Abstract
Each cotton fiber is a single-celled seed trichome or hair, and over 20,000 fibers may develop semi-synchronously on each seed. The molecular basis for seed hair development is unknown but is likely to share many similarities with leaf trichome development in Arabidopsis. Leaf trichome initiation in Arabidopsis thaliana is activated by GLABROUS1 (GL1) that is negatively regulated by TRIPTYCHON (TRY). Using laser capture microdissection and microarray analysis, we found that many putative MYB transcription factor and structural protein genes were differentially expressed in fiber and non-fiber tissues. Gossypium hirsutum MYB2 (GhMYB2), a putative GL1 homolog, and its downstream gene, GhRDL1, were highly expressed during fiber cell initiation. GhRDL1, a fiber-related gene with unknown function, was predominately localized around cell walls in stems, sepals, seed coats, and pollen grains. GFP:GhRDL1 and GhMYB2:YFP were co-localized in the nuclei of ectopic trichomes in siliques. Overexpressing GhRDL1 or GhMYB2 in A. thaliana Columbia-0 (Col-0) activated fiber-like hair production in 4–6% of seeds and had on obvious effects on trichome development in leaves or siliques. Co-overexpressing GhRDL1 and GhMYB2 in A. thaliana Col-0 plants increased hair formation in ∼8% of seeds. Overexpressing both GhRDL1 and GhMYB2 in A. thaliana Col-0 try mutant plants produced seed hair in ∼10% of seeds as well as dense trichomes inside and outside siliques, suggesting synergistic effects of GhRDL1 and GhMYB2 with try on development of trichomes inside and outside of siliques and seed hair in A. thaliana. These data suggest that a different combination of factors is required for the full development of trichomes (hairs) in leaves, siliques, and seeds. A. thaliana can be developed as a model a system for discovering additional genes that control seed hair development in general and cotton fiber in particular.
Introduction
Cotton fiber is the largest renewable source of textile materials, a sustainable alternative to petroleum-based synthetic fibers. Cotton fiber is derived from seed protodermal cells and among the premier biological systems for studying cell differentiation and development. Cotton seed hair development share many similarities with Arabidopsis leaf trichome development [1], which is mediated by a “trichome activation complex”. Leaf trichome initiation in Arabidopsis thaliana is promoted by the positive transcription regulators GLABROUS1 (GL1), TRANSPARENT TESTA GLABRA1 (TTG1), GLABRA3 (GL3), and ENHANCER of GL3 (EGL3) that are counteracted by the negative regulators TRIPTYCHON (TRY), CAPRICE (CPC), and ENHANCER of TRY and CPC1 (ETC1, 2, and 3) that encode single MYB-domain protein families [2], [3], [4], [5], [6]. GLABROUS2 (GL2) functions downstream of the GL1/TTG/GL3 complex and also plays a role in leaf trichome development [3], [7]. Some MYB factors such as AtMYB5 and AtMYB23 have minor effects on trichome initiation but regulate mucilage biosynthesis and seed coat development [8]. Moreover, trichome genes such as TTG1 and GL2 affect mucilage biosynthesis and columella cell formation [9], [10], suggesting a role of these trichome genes in seed coat development [11].
Several studies using cotton fiber-related genes have demonstrated a close relationship between cotton seed fibers and Arabidopsis leaf trichomes. Gossypium arboreum MYB2 (GaMYB2) encoding a putative homolog of GL1 MYB transcription factor complements the trichomeless gl1 mutant and induces occasional hair formation in A. thaliana seeds [12]. GaHOX1, a homeobox gene, encodes a HD-ZIP IV transcription factor, and is a functional homologue of the A. thaliana GL2 gene [13]. Two WD-repeat genes from Gossypium hirsutum (GhTTG1) restore trichome formation in A. thaliana ttg1 mutant plants and complement anthocyanin defects in Matthiola incana ttg1 mutants [14]. Moreover, microrarray and gene expression analyses have uncovered many cotton fiber-related genes, including those encoding MYB transcription factors and phytohormonal regulators [15], [16]. For example, differential expression of six MYB genes is observed in allotetraploid cotton (G. hirsutum L.) [17]. Several MYB and RDL genes are expressed in fiber initials through microarray analysis [18]. GhMYB25 regulates early fiber and trichome development in cotton [19]. The data collectively suggest that Arabidopsis and cotton use similar transcription factors for the development of leaf trichomes and seed hairs. However, the mechanisms responsible for the differentiation of branched trichomes in vegetative tissues (leaves) and unbranched hairs in reproductive organs (seeds) may not be the same, and many seed plants including Arabidopsis do not produce seed hairs.
In this study, we employed microarray analysis of gene expression in fiber and ovular cells captured by laser micro-dissection and found differential expression of several hundred genes. A subset of genes, including G. hirsutum MYB2 (GhMYB2), GhMYB112b, GhRDL1, and Fb37, were validated by quantitative RT-PCR analysis and RNA in situ hybridization. Furthermore, we chose GhMYB2, a GL1-like MYB transcription factor gene, and a downstream gene GhRDL1 to test the hypothesis that cotton fiber-related genes can program seed hair development in A. thaliana. GFP:GhRDL1 and GhMYB2:YFP fusion proteins were studied in A. thaliana seed coat and ectopic trichomes. The functions of GhMYB2, a putative homolog of GL1, and GhRDL1, a putative homolog of RD22 in A. thaliana, were tested by overexpressing each cotton gene alone or together in A. thaliana wild-type (Col-0) or try mutant plants. Our data revealed novel roles of cotton fiber genes in the formation of seed hairs and ectopic trichomes inside and outside of siliques in A. thaliana.
