Abstract
Exo-endocytotic cycling of synaptic vesicles (SVs) is one of the most intensely studied membrane trafficking pathways. It is governed by sets of conserved proteins including Rab GTPases. Long considered to define the identity and composition of a subcellular organelle, it has become increasingly evident that multiple Rabs co-exist on intracellular compartments, each contributing to its membrane organization and specialised function. Indeed, we have recently demonstrated that at least 11 distinct Rab proteins co-exist on highly purified SVs. These include Rabs involved in exocytosis (Rab3a/b/c and Rab27b) and intermediates of SV recycling such as early endosomes (Rab4, Rab5, Rab10, Rab11b and Rab14). Interestingly, we found that while two of these proteins, namely Rab3a and Rab27b, exhibited differential cycling dynamics on SV membranes; they played complementary roles during Ca2+-triggered neurotransmitter release. The implications of these findings in the SV trafficking cycle are discussed.
Key words: synaptic vesicle, Rab, quantitative proteomics, exocytosis, endocytosis, vesicle cycle, GTPases
Synaptic vesicles (SVs) store neurotransmitters that are released by exocytosis upon the receipt of an action potential at the presynaptic nerve terminal. Following fusion with the presynaptic plasma membrane, SVs are rapidly internalised by endocytosis and recycled, possibly via endosomal intermediates.1 To achieve this, SVs are endowed with a set of unique proteins which cooperate with other cytoplasmic residents to regulate the exo-endocytotic membrane trafficking cycle. Recently, the convergence of biochemical and proteomic studies in rodents has unveiled the composition of purified SVs.2,3 While these findings greatly advanced our understanding of the core molecular constituents and lipid composition of SVs, they also revealed unexpected diversity among several protein families, particularly those involved in membrane trafficking.
Small regulatory Rab GTPases belong to the group of conserved trafficking proteins that govern membrane traffic in all eukaryotes.4 Since their discovery during genetic screens of vesicular trafficking defects in yeast (Ypt1/Sec4), over 60 Rab family members have been identified in humans. By virtue of prenylation moieties anchored to their C-terminus, Rabs bind intracellular membranes and function as molecular “on/off ” switches in line with their GTP/GDP cycle. In their GTP-bound state, Rabs recruit diverse effector complexes on membrane micro-domains, which usually allow the organelles to connect to motor proteins for organelle transport or to target membranes in preparation for SNARE-coupled membrane fusion.5 Each trafficking organelle appears to contain its own unique set of Rab proteins. Thus, Rabs are widely considered “deciphers of organelle identity”, with the number of isoforms residing on a particular organelle not only reflecting its complexity but also the nature of the trafficking step(s) within which it operates. Elucidating complete and accurate organelle-specific Rab sets is therefore an important first step towards defining the composition and function of any intracellular organelle. We have recently resolved the identity and number of Rab proteins enriched on highly purified SVs.6 Here we revisit these findings alongside past and present studies in pursuit of a “Rabcentric” view of SV trafficking.
Rabs on SVs: Gearing Up the Exo-Endocytotic Trafficking Cycle
For many years it has been known that Rab3 proteins (Rab3a/b/c) are specifically associated with SVs.7–9 Furthermore, Rab5 was also found to be highly enriched on SVs,10 supporting the view that an endosomal intermediate is involved in the SV recycling pathway. Therefore, it was surprising when 33 distinct Rabs were identified by proteomic analyses in highly purified SV fractions, thus accounting for more than half the mammalian Rab inventory.3 Such diversity was difficult to reconcile with the exquisitely specific role that Rabs are thought to play in individual trafficking steps. Furthermore, only a minority of the Rabs identified (i.e., Rab3a/b/c, Rab5 and Rab11b) had been previously assigned to SV transport.10–12 Therefore, questions remained as to whether a lot more Rabs function in the SV cycle than had once been assumed, or whether the bulk of Rabs uncovered were derived from contaminating organelles or else, simply picked up during the SV isolation procedure.
We recently addressed this issue by systematically revising Rab occupancy on highly purified SVs isolated from rat brain.6 Combining chemical labelling (iTRAQ™) with state-of-the-art proteomics, we were able to distinguish between those Rabs that were specifically enriched on SVs and those which were not. This approach enabled us to quantitatively monitor individual Rab enrichment profiles over multiple membrane and cytosolic fractions obtained at specific stages of the SV purification procedure (thereby circumventing problems that are inherent in proteomic data sets from single fractions). To complement our proteomic data, we performed immunoanalytical and bioinformatic analyses to validate specific Rab enrichment profiles and probe for phylogenetically-related Rabs that might have been overlooked in our proteomic catchment due to peptide redundancy. As a result, we identified several distinct Rab isoforms (at least 11 family members) that are specifically enriched on SVs encompassing those whose roles are well established in exocytic (Rab3a/b/c and Rab27b) and endocytic (Rab4b, Rab5a, Rab10, Rab11b and Rab14) transport. Intriguingly, many other Rabs were also found to be moderately enriched on purified SVs although not to the same extent as those discussed above. The latter include Rabs such as Rab1 and Rab2, which have been implicated in ER-Golgi transport.13,14 Therefore, it cannot be excluded that these Rabs were picked up from the soluble Rab-GDI pool during vesicle purification or else, that the SV fraction—despite its high degree of purity—is contaminated by Golgi-derived trafficking vesicles. Indeed, we confirmed this position by expressing each of the SV Rabs as green fluorescent protein (GFP) fusion chimeras in rat hippocampal neurons. We found that whereas all exoendocytotic SV Rabs localised to a subset of presynaptic nerve terminals, Rab1 was almost exclusively retained in the perinuclear/Golgi vicinity and on nascent SVs. Interesting, we also showed that two inter-related proteins, namely Rab3a and Rab27b, closely co-occupied SV membranes and played unique yet intersecting roles during Ca2+-triggered exocytosis. In all, these findings documented the first organellar Rab proteome (Rabome) and reaffirmed the position that multiple exoendocytic Rabs reside on SV membranes, acting in concert to bestow SVs with both flexible and robust features during neurotransmitter release.
Overlapping Roles of Rab3a and Rab27b on SVs
To ensure the fidelity of membrane targeting and fusion during exocytosis, secretory organelles have evolved specialised adaptations and redundancies within trafficking protein families in higher eukaryotic cells. Such redundancy is, perhaps, best exemplified among Rab GTPases which exhibit remarkable sequence conservation and functional compensation within subfamilies.15 For instance, the Rab3 subfamily includes four highly homologous isoforms (Rab3a, Rab3b, Rab3c and Rab3d) which play largely overlapping yet redundant functions in various secretory cells including neurons.16 Of these, Rab3a is by far the most abundant variant at the synapse, accounting for some 25% of total brain Rab expression and dominating up to 2.5% of the SV surface (equating to ∼10 copies/vesicle).3 Therefore, it was not unexpected that Rab3a headed our list of Rabs highly enriched on purified SVs. Accordingly, we found that Rab3b and Rab3c, but the not non-neuronal isoform Rab3d, were also markedly enriched on SV membranes, albeit to a lesser degree to Rab3a, consistent with their documented involvement in a subset of synapses.9,12
Despite overwhelming evidence supporting a role for Rab3s and Rab3-effectors (namely raphilin and RIM) in SV exocytosis, it has been surprisingly difficult to assign their function(s) (i.e., docking and/or tethering) at SV release sites.16 On one hand, Rab3a has been shown to cycle “on/off” SVs concomitant with neurotransmitter release8 and engage with the presynaptic scaffolding partners rabphilin, RIM and Munc-13,17 each an executive component of the tethering/release machinery. On the other hand, genetic ablation of either rabphilin or RIM results in only mild exocytotic deficiencies without affecting Rab3 SV positioning.18,19 Even more confounding is the modest phenotype observed in quadruple knockout of all Rab3 isoforms in mice which exhibit, at best, a 30% reduction in the probability of Ca2+-triggered neurotransmitter release,20 re-enforcing that multiple Rabs must exist on SVs to compensate and ensure the fidelity of neurotransmitter release.
Of the list of Rabs known to participate along the regulated secretory pathway, considerable attention has recently focused on Rab27 proteins Rab27a/b.21 Like Rab3s, Rab27 isoforms have been shown to differentially localise to a variety of secretory vesicles and regulate specific but complementary stages during exocytosis in specialised cell types. More importantly, Rab27 and Rab3 proteins share the closest branch of the Rab evolutionary tree and acquire common activators and effector proteins, making them attractive “co-modulators” of SV cycling. Indeed, we demonstrated that Rab27b, but not Rab27a, was also abundantly enriched on SVs by immuno-analyses.6 This exquisite expression pattern followed that of previous studies demonstrating that Rab27a is widely expressed in more peripheral tissues, whereas Rab27b is largely restricted to the CNS.22 In fact, Rab27b was initially overlooked in our proteomic analyses because many of the resulting peptides were identical with those derived from Rab3 family members following tryptic digestion.
Like Rab3s, the enrichment profile of Rab27b closely resembled that of other bona fide SV constituents such as synaptophysin and synaptotagmin. Furthermore, using a series of complementary biochemical and morphological assays we demonstrated that Rab27b co-exists with Rab3a on a subset of SVs, approximating to half of the Rab3a-bearing SV pool. However, while Rab3a and Rab27b share interconnected SV populations, they diverge in membrane cycling dynamics, with Rab3a spontaneously dissociating from SVs during Ca2+-triggered exocytosis, while Rab27b persists on SV membranes. This disunity is supported by recent findings in neuroendocrine PC12 cells which demonstrated that Rab3 and Rab27 proteins differentially cycle on dense-core granules to co-operatively regulate vesicle docking.23,24 Furthermore, we found that while the pre-synaptic cytosol houses an abundant reservoir of soluble GDI-bound Rab3a, cytosolic reserves of Rab27b were minimal. Consistently, Rab3a, but not Rab27b, could be removed from SV membranes upon incubation with GDI. The exact reason for this disparity remains unclear, although it is worth mentioning that Rab27b exists predominantly in its active (GTP)/membrane-bound conformation on a variety of secretory organelles.22,25 In this regard, Rab27b is largely membrane-bound since only the GDP-form of Rab proteins can be complexed with GDI and thus be extracted from the membrane. Furthermore, the recent published crystal structure of the GDP-Rab27b complex revealed a swapped homodimer, sharing overlapping switch regions.26 Moreover, this inactive Rab27b dimer is predicted to cause a steric clash with GDI, thereby precluding GDI-Rab binding and extraction, i.e., even if GTP is hydrolyzed, the protein cannot be accessed by GDI. Finally, recent evidence suggests that the prenylation/membrane delivery of Rab27 is different from that of other Rabs as it does not appear to involve the conventional REP1/GDI-mediated pathways.27 Irrespective of the precise mechanism, our results firmly place Rab3a and Rab27b as dual SV residents.
Rab3a and Rab27b: Working Together to Safe-Guard Synaptic Transmission
By overexpressing selective Rab27b mutants deficient in either GDP/GTP exchange and/or hydrolysis we demonstrated that Rab27b participates in the recycling of SVs in rat hippocampal neurons. In this instance, both GTP- and GDP-deficient Rab27b mutants potently blocked evoked endo-exocytotic cycling of synaptic vesicles, suggesting that, like Rab3a, Rab27b functions as a core component of the SV cycling machinery. This synergy was not unexpected given the recent attention focused on Rab27 in SV docking and release in invertebrate systems.28,29 Interestingly, we found that along with its inhibitory effect on uptake of a dye marker for endocytosis, dominant-negative Rab27b overexpression correlated with a depletion of endogenous Rab3a. As Rab3/Rab27b proteins share evolutionary conserved GTP-exchange factors i.e., Rab3GEF28,30 together with the knowledge that GDP-mutants sequester cognate RabGEFs,31 we reasoned that the GDP-preferring Rab27b mutants compete out Rab3a activation, thus accounting for the more pronounced SV recycling defect. Furthermore, we postulated that this mechanism may also underscore the phenotypic disparity between Rab3/Rab27 and Rab3GEF/AEX3 knock-out models, with the latter notably indispensible for SV neurotransmission.20,32
How might Rab3a and Rab27b co-ordinate the SV trafficking cycle? Considering the role(s) of Rab3/Rab27 in other secretory systems, it is likely that Rab3a and Rab27b function in complementary yet successive steps of the SV exocytotic cycle with Rab specificity preserved through the differential recruitment of Rab3/Rab27 effectors. Indeed, Rab3a and Rab27b share a gamut of docking and tethering partners including Noc2, rabphilin, RIM and Munc-13 (for a comprehensive review, see ref. 19), thus it is conceivable that SV docking and priming is guided by the timely and co-ordinated acquisition of the rabphilin/RIM/Munc-13 triad between Rab3a/Rab27b during exocytosis (Fig. 1). Since Rab3a dissociates from SVs upon receipt of a Ca2+-trigger, Rab27b may persist on SV membranes to “safe-guard” docking and/or fusion at the active zone. Functional diversity might thus be achieved through the recruitment of Slps (synaptotagmin-like proteins) and/or Slac2 (Slp homologue lacking C2 domains), both of which are Rab27 effectors.21 In fact, recent structural insights into the key determinants of Rab3/Rab27 effector specificity would support such a position, with surprisingly subtle structural variations shown to underlie differential specificities between Rab3a and Rab27a/b for rabphilin and Slac2/Spl2 effectors, respectively (in ref. 33). Such simplicity would therefore enable rapid switching between Rab3/Rab27b Rab-effector complexes, thereby providing insurance for timely and efficient docking and fusion during neurotransmitter release. Whatever the mechanism involved, this model represents an attractive account for this paradigm.
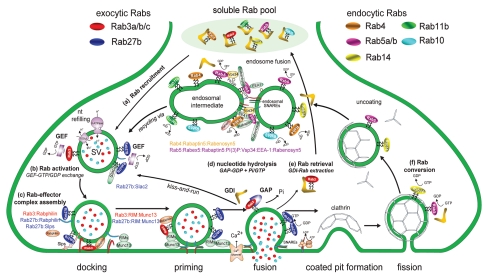
Figure 1.
Proposed involvement of exo-endocytotic Rabs and effector complexes in the synaptic vesicle cycle. (A) Following their synthesis and/or recruitment from the presynaptic cytosolic Rab pool, prenylated exocytotic Rabs (Rab3a/b/c and Rab27b) are delivered to- and inserted into the phospholipid membranes of recently formed synaptic vesicles (SVs) laden with neurotransmitters (nt). (B) Here, Rab GTP-activation is catalysed via Rab guanine nucleotide exchange factors (GEFs) which initiates the recruitment of Rab3/Rab27 effectors (C) required for specific stages of the SV exocytotic cycle i.e., motility (Slac2), SV docking (rabphilin and Slps) and priming (RIMs and Munc-13). Upon receipt of a Ca2+-signal, Rab3s are inactivated by a GTPase activating protein (GAP) which drives nucleotide hydrolysis (D), thus enabling GDI-mediated Rab-extraction (E) prior to SNARE-mediated exocytotic fusion at the presynaptic active zone. During SV fusion, Rab27b persist on SV membranes, possibly as an inactive swapped homodimer. Fused SVs then rapidly recycle via “kiss-and-run” or clathrin-mediated endocytotic pathways. Following the formation of clathrin-coats, Rab5 and Rab14 are stage dependently recruited to recently recycled vesicles undergoing dynamic Rab conversion upon Rab-activation (F). During this transition, Rab5 mediates the assembly of a macromolecular effector complex (i.e., Rabaptin5/Rabex5/PI(3)P/Vsp34/EEA-1/Rabenosyn5) required for fusion with endosomal sorting intermediates. Rab4, Rab10 and Rab11b are then stage-dependently recruited to specific membrane micro-domains on endosomal intermediates, the latter two representing prime candidates of SV recycling. Image adapted from reference 44.
Returning to Base: Possible Involvement of Endosomal Rabs in SV Recycling
Along with exocytotic fusion, recycling of SVs via clathrin-coated vesicles is obligatory but the involvement of endosomal intermediates is still controversial.1 While coated vesicles interconnected with endosome-like structures were discovered by electron microscopy more than three decades ago as intermediates of SV cycling,34 it has only recently become clear that SVs, in addition to their exocytotic machinery, are equipped with complementary endocytic proteins (Rabs and SNAREs inclusive).3,10,35 Attesting to this, we detected several endocytotic Rabs including Rabs 4, 5, 10, 11b and Rab14 enriched on the surface of purified SV membranes. Of these, Rab5 has been most intensively characterized with respect to early endosomes, orchestrating a step-wise effector recruitment cascade (i.e., Rabaptin5/Rabex5/PI(3)P/Vps34/ EEA-1/Rabenosyn5) to endosomal microdomains upon activation (reviewed in ref. 4). We envisage a similar scenario at the mammalian synapse (Fig. 1). Indeed, we and others10,36–39 have previously shown that Rab5 localizes to a subset of SVs where it is has been implicated in the regulation of SV recycling38,39 and SV uniformity.37 A functional role for Rab5 in SV recycling is also favoured by recent studies employing super-resolution stimulation emission depletion (STED) microscopy.40 Here recently endocytosed SVs were shown to recycle via Rab5-bearing intermediates. While the precise nature of these intermediates remains unclear, the authors postulated that they may in fact represent endosomal sorting reservoirs to facilitate the removal of contaminating plasma membrane constituents picked up during SV fusion.
Based on other endomembrane systems, it appears unlikely that Rab5 acts as a lone protagonist during SV recycling and sorting. For instance, Rab4, Rab5 and Rab11 have been shown to collaboratively regulate endosome recycling in several cell types, with each localizing to a distinct membrane-domain.41 Similarly, Rab10 and Rab14 have been implicated in clathrin-coated trafficking and recycling pathways.42,43 Thus, an attractive model might entail a guided transition of recently endocytosed clathrin-coated vesicles from one pool to the next by the collaborative acquisition of Rab5/Rab14/Rab4/Rab10 and Rab11 (Fig. 1).
Perspectives
A paramount challenge in the future remains to functionally integrate the SV Rab cache into a coherent picture of Rab-association/dissociation during SV cycling at the neuronal synapse. Along with assigning each Rab to a specific phase of the exo-endocytic SV trafficking cycle it will be crucial to identify specific Rab-effector repositories which are stage-dependently recruited to SV membranes and through which Rabs exert their regulatory function(s). Finally, it is also necessary to resolve which local or recycling SV subpopulations contain or lack individual Rabs, a monumental task when considering that the majority of SVs at the synapse are resting. The recent advent of high-end super-resolution STED microscopy coupled with refinements in the isolation of specific SV subsets will undoubtedly prove instrumental in deciphering this ambiguity.
Acknowledgements
This work was supported in part by a grant from the EU-commission (Project EU-Synapse, FP6). N.J.P. is supported by a National Health and Medical Research Council (Aust) CJ Martin Fellowship ID: 463911.
Abbreviations
- SV
synaptic vesicle
- GDI
GDP-dissociation inhibitor
- STED
stimulation emission depletion
- EEA1
early endosome antigen 1
Extra View to: Pavlos NJ, Grønborg M, Riedel D, Chua JJ, Boyken J, Kloepper TH, Urlaub H, Rizzoli SO, Jahn R. Quantitative analysis of synaptic vesicle Rabs uncovers distinct yet overlapping roles for Rab3a and Rab27b in Ca2+-triggered exocytosis. J Neurosci. 2010;30:13441–13453. doi: 10.1523/JNEUROSCI.0907-10.2010.
References
- 1.Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.Morciano M, Burre J, Corvey C, Karas M, Zimmermann H, Volknandt W. Immunoisolation of two synaptic vesicle pools from synaptosomes: a proteomics analysis. J Neurochem. 2005;95:1732–1745. doi: 10.1111/j.1471-4159.2005.03506.x. [DOI] [PubMed] [Google Scholar]
- 3.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 5.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlos NJ, Gronborg M, Riedel D, Chua JJ, Boyken J, Kloepper TH, et al. Quantitative analysis of synaptic vesicle Rabs uncovers distinct yet overlapping roles for Rab3a and Rab27b in Ca2+-triggered exocytosis. J Neurosci. 2010;30:13441–13453. doi: 10.1523/JNEUROSCI.0907-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geppert M, Goda Y, Stevens CF, Sudhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- 8.Fischer von Mollard G, Mignery GA, Baumert M, Perin MS, Hanson TJ, Burger PM, et al. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci USA. 1990;87:1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer von Mollard G, Stahl B, Khokhlatchev A, Sudhof TC, Jahn R. Rab3C is a synaptic vesicle protein that dissociates from synaptic vesicles after stimulation of exocytosis. J Biol Chem. 1994;269:10971–10974. [PubMed] [Google Scholar]
- 10.Fischer von Mollard G, Stahl B, Walch-Solimena C, Takei K, Daniels L, Khoklatchev A, et al. Localization of Rab5 to synaptic vesicles identifies endosomal intermediate in synaptic vesicle recycling pathway. Eur J Cell Biol. 1994;65:319–326. [PubMed] [Google Scholar]
- 11.Khvotchev MV, Ren M, Takamori S, Jahn R, Sudhof TC. Divergent functions of neuronal Rab11b in Ca2+-regulated versus constitutive exocytosis. J Neurosci. 2003;23:10531–10539. doi: 10.1523/JNEUROSCI.23-33-10531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlüter OM, Khvotchev M, Jahn R, Sudhof TC. Localization versus function of Rab3 proteins. Evidence for a common regulatory role in controlling fusion. J Biol Chem. 2002;277:40919–40929. doi: 10.1074/jbc.M203704200. [DOI] [PubMed] [Google Scholar]
- 13.Plutner H, Cox AD, Pind S, Khosravi-Far R, Bourne JR, Schwaninger R, et al. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115:31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tisdale EJ, Balch WE. Rab2 is essential for the maturation of pre-Golgi intermediates. J Biol Chem. 1996;271:29372–29379. doi: 10.1074/jbc.271.46.29372. [DOI] [PubMed] [Google Scholar]
- 15.Pereira-Leal JB, Seabra MC. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J Mol Biol. 2000;301:1077–1087. doi: 10.1006/jmbi.2000.4010. [DOI] [PubMed] [Google Scholar]
- 16.Jahn R. Principles of exocytosis and membrane fusion. Ann N Y Acad Sci. 2004;1014:170–178. doi: 10.1196/annals.1294.018. [DOI] [PubMed] [Google Scholar]
- 17.Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, et al. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 19.Schlüter OM, Schnell E, Verhage M, Tzonopoulos T, Nicoll RA, Janz R, et al. Rabphilin knock-out mice reveal that rabphilin is not required for rab3 function in regulating neurotransmitter release. J Neurosci. 1999;19:5834–5846. doi: 10.1523/JNEUROSCI.19-14-05834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlüter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao S, Torii S, Yokota-Hashimoto H, Takeuchi T, Izumi T. Involvement of Rab27b in the regulated secretion of pituitary hormones. Endocrinology. 2002;143:1817–1824. doi: 10.1210/en.143.5.1817. [DOI] [PubMed] [Google Scholar]
- 23.Handley MT, Haynes LP, Burgoyne RD. Differential dynamics of Rab3A and Rab27A on secretory granules. J Cell Sci. 2007;120:973–984. doi: 10.1242/jcs.03406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuboi T, Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J Cell Sci. 2006;119:2196–2203. doi: 10.1242/jcs.02962. [DOI] [PubMed] [Google Scholar]
- 25.Kondo H, Shirakawa R, Higashi T, Kawato M, Fukuda M, Kita T, et al. Constitutive GDP/GTP exchange and secretion-dependent GTP hydrolysis activity for Rab27 in platelets. J Biol Chem. 2006;281:28657–28665. doi: 10.1074/jbc.M603227200. [DOI] [PubMed] [Google Scholar]
- 26.Chavas LM, Torii S, Kamikubo H, Kawasaki M, Ihara K, Kato R, et al. Structure of the small GTPase Rab27b shows an unexpected swapped dimer. Acta Crystallogr D Biol Crystallogr. 2007;63:769–779. doi: 10.1107/S0907444907019725. [DOI] [PubMed] [Google Scholar]
- 27.Leung KF, Baron R, Ali BR, Magee AI, Seabra MC. Rab GTPases containing a CAAX motif are processed post-geranylgeranylation by proteolysis and methylation. J Biol Chem. 2007;282:1487–1497. doi: 10.1074/jbc.M605557200. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney TR, Liu Q, Itoh T, Luo S, Hadwiger G, Vincent R, et al. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol Biol Cell. 2006;17:2617–2625. doi: 10.1091/mbc.E05-12-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu E, Kanno E, Choi S, Sugimori M, Moreira JE, Llinas RR, et al. Role of Rab27 in synaptic transmission at the squid giant synapse. Proc Natl Acad Sci USA. 2008;105:16003–16008. doi: 10.1073/pnas.0804825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figueiredo AC, Wasmeier C, Tarafder AK, Ramalho JS, Baron RA, Seabra MC. Rab3GEP is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes. J Biol Chem. 2008;283:23209–23216. doi: 10.1074/jbc.M804134200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burstein ES, Brondyk WH, Macara IG. Amino acid residues in the Ras-like GTPase Rab3A that specify sensitivity to factors that regulate the GTP/GDP cycling of Rab3A. J Biol Chem. 1992;267:22715–22718. [PubMed] [Google Scholar]
- 32.Gomi H, Mori K, Itohara S, Izumi T. Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol Biol Cell. 2007;18:4377–4386. doi: 10.1091/mbc.E07-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itzen A, Goody RS. Key determinants of Rab specificity. Structure. 2008;16:1437–1439. doi: 10.1016/j.str.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzoli SO, Bethani I, Zwilling D, Wenzel D, Siddiqui TJ, Brandhorst D, et al. Evidence for early endosome-like fusion of recently endocytosed synaptic vesicles. Traffic. 2006;7:1163–1176. doi: 10.1111/j.1600-0854.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 36.de Hoop MJ, Huber LA, Stenmark H, Williamson E, Zerial M, Parton RG, et al. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu H, Kawamura S, Ozaki K. An essential role of Rab5 in uniformity of synaptic vesicle size. J Cell Sci. 2003;116:3583–3590. doi: 10.1242/jcs.00676. [DOI] [PubMed] [Google Scholar]
- 38.Star EN, Newton AJ, Murthy VN. Real-time imaging of Rab3a and Rab5a reveals differential roles in presynaptic function. J Physiol. 2005;569:103–117. doi: 10.1113/jphysiol.2005.092528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoopmann P, Punge A, Barysch SV, Westphal V, Buckers J, Opazo F, et al. Endosomal sorting of readily releasable synaptic vesicles. Proc Natl Acad Sci USA. 2010;107:19055–19060. doi: 10.1073/pnas.1007037107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, Dunn KW. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Junutula JR, De Maziere AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, et al. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell. 2004;15:2218–2229. doi: 10.1091/mbc.E03-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chua JJ, Kindler S, Boyken J, Jahn R. The architecture of an excitatory synapse. J Cell Sci. 2010;123:819–823. doi: 10.1242/jcs.052696. [DOI] [PubMed] [Google Scholar]