarize the immune response in favor of Th17 cells.
Acknowledgements
Joseph Holoshitz has been supported by the US National Institutes of Health (AR55170, GM88560, AR20557, AR48310, AR56786), the University of Michigan Global Reach Collaborative Research Fund, by a Basic Research Grant from the Arthritis Foundation and an Innovative Basic Science Award from the American College of Rheumatology Research and Education Foundation. We thank Dr. Song Ling for his graphics assistance.
References
- 1.Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA, et al. Nomenclature for factors of the HLA system. Tissue Antigens. 2010;75:291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinkernagel RM, Doherty PC. The discovery of MHC restriction. Immunol Today. 1997;18:14–17. doi: 10.1016/s0167-5699(97)80008-4. [DOI] [PubMed] [Google Scholar]
- 3.Rosloniec EF, Brand DD, Myers LK, Whittington KB, Gumanovskaya M, Zaller DM, et al. An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen. J Exp Med. 1997;185:1113–1122. doi: 10.1084/jem.185.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ollier WE, Kennedy LJ, Thomson W, Barnes AN, Bell SC, Bennett D, et al. Dog MHC alleles containing the human RA shared epitope confer susceptibility to canine rheumatoid arthritis. Immunogenetics. 2001;53:669–673. doi: 10.1007/s002510100372. [DOI] [PubMed] [Google Scholar]
- 5.Nishino S, Okuro M, Kotorii N, Anegawa E, Ishimaru Y, Matsumura M, et al. Hypocretin/orexin and narcolepsy: new basic and clinical insights. Acta Physiol. 2010;198:209–222. doi: 10.1111/j.1748-1716.2009.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shepherd CE, Piguet O, Broe GA, Creasey H, Waite LM, Brooks WS, et al. Histocompatibility antigens, aspirin use and cognitive performance in non-demented elderly subjects. J Neuroimmunol. 2004;148:178–182. doi: 10.1016/j.jneuroim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Jawaheer D, Thomson W, MacGregor AJ, Carthy D, Davidson J, Dyer PA, et al. “Homozygosity” for the HLA-DR shared epitope contributes the highest risk for rheumatoid arthritis concordance in identical twins. Arthritis Rheum. 1994;37:681–686. doi: 10.1002/art.1780370511. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso CS, de Sousa M. HFE, the MHC and hemochromatosis: Paradigm for an extended function for MHC class I. Tissue Antigens. 2003;61:263–275. doi: 10.1034/j.1399-0039.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 9.Loconto J, Papes F, Chang E, Stowers L, Jones EP, Takada T, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–618. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 10.Simister NE, Jacobowitz Israel E, Ahouse JC, Story CM. New functions of the MHC class I-related Fc receptor, FcRn. Biochem Soc Trans. 1997;25:481–486. doi: 10.1042/bst0250481. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 12.Klein J. Origin of major histocompatibility complex polymorphism: The trans-species hypothesis. Hum Immunol. 1987;19:155–162. doi: 10.1016/0198-8859(87)90066-8. [DOI] [PubMed] [Google Scholar]
- 13.Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, et al. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364:33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 14.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 15.Boyington JC, Motyka SA, Schuck P, Brooks AG, Sun PD. Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC ligand. Nature. 2000;405:537–543. doi: 10.1038/35014520. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser BK, Pizarro JC, Kerns J, Strong RK. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc Natl Acad Sci USA. 2008;105:6696–6701. doi: 10.1073/pnas.0802736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett MJ, Lebron JA, Bjorkman PJ. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 2000;403:46–53. doi: 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- 18.Olson R, Huey-Tubman KE, Dulac C, Bjorkman PJ. Structure of a pheromone receptor-associated MHC molecule with an open and empty groove. PLoS Biol. 2005;3:257. doi: 10.1371/journal.pbio.0030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling S, Lai A, Borschukova O, Pumpens P, Holoshitz J. Activation of nitric oxide signaling by the rheumatoid arthritis shared epitope. Arthritis Rheum. 2006;54:3423–3432. doi: 10.1002/art.22178. [DOI] [PubMed] [Google Scholar]
- 20.Ling S, Li Z, Borschukova O, Xiao L, Pumpens P, Holoshitz J. The rheumatoid arthritis shared epitope increases cellular susceptibility to oxidative stress by antagonizing an adenosine-mediated anti-oxidative pathway. Arthritis Res Ther. 2007;9:5. doi: 10.1186/ar2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holoshitz J, Ling S. Nitric oxide signaling triggered by the rheumatoid arthritis shared epitope: a new paradigm for MHC disease association? Ann NY Acad Sci. 2007;1110:73–83. doi: 10.1196/annals.1423.009. [DOI] [PubMed] [Google Scholar]
- 22.Ling S, Pi X, Holoshitz J. The rheumatoid arthritis shared epitope triggers innate immune signaling via cell surface calreticulin. J Immunol. 2007;179:6359–6367. doi: 10.4049/jimmunol.179.9.6359. [DOI] [PubMed] [Google Scholar]
- 23.De Almeida DE, Ling S, Pi X, Hartmann-Scruggs AM, Pumpens P, Holoshitz J. Immune dysregulation by the rheumatoid arthritis shared epitope. J Immunol. 2010;185:1927–1934. doi: 10.4049/jimmunol.0904002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holoshitz J, De Almeida DE, Ling S. A role for calreticulin in the pathogenesis of rheumatoid arthritis. Ann NY Acad Sci. 2010;1209:91–98. doi: 10.1111/j.1749-6632.2010.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling S, Cheng A, Pumpens P, Michalak M, Holoshitz J. Identification of the rheumatoid arthritis shared epitope binding site on calreticulin. PLoS One. 2010;5:11703. doi: 10.1371/journal.pone.0011703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Almeida DE, Ling S, Holoshitz J. New insights into the functional role of the rheumatoid arthritis shared epitope. FEBS Lett. 2011 doi: 10.1016/j.febslet.2011.03.035. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holoshitz J. The rheumatoid arthritis HLA-DRB1 shared epitope. Curr Opin Rheumatol. 2010;22:293–298. doi: 10.1097/BOR.0b013e328336ba63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mewar D, Marinou I, Coote AL, Moore DJ, Akil M, Smillie D, et al. Association between radiographic severity of rheumatoid arthritis and shared epitope alleles: differing mechanisms of susceptibility and protection. Ann Rheum Dis. 2008;67:980–983. doi: 10.1136/ard.2007.075382. [DOI] [PubMed] [Google Scholar]
- 29.Wucherpfennig KW, Strominger JL. Selective binding of self peptides to disease-associated major histocompatibility complex (MHC) molecules: a mechanism for MHC-linked susceptibility to human autoimmune diseases. J Exp Med. 1995;181:1597–1601. doi: 10.1084/jem.181.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Cava A, Nelson JL, Ollier WE, MacGregor A, Keystone EC, Thorne JC, et al. Genetic bias in immune responses to a cassette shared by different microorganisms in patients with rheumatoid arthritis. J Clin Invest. 1997;100:658–663. doi: 10.1172/JCI119577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhayani HR, Hedrick SM. The role of polymorphic amino acids of the MHC molecule in the selection of the T cell repertoire. J Immunol. 1991;146:1093–1098. [PubMed] [Google Scholar]
- 32.Tait BD, Drummond BP, Varney MD, Harrison LC. HLA-DRB1*0401 is associated with susceptibility to insulin-dependent diabetes mellitus independently of the DQB1 locus. Eur J Immunogenet. 1995;22:289–297. doi: 10.1111/j.1744-313x.1995.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 33.Korendowych E, Dixey J, Cox B, Jones S, McHugh N. The Influence of the HLA-DRB1 rheumatoid arthritis shared epitope on the clinical characteristics and radiological outcome of psoriatic arthritis. J Rheumatol. 2003;30:96–101. [PubMed] [Google Scholar]
- 34.Chan MT, Owen P, Dunphy J, Cox B, Carmichael C, Korendowych E, et al. Associations of erosive arthritis with anti-cyclic citrullinated peptide antibodies and MHC Class II alleles in systemic lupus erythematosus. J Rheumatol. 2008;35:77–83. [PubMed] [Google Scholar]
- 35.Doherty DG, Donaldson PT, Underhill JA, Farrant JM, Duthie A, Mieli-Vergani G, et al. Allelic sequence variation in the HLA class II genes and proteins in patients with autoimmune hepatitis. Hepatology. 1994;19:609–615. doi: 10.1002/hep.1840190311. [DOI] [PubMed] [Google Scholar]
- 36.Dorak MT, Machulla HK, Hentschel M, Mills KI, Langner J, Burnett AK. Influence of the major histocompatibility complex on age at onset of chronic lymphoid leukaemia. Int J Cancer. 1996;65:134–139. doi: 10.1002/(SICI)1097-0215(19960117)65:2<134::AID-IJC2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Wen L, Chen NY, Tang J, Sherwin R, Wong FS. The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model. J Clin Invest. 2001;107:871–880. doi: 10.1172/JCI11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forsthuber TG, Shive CL, Wienhold W, de Graaf K, Spack EG, Sublett R, et al. T cell epitopes of human myelin oligodendrocyte glycoprotein identified in HLA-DR4 (DRB1*0401) transgenic mice are encephalitogenic and are presented by human B cells. J Immunol. 2001;167:7119–7125. doi: 10.4049/jimmunol.167.12.7119. [DOI] [PubMed] [Google Scholar]
- 39.Lundberg AS, McDevitt HO. Evolution of major histocompatibility complex class II allelic diversity: direct descent in mice and humans. Proc Natl Acad Sci USA. 1992;89:6545–6549. doi: 10.1073/pnas.89.14.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang L, Baban B, Johnson BA, 3rd, Mellor AL. Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int Rev Immunol. 2010;29:133–155. doi: 10.3109/08830180903349669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas SR, Mohr D, Stocker R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J Biol Chem. 1994;269:14457–14464. [PubMed] [Google Scholar]
- 43.Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 45.Wucherpfennig KW, Call MJ, Deng L, Mariuzza R. Structural alterations in peptide-MHC recognition by self-reactive T cell receptors. Curr Opin Immunol. 2009;21:590–595. doi: 10.1016/j.coi.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]