Results
Differential gene expression in cotton fiber cell initials and ovular cells captured by laser microdissection
Using the cotton spotted long oligonucleotide microarrays with probes of many fiber ESTs developed in previous studies [16], [20], [21], we studied expression of fiber-related genes in ovules, protodermal fiber cells, and fiber cell initials by laser capture microdissection (LCM) (Figure S1, A and B). Several hundred genes in each of ten comparisons were differentially expressed between fibers and non-fiber tissues (Table S1, Table S2, Figure S1C). In the comparison between inner integuments and protodermal cells (−2 and 0 DPA), fiber cell initials (2 DPA), or fibers (7 DPA) (Table S1), the number of differentially expressed genes during fiber initiation (−2, 0, and 2 DPA) was higher than that in early stages of fiber cell elongation (7 DPA) (Figure S2A). The overlap between the differentially expressed genes in different stages (−2, 0, and 2 DPA) was relatively small (2–16%) and statistically insignificant (Table S3, Figure S2A), suggesting coordinated regulation of gene expression changes during early stages of fiber development [16]. The numbers of differentially expressed genes between ovule integument versus protodermal cells (−2 DPA) or fiber cell initials (2 DPA) were statistically significantly different, suggesting that a different set of genes is expressed during ovule and fiber development (Table S4).
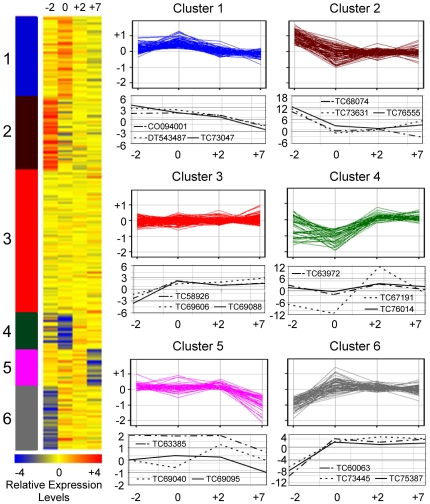
Using gene ontology classification of molecular function, the differentially expressed genes in different stages of ovule and fiber development were clustered by K-means clustering [22]. Six groups of gene expression patterns and their representative genes were identified (Figure 1, Table S5). Expression of the genes related to transcription activity (cluster 4) decreased from −2 to 0 DPA, but increased in fiber cells initials and fibers from 2 to 7 DPA. These include genes encoding putative transcription factors (TC67191, TC75739, TC76014), phytohormone responsive factors, and a germin-like protein (TC72843), which is consistent with the previous data [21], [23]. The genes encoding putative MYB2 and MYB25 transcription factors and germin-like proteins play roles in cotton fiber cell development [12], [19]. The genes related to DNA or RNA binding activity (cluster 5) were upregulated in the protodermal cells during fiber initiation, but the expression levels decreased at 7 DPA. Two gibberellin-responsive genes (TC65219 and TC79957) and a sucrose synthase gene (Sus) (TC73327) belonged to this group. SUS3 is a fiber-related protein and localized in fiber cells. Suppression of SUS3 expression inhibits cotton fiber initiation and development [24]. The genes encoding structural molecules such as ribosomal proteins and tubulin factors (cluster 6) were highly expressed in the ovules at −2 DPA and during fiber cell development, consistent with preferential accumulation of tubulins during fiber cell elongation [23], [25] and a large proportion of ribosomes produced during rapid cell elongation [26].
Figure 1. Comparative and K-means analyses of differentially expressed genes detected by microarrays.
A heat map of K-means cluster analysis of differentially expressed genes in protodermal cells (−2 DPA), fiber cell initials (0 and +2 DPA), and fibers (+7 DPA). Changes of gene expression in six Gene Ontology (GO) categories are shown with the expression patterns of three representative genes shown below each cluster graph. Cluster 1: kinase activity; 2: hydrolase activity; 3: transferase activity; 4: transcription factor; 5: DNA/RNA binding activity; and 6: structural molecule. Expression of representative genes was validated by qRT-PC. ID in parenthesis is updated from ICG10.
Enriched expression of GhMYB2 and GhRDL1 in cotton fiber
Expression patterns for a subset of genes detected by microarrays were validated by quantitative RT-PCR (qRT-PCR) analysis (Table S6, Figure S2B-G). AI730621 encoding a predicted ring zinc finger protein and TC62849 encoding a putative fiber protein 37 (Fb37) were abundantly expressed in the protodermal cells and fiber cells. The transcript levels of TC75739 (similar to GhMYB25 and GhMYB112b) increased from −2 DPA to +2 DPA, while GhMYB2 was actively expressed in young fibers (3 and 5 DPA). Transcript levels of AI072821 were high in the protodermal cells and increased in elongating fibers (7 DPA).
AI072821 is a G. hirsutum homolog (GhRDL1) of A. thaliana RESPONSIVE TO DESSICATION 22 (RD22)-like1. A. thaliana RD22 is responsive to dehydration stress and expressed in seeds [27]. A putative homolog of seed coat BURP-domain protein1 (SCB1) gene in soybean is expressed within cell walls, suggesting a role in the differentiation of the seed coat and columella cells [28]. In cotton, G. arboreum RDL1 (GaRDL1) was expressed in developing fiber cells [29], and the promoter of GaRDL1 fused with a β-glucuronidase (GUS) displayed trichome-specific expression in A. thaliana leaves [12]. GaMYB2 activated expression of GaRDL1 probably through direct binding of the L1 box and MYB motif in the promoter. GaMYB2 affects seed hair formation in A. thaliana [12], and GhMYB25 affects trichome and fiber development in cotton [19], but their effects are moderate, suggesting that it may require additional downstream genes to stimulate the activities. Here we chose GhMYB2, a GL1-like MYB transcription factor gene, and GhRDL1, a gene that contains GaMYB2 binding motifs in its promoter to test the hypothesis that cotton fiber-related genes can program seed hair development in A. thaliana.
Expression of GhRDL1 in cotton fiber cell initials and seed coat in A. thaliana seeds
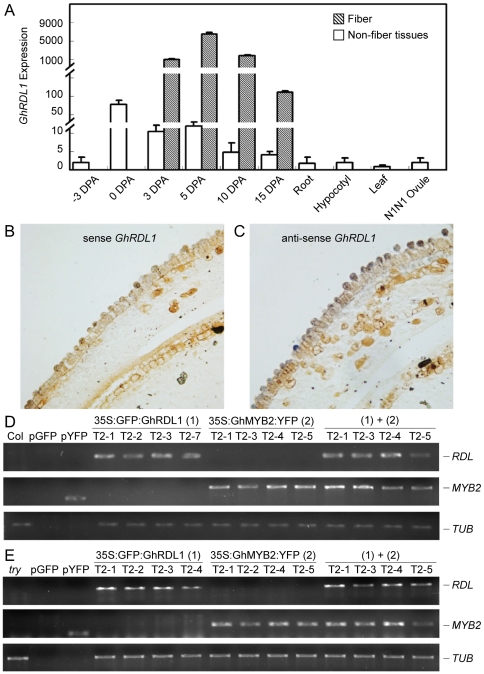
The predicted protein of a cloned full-length GhRDL1 cDNA shared 97% amino-acid sequence identity with GaRDL1 and had a major deletion relative to A. thaliana RD22 (AtRD22) (Figure S3). GhRDL1 was expressed at higher levels in fiber cells and elongating fibers than in ovules and non-fiber tissues, including roots and hypocotyls (Figure 2A). Interestingly, GhRDL1 expression was low in ovules of the G. hirsutum naked seed mutant (N1) that produces little or no fiber.
Figure 2. GhRDL1 expression patterns in cotton and GFP:GhRDL1 and GhMYB2:YFP transgene expression in A. thaliana Col and try mutant transgenic plants.
(A) Quantitative RT PCR (qRT-PCR) analysis of GhRDL1 expression in ovules, fibers, and non-fiber tissues of TM-1 and ovules (0 DPA) of N1N1. (B–C) RNA in situ hybridization in cotton ovules (0 DPA) using sense (B) and anti-sense (C) probes showing accumulation of GhRDL1 transcripts in fiber cell initials (C). (D) RT-PCR analysis of transgene expression in 35S:GFP:GhRDL1 transgenics, 35S:GhMYB2:YFP transgenics, and 35S:GFP:GhRDL1 and 35S:GhMYB2:YFP double transgenics. Amplification of Tublin (beta 6) gene (TUB) was used as an RNA loading control. (E) RT-PCR analysis of the transgene expression as in (D), except that the transgenic plants were in the try mutant background.
RNA in situ hybridization assays indicated that GhRDL1 transcripts were localized specifically in fiber cell initials of cotton ovules, compared to the background signals detected using a sense RNA probe (Figure 2B). The data suggest that GhRDL1 is a fiber-related gene in tetraploid cotton.
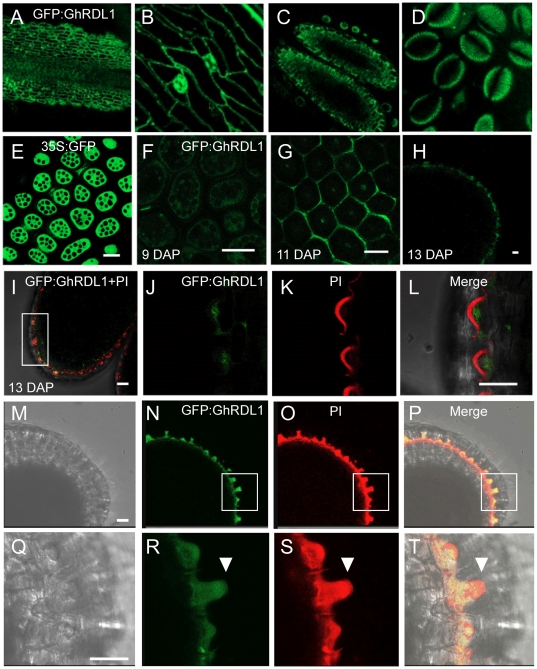
A. thaliana transgenic plants expressing 35S:GFP:GhRDL1 fusion protein construct were generated to test cellular localization of GhRDL1 in A. thaliana plants and seed coat. The transgenic plants were selected using 3∶1 segregation ratio based on antibiotic selection in T2 generation. Among ∼30 independent transgenic lines, five (GhRDL1At1–5) were genotyped, and four lines were used for further analysis. GhRDL1 was uniformly expressed in four independently-derived T2 transgenic plants (Figure 2D). GFP:GhRDL1 was found around cell walls in stems, sepals, stamens, and pollen grains (Figure 3A–D). In the control plants expressing 35S:GFP, the GFP signals dispersed in the protodermal cells in all stages examined (Figure 3E). At cellular levels, the GFP:GhRDL1 signals (green) in the GFP:GhRDL1 transgenic plants appeared 5 days after pollination (DAP), clearly visible at 9 DAP (Figure 3F), and accumulated at high levels within cell walls of seed coat at 11 and 13 DAP, respectively (Figure 3G and 3H). The GFP:GhRDL1 signals were distributed in the epidermal cells at 13 DAP (Figure 3J). Propidium iodide (PI) is normally used to stain DNA and RNA as well as cell walls. At subcellular levels, GFP:GhRDL1 proteins (green) (Figure 3I and 3L) were localized underneath the cell walls that were stainable with PI (red) (Figure 3K and 3L), suggesting that GhRDL1 proteins accumulate in columella cells of A. thaliana seed coat. Indeed, localization of GhRDL1:GFP and PI aggregated at 13 DAP (Figure 3N–O), and a cell initial elongated under high magnification (arrows, Figure 3R–T). Because endogenous RDL1 promoter had a weak activity in our hands, these data did not preclude localization of RDL1 in other cell types in addition to cell walls.
Figure 3. Localization of 35S:GFP:GhRDL1 in A. thaliana transgenic plants.
(A–D) Localization of 35S:GFP:GhRDL1 in stem (A), sepal cell walls (B), stamen (C) and pollen grains (D). (E) 35S:GFP alone in the seed coat at 9 days after pollination (DAP). (F–H) Localization of 35S:GFP:GhRDL1 in cell walls of seed coat in A. thaliana: 35S:GFP:GhRDL1 at 9 DAP (F), 11 DAP (G), and longitudinal section of seed coat at 13 DAP (H). (I) Merged image of 35S:GFP:GhRDL1 and propidium iodide (PI, red) at 13 DAP. (J–L) Enlarged views of a section in (I) stained with GFP:GhRDL1 (J), PI (K), and merged (L). (M–P) Unstained (M), GFP:GhRDL1 (N), PI (O), and merged images (P) of 35:GFP:GhRDL in columella cells of seed coat. (Q–T) Enlarged views of the selected areas (boxed in I) showing unstained (Q), GFP:GhRDL1 (R), PI (S), and merged (T) of a fiber-like cell initial (arrowheads). Scale bars represent 50 mm.
Activation of seed hair development by overexpression of GhRDL1, GhMYB2 or both
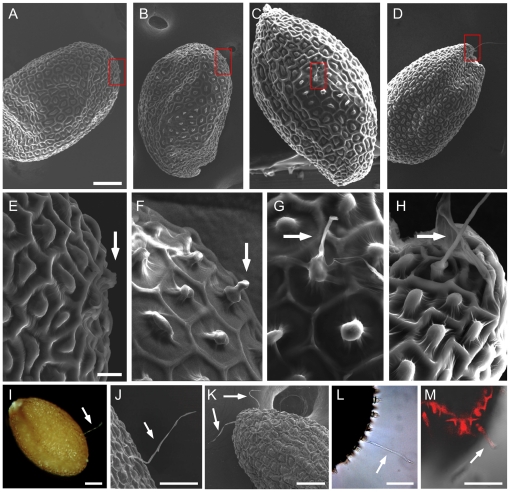
Under the electron microscope (SEM), the fiber-like initial emerged at 13 DAP (Figure 4A and 4E) and 15 DAP (Figure 4B and 4F). The elongating fiber-like hair was clearly visible at 17 DAP (Figure 4C and 4D) and 21 DAP (Figure 4D and 4H). Consequently, fiber-like hairs were found in some mature seeds (Figure 4I). SEM images showed that the hairs in mature A. thaliana seeds were unbranched single cells (Figure 4J), similar to the fibers grown on cotton seeds [30]. The seed hair was expandable in water (Figure 4L) and stainable with PI (Figure 4M), suggesting a wall-like structure, probably similar to the secondary wall materials in cotton fiber. The seed hairs were distributed in different (chalazal, micropylar, and middle) parts of seed coat (Figure 4A–4B). Approximately ∼6% of transgenic seeds had one or two hairs (Figure 4K) with an average hair length of ∼100 µm (Table 1).
Figure 4. Seed hair production in the transgenic plants overexpressing GhRDL1 alone or GhRDL1 and GhMYB2 together.
(A–D) Initiation of seed hair in the try transgenic plants (seeds) expressing 35S:GFP:GhRDL1 alone. Scanning electron microscope (SEM) images of developing seeds at 13 days after pollination (DAP) (A), 15 DAP (B), 17 DAP (C), and 21 DAP (D). Hair-like initial was observed as early as 11 DAP. The boxed areas were zoomed in (E–H). Scale bar represents 100 µm. (E–H) Enlarged SEM images of corresponding boxed regions in (A–D). Scale bar represents 10 µm. (I–J) A single seed hair in a mature seed observed using light microscope (I) and SEM (J). (K) Multiple seed hairs (two shown) produced in a seed of the transgenic plants overexpressing both GhRDL1 and GhMYB2. (L–M) Seed hair without staining (L) and PI staining (M) in GhRDL1 transgenic plants. PI stained cell walls of seed coat and seed hair. Scale bars in (I–M) represent 50 µm. Arrows in (E–M) indicate seed hair or fiber.
Table 1. Percentage and length of seed hair in Arabidopsis thaliana Col-0 or Col-0 try mutant plants overexpressing GhRDL1, GhMYB2, or both.
| Line | Hairy seed (%) | Seed hair length (µm) |
| Col-0 | 0.40±0.02a | 43.35±18.55a |
| Col-0 35S::GFP-GhRDL1 | 5.57±1.96b | 93.53±11.28b |
| Col-0 35S::GhMYB2-YFP | 5.88±1.78b | 90.31±6.08b |
| Col-0 35S::GFP-GhRDL1 35S::GhMYB2_YFP | 7.73±2.33c | 113.46±25.28c |
| Col-0 try | 5.89±1.66b | 116.58±41.94c |
| Col-0 try 35S::GFP-GhRDL1 | 5.14±2.12b | 115.78±8.08c |
| Col-0 try 35S::GhMYB2-YFP | 5.95±1.45b | 114.30±11.51c |
| Col-0 try 35S::GFP-GhRDL1 & 35S::GhMYB2-YFP | 10.04±2.26d | 138.91±45.08c |
P<0.05, a, b, c and d indicate significant different groups.
GaMYB2 binds the promoter of GaRDL1 in yeast one-hybrid assays and activates GaRDL1 expression [12]. To test if GhMYB2 and GhRDL1 act additively or synergistically to promote seed hair development, a genomic DNA fragment containing full-length GhMYB2 was cloned into an YFP cassette. We transformed 35S:GhMYB2:YFP or 35S:GFP:GhRDL1 and 35S:GhMYB2:YFP together into Col-0. Four of ten independent T2 transgenic plants were analyzed in each event. The transgenic plants expressing 35S:GhMYB2:YFP or 35S:GFP:GhRDL1 (Figure 2D) did not alter the distribution or density of trichomes in leaves, stems (data not shown), or pedicels (Figure 5A). In the co-transformed transgenic plants, both GhMYB2 and GhRDL1 were equally expressed (Figure 2D). Expressing either GhRDL1 or GhMYB2 in the transgenic plants produced hairs in ∼6% of seeds. In the double-gene transgenic plants, hairs were found in ∼8% of seeds (Table 1). Moreover, a few ectopic trichomes appeared in the pedicels of the double-gene transgenic plants (Figure 5A), but not in other parts of plants examined (data not shown). The data suggest additive effects of GhMYB2 and GhRDL1 on seed hair development in A. thaliana.
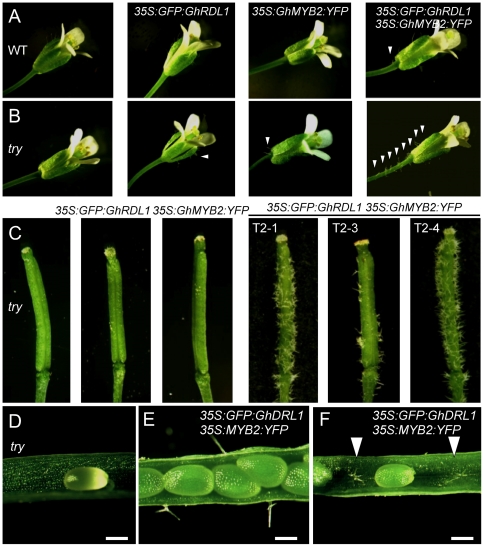
Figure 5. Ectopic trichome production in the transgenic plants overexpressing GhRDL1, GhMYB2, or GhRDL1 and GhMYB2 together.
(A) Flowers of A. thaliana Col (WT, left) and transgenic plants overexpressing GhRDL1, GhMYB2, and both (right). (B) Flowers of A. thaliana try mutant (left) and transgenic plants overexpressing GhRDL1, GhMYB2, and both (right). Arrows indicate ectopic trichomes. (C) Ectopic trchomes outside siliques produced in three try transgenic plants (T2-1 to T2-3) overexpressing both GhRDL1 and GhMYB2. (D) No ectopic silique trichome was observed in the try mutant. (E–F) Ectopic trichomes produced outside (E) and inside (F) siliques in the try transgenic plants ovexpressing GhRDL1 and GhMYB2. Scale bars (D–F) represent 300 µm.
Synergistic effects of GhMYB2 and GhRDL1 on seed hair development in the try mutant
These data suggest that similar to A. thaliana GL1, cotton GhMYB2 and GhRDL1 may play positive roles in trichome development, which is counterbalanced by the negative regulators such as TRY [3], [5], [31]. Constitutive expression of cotton GaMYB2 or GaHOX1 suppresses the leaf trichome development in A. thaliana [12], [13]. Removal of negative regulators such as TRY altered cell patterning of leaf trichomes and root hairs [4], [31] and might also affect seed trichome development. Indeed, gl1, try, etc, gl3/egl3, and 35S:GhRDL1 affect the formation of mucilage and columella cells. The seed surface was very rough, and some hair-like structure was observed (Figure S4). Seed hairs were found in ∼6% of seeds of A. thaliana try mutant plants (Table 1), suggesting a role of try in seed hair development. The presence of seed hair in the try mutants was not previously reported probably because it was not closely examined. On the contrary, seed surface in the gl2-2 mutant was extremely smooth.
To test the effects of GhMYB2 and GhRDL1 on trichome and seed hair development in try background, 35S:GFP:GhRDL1 or 35S:GhMYB2:YFP was transformed into A. thaliana Col-0 try mutant plants alone or together. Both GhRDL1 and GhMYB2 were expressed at similar levels in multiple independent transgenic plants (Figure 2E). Overexpressing GhMYB2 or GhRDL1 alone in the try mutants did not have obvious effects on trichome development in leaves or stems, except for a few ectopic trichomes developed in the pedicels and outside sepals (Figure 5B). Seed hair frequency (5–6%) in 35S:GFP:RDL1 or 35:MYB2:YFP transgenic plants was similar to that of try mutant seeds (Table 1). However, expressing both GhMYB2 and GhRDL1 in the try mutant plants increased seed hair production to ∼10% of seeds and produced long seed hairs (∼120 µm) (Table 1) and multiple hairs (Figure 4K). Moreover, dense ectopic trichomes developed on sepals and pedicels (Figure 5B) as well as outside (Figure 5C and 5E) and inside (Figure 5F) siliques. The trichomes were clustered outside siliques (Figure 5C and Figure 6A). Cluster- and multi-branched trichomes are characteristic of try and cpc mutant phenotypes in the leaves but not in the siliques [3], [4], [31]. Moreover, ectopic trichomes, either branched or unbranched, were produced in the inner wall of siliques of the try mutant plants that expressed both GhMYB2 and GhRDL1 (Figure 6B). No ectopic trichomes were found on the siliques in the try mutants or the try mutants over-expressing GhMYB2 or GhRDL1 alone (data not shown). These data suggest that cotton fiber-related genes GhRDL1 and GhMYB2 act synergistically and in concert with try to promote trichome development in siliques and seeds in A. thaliana.
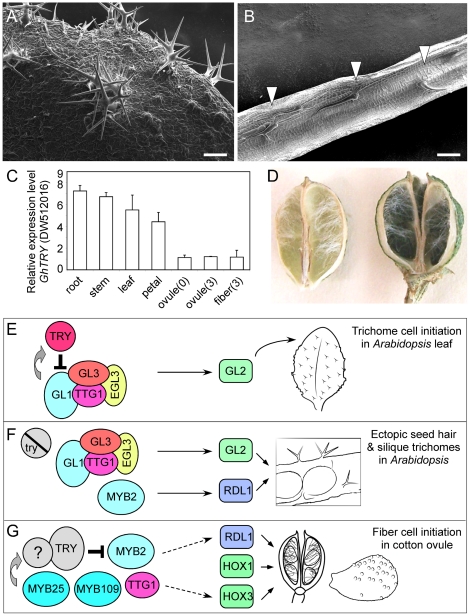
Figure 6. Trichome production inside siliques and cotton bolls.
(A) An SEM image showing clustered silique trichomes in try transgenic plants overexpressing GhRDL1 and GhMYB2. (B) An SEM image of branched and unbranched trichomes inside siliques of the transgenic plants. Scale bars in (A and B) represent 100 µm. (C) qRT-PCR analysis of a putative cotton cpc gene (DW512016) in cotton tissues (n = 3). (D) Unbranched trichomes (white) produced inside cotton bolls. (E) A model of leaf trichome development. (F) A model of ectopic trichome production in Arabidopsis silique and seed coat. (G) A model of seed hair production in cotton. See text for detailed explanations.
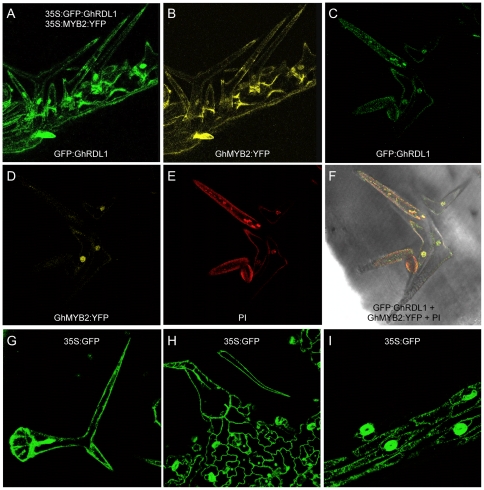
GFP:GhRDL1 was present primarily in cell walls of the silique trichomes in the try transgenic A. thaliana plants (Figure 7A). GhMYB2:YFP was primarily localized in nuclei but also in cell walls (Figure 7B). At subcellular levels, GFP:GhRDL1 was predominately localized around the cell walls and nuclei of the ectopic trichome cells in siliques (Figure 7C), whereas GhMYB2:YFP was exclusively localized in nuclei (Figure 7D). This may suggest that TRY prevents GhMYB2 from entering nuclei, and the interaction between GhMYB2:YFP and GFP:GhRDL1 promotes GFP:GhRDL1 localization in nuclei. The images of propidium iodide (Figure 7E) and GFP:GhRDL1 overlapped (Figure 7F), expect for those in the nuclei, suggesting that they are co-localized in cell walls. Co-localization of GhRDL1 and GhMYB2 in nuclei (Figure 7F) may suggest interaction of GhMYB2 with GhRDL1 in the same nuclei of the trichome cell. Whether or not GhMYB2 and GhRDL1 function in the nuclei of trichome cells remained to be tested. As controls, the staining patterns of 35S:GFP in silique trichomes (Figure 7G), epidermal cells (Figure 7H), and siliques (Figure 7I) were dispersed.
Figure 7. Subcellular localization of GFP:GhRDL1 and GhMYB2:YFP.
(A–B) Localization of GFP:GhRDL1 (A) and YFP:GhMYB2 (B) in silique trichomes. (C–F) Silique trichomes in the try transgenic plants overexpressing GhRDL1 and GhMYB2 stained with GFP (C), YFP (D), PI (E, red), and merged (F). (G–I) Diffusion of GFP in leaf trichomes (G), epidermal cells (H), and siliques (I) in 35S:GFP transgenic plants. GFP donuts in (I) indicate guard cells of stomata.
Single MYB-domain proteins such as TRY and CPC are negative regulators of GL1 and GL3 in the feedback regulation of trichome formation [3],[4]. In A. thaliana, a mutation in TRY partially suppresses the GL1 phenotype and leads to increased numbers of epidermal and mesophyll cells and increased levels of endoreduplication in trichomes [6], [32]. A cotton EST (DW512016) encoding a putative GhCPC-like protein was expressed 5–8 fold higher in roots, stems, leaves, and petals than in ovules (0 and 3 DPA) or fibers (3 DPA) (Figure 6C). Different levels of putative GhCPC expression in fibers and roots suggest similar but different inhibitory effects of CPC on de novo patterning of trichome initiation and position-dependent cell determination during root hair development [4], [33]. Interestingly, trichomes were also present inside cotton bolls in some cotton species including Gossypium thurberi (Figure 6D), which is reminiscent of the ectopic trichomes developed inside siliques in the GhMYB2 and GhRDL1 overexpression lines in the try background (Figure 6B).
We proposed models to explain the development of trichomes in leaves, siliques, and seeds. In A. thaliana, genetic studies indicate that GL1, TTG1, GL3, and ENHANCER of GL3 (EGL3) are in the same pathway [3], [34]. Yeast two-hybrid studies showed that GL3 interacts with GL1 and TTG1. Similarly, EGL3 interacts with TTG1 and GL1 to form heterodimers with GL3 [10], [35], [36]. This complex promotes leaf trichome initiation through homeobox domain protein GL2 [4], [7] (Figure 6E). The positive effects of GL1 complex on trichome formation are counterbalanced by negative regulators TRY and CPC that are redundant but have incomplete overlapping functions [4], [5], [6], [32], [33]. When the negative regulator TRY is eliminated by mutation, overexpression of cotton GhMYB2, a putative GL1 homologue, and GhRDL1, a gene expressed in the seed coat, induces regulatory perturbation of the trichome pathway, leading to the production of ectopic trichomes inside siliques and on the seed coat (Figure 6F). In cotton, several putative homologues of GL1 genes such as MYB2 and MYB109 and MIXTA-like gene such as MYB25 are shown to affect seed fiber development [12], [19], [37] (Figure 6G). Although many factors are still missing, the interactions among MYB2, RDL1, GhTTG1, and GL2-like homeobox domain proteins such as GaHOX1 and GaHOX3 [13] promote the development of trichomes inside and outside siliques in A. thaliana, which may suggest the roles of these genes in cotton bolls and fibers (Figure 6D). Moreover, low expression levels of a putative GhCPC gene in cotton ovules and fibers is negatively correlated with cotton fiber development, similar to the negative role of CPC in Arabidopsis trichome development [3], [4].
Discussion
Enrichment of transcripts in several important pathways in cotton fiber development
Studying cotton gene expression during fiber cell initiation is challenging because of technical difficulties in isolating fiber initials from the whole ovules. Laser capture microdissection (LCM) has been widely used in the study of cell-type specific gene expression in plants [38], [39] including cotton [15], [40]. The most highly up-regulated genes in cotton fiber initials include those encoding proteins for the synthesis of enzymes and cell wall proteins, carbohydrates, and lipids [40]. In this study, we expanded to include ten different comparisons.
Many differentially expressed genes cannot be directly compared among different experiments. Among top 50 highly expressed genes in cotton fiber initial cells in the previous studies [15], [40], 32 (64%) were also up-regulated in the similar tissues during fiber initiation in this study. The overlapped genes include a large number of transcription factor genes, such as GhMYB25, GhMYB2, GhMYB36, GhMYB38, and GhGL2-like, as well as structural genes, such as cellulase synthetase gene (CESA8), sucrose synthase gene, fiber protein genes Fb27 and Fb34, and GhRDL1. The identification of these MYB-related genes in multiple independent experiments suggests an important role for MYB transcription factors in the early stages of cotton fiber development [12], [19], consistent with their roles in trichome cell differentiation in A. thaliana [3], [6]. AtMYB106, a MIXTA-like transcription factor and homolog of cotton GhMYB25, functions as a repressor of cell outgrowth in A. thaliana [41]. GaMYB2 affects seed hair formation in A. thaliana [12], and GhMYB25 affects trichome and fiber development in cotton [19], but the effects are moderate, suggesting that it may require additional downstream genes to stimulate the activities. Co-regulation of a MYB transcription factor gene (GhMYB2) and an interacting gene GhRDL1 whose function is unknown but highly expressed in fiber cells suggests additive effects of these two genes on fiber cell initiation. As a result, these two genes were subsequently selected for further study of their roles in parallel trichome and seed hair pathways in A. thaliana.
Transcriptome analysis also revealed enrichment of auxin responsive factor genes such as ARF2 and ARF6 and gibberellin synthetic factor transcripts in the epidermis, consistent with the requirement of gibberellin and auxin during fiber cell initiation [1], [42]. In addition, ATP binding cassette (ABC) transporter genes are involved in transporting diverse substrates including auxin across membranes [43]. Several genes encoding ABC transporters that were enriched in the fiber in this study were also abundantly expressed in a previous study [16]. Apyrase (nucleoside triphosphate-diphosphohydrolase) genes are influenced by auxin and their expression is closely related with plant growth. Expression of two A. thaliana apyrase (APY1 and APY2) genes showed the highest expression in the tissues that accumulate high auxin levels [44]. Two putative homologs of cotton APY genes were low abundant during fiber initiation but highly induced during fiber elongation (at 7 DPA), indicating a role of apyrases in cotton fiber cell elongation, which was confirmed in a recent study [45].
Transcripts of the genes encoding several peroxidase and cytochrome P450 family members were enriched in elongating fibers, consistent with the enrichment of stress responsive genes during fiber development in cotton related species [46]. The biological effects of these stress responsive genes on fiber cell development remains to be tested. Comparative analysis of ten microarray datasets also supported the previous notion of temporal and spatial regulation of gene expression during fiber cell development [1], [16]. These microarray data will provide some guidance for future studies on fiber cell development in cotton and possibly trichome and seed hair development in A. thaliana.
Roles of cotton fiber-related genes in A. thaliana seed hair development
Little is known about why some plant seeds are hairy, whereas others do not have hair at all. The gene expression and subcellular localization data suggest positive roles of GhMYB2 and GhRDL1 in the development of silique trichomes and seed hairs in A. thaliana and cotton.
Arabidopsis plants rarely produce seed hairs. A mutation in TRY induces seed hair formation. This suggests that Arabidopsis has the basic machinery for seed hair production, but the hair frequency is too low to be readily detected probably because other genes important to seed hair initiation are repressed, inactive or absent in the seed coat. GaMYB2 by analogy GhMYB2, a putative homolog of A. thaliana GL1, mediates GaRDL1 expression through direct interaction with its promoter [12]. Similar to seed-specific expression of RD22 in A. thaliana [27] and SCB1 expression in parenchyma cells of soybean [28], GhRDL1 is predominately expressed around cell walls and columella cells of A. thaliana seed coat and fiber cell initials of cotton ovules, which may determine the fate of protodermal cells into seed trichome or hair. Although the function of A. thaliana RD22 is largely unknown, localization of cotton RDL1 in A. thaliana columella cells of seed coat suggest that GhRDL1 alone or in combination with GhMYB2 increases a potential of transforming protodermal cells into seed hairs. A soybean homolog of AtRD22 is a seed coat BURP-domain protein1 (SCB1) and is expressed within cell walls [28]. GhRDL1 and AtRD22 share 63% amino acid sequence similarities in the BURP domain. AtRD22 is a seed dehydration responsive protein and is inducible by abscisic acid [27]. High levels of GhRDL1 expression during cotton fiber initiation and elongation suggest a dehydration-like condition during fiber cell development, probably resulting from rapid accumulation of glucose and the other secondary metabolites.
Notably, temporal regulation of RDL1 is different in A. thaliana seed coat and cotton ovules. GFP:GhRDL1 was expressed in A. thaliana ovules 5 days after pollination, whereas GhRDL1 expression commenced immediately on the day of fiber initiation (0 DPA) and peaked during early stages of fiber elongation (5–7 DPA), suggesting a role of GhRDL1 in both fiber cell initiation and elongation. The time delay is probably related to the absence of some factors in protodermal cells in the seed coat of A. thaliana, and accumulation of GhMYB2 and GhRDL1 in the absence of try has pushed columella cells to form elongating fibers. Hair-like spikes in try and etc mutants and 35S:GhRDL1 lines were evenly distributed on the seed surface (Figure S4), suggesting that all seeds are affected, and many cells are potentiated to become fiber cells. It is conceivable that additional genes are required to fully activate the regulatory network of A. thaliana trichome development [47], [48] and seed hair production. Seed hair development in A. thaliana may be similar to fiber development in cotton, which requires two major steps: fiber cell initiation and fiber cell elongation. Like trichome development, initiation of a seed hair cell requires positive regulators such as GL1 or GhMYB2. Elongation of seed hair in A. thaliana results from the elongation of columella cells, which consists of cell wall materials. This is reminiscent of primarily secondary cell wall formation in the late stage of fiber cell elongation in cotton [30], [49]. The current A. thaliana lines may not have fiber elongation factors. As a result, the seed hair may be very short (∼100 µm), and many short hairs are lost during sample preparation or simply invisible using regular optical microscope. The A. thaliana try transgenic plants overexpressing GhMYB2 and GhRDL1 can be used to screen for additional cotton fiber genes during seed hair development. This will provide a novel genetic system for the discovery and functional analysis of the cotton genes that fully activate seed fiber development in A. thaliana, cotton and other plants.
Methods
Plant materials
Gossypium hirsutum L. cv. TM-1, isogenic naked seed mutant (N1N1) (>6 generations of selfing) and Gossypium thurberi were grown in a greenhouse. Flowers were tagged on the day of anthesis, and ovules were harvested at 0 and 2 DPA. Fibers (7 DPA) were dissected from the cotton bolls. For each genotype, three biological pools each with ten plants were grown. Ovules or fibers were dissected, and the fresh tissues were frozen in liquid nitrogen and stored in a −70°C freezer and subjected to RNA extraction.
Seeds of Arabidopsis thaliana Columbia-0 (Col-0), try, gl1, try, etc, gl3/egl3 (all in Col-0 background) were sterilized with 10% (v/v) bleach for 10 min, followed by two washes in sterile water. Seedlings were germinated on germination medium, and seedlings were transferred to soil (MetroMix 200, Sungro, Bellevue, WA) and grown in 16 h photoperiod at 22°C. T-DNA insertion mutant of try (SALK_029760) was obtained from Arabidopsis Biological Resource Center.
EST selection and 70-mer oligonucleotide design for microarrays
The cotton oligonucleotide microarray was designed from several cotton EST libraries, which included three sets (1,154, 12,006, and 9,629) of oligonucleotide probes, making a total 22,789 of oligonucleotides on a single chip [20]. The two sets were derived from enriched ESTs in ovules and fibers [21] or oligonucleotides with known function genes [16].
Laser capture microdissection (LCM)
Cotton ovules collected at −2, 0, and 2 DPA were subjected to tissue fixation [40]. The fixed ovules were embedded using Tissue-Tek® Optimal Cutting Temperature in cryo-mold (Sakura Finetek U.S.A., Torrance, CA). The embedded ovules were frozen immediately in liquid nitrogen and stored at −80°C.
Cryosectioning was performed using a Leica Cryostat (Leica Microsystems, Bannockburn, IL). The block was equilibrated at −20°C for 1 hour and cryosectioned at 10 µm. The slides with cryosectioned ovules were dehydrated in a series of ethanol (70%, 95%, and 100%) for 2 min each on ice and transferred to histoclear (National Diagnostics, Atlanta, GA).
Individual epidermis (−2 DPA) or fiber cell initials (2 DPA) were catapulted (Figure S1B) using a PALM laser capture system (P.A.L.M. Microlaser Technologies AG Inc., Bernried, Germany), and approximately 1,000 cells were collected into a tube containing 45 µl of RNALater (Ambion, Austin, TX) for RNA extraction.
Microarray experimental design
All data is MIAME compliant and that the raw data has been deposited in a MIAME compliant database (E.g. ArrayExpress, GEO), as detailed on the MGED Society website http://www.mged.org/Workgroups/MIAME/miame.html. The microarray data are under the series accession number GSE17378.
A combination of loop and reference designs was used for microarray analysis of gene expression changes in four developmental stages (Figure S1). LCM was used to separate epidermis (−2 DPA) and fiber cell initials (0 DPA and 2 DPA) form ovules at −2 DPA, 0 DPA, and 2 DPA. Fibers were also manually dissected from ovules at 7 DPA. One loop included gene expression comparisons between epidermis (−2 DPA), fiber cell initials (0 DPA), ovules (0 DPA), and ovules (−2 DPA), while the other loop was used to compare gene expression changes between fiber cell initials at 0 and 2 DPA and ovules at 0 and 2 DPA. Gene expression changes were also compared between fiber cell initials at 0DPA and fibers at 7 DPA and between the ovules at 0 and 7 DPA.
Microarray hybridization and statistical analysis
Captured cells were homogenized in 500 µl of RNA extraction buffer for RNA extraction [21]. Total RNA was amplified using an Amino Allyl MessageAmp™ aRNA amplification Kit (Ambion, Austin, TX) in two rounds of amplification. Amplified RNA was coupled with Cy3 or Cy5 dyes (Amersham Biosciences, Piscataway, NJ) and purified using Qiagen RNeasy mini column (Qiagen, Germantown, MD). About 1 µg of fragmented Cy3- and Cy5-labeled aRNA probes was used for hybridization. In each comparison, two dye-swaps with two biological replications were performed, resulting in a total of eight slide hybridizations. Hybridization and washing were performed as previously described [16].
Microarray data were analyzed as previously described [50]. A standard t-test statistics was used for this comparison, based on the normality assumption for the residuals. A false discovery rate (FDR) of a = 0.05 [51] was employed to control the family-wise error rate. The genes with a significant p value were further analyzed by fold-expression changes. K-means clustering using Euclidean distance measurement [22] was employed to compare differentially expressed genes across different developmental stages and in different tissue types. Wilcoxon rank-sum test [52] was performed to examine how each set of differentially expressed genes is different from each other (Table S3).
RT-PCR and quantitative RT-PCR (qRT-PCR) analyses
qRT-PCR reaction was carried out in a final volume of 20 µl containing 10 µl SYBR Green PCR master mix (Applied Biosystems, Foster City, CA), 1 µM forward and reverse primers (Table S6), and 0.1 µM cDNA template in a ABI7500 Real-Time PCR system (Applied Biosystems, Foster City, CA). Cotton HISTONE H3 (AF024716) was used to normalize the amount of RT-PCR products [12]. All reactions were performed in three replications using a dissociation curve as a control for the primer dimers.
Semi-quantitative RT-PCR was used to detect the expression levels of GhRDL1 and GhMYB2 in single-gene and double-gene transgenic plants using the primer pairs shown in Table S6. Expression of TUB (AT5G12250, BETA-6 tublin) was used as control.
Cloning, transformation, and transgenic plants
A full-length cDNA of GhRDL1 (AY072821) was amplified in the epidermis of 0 DPA ovules using a gene specific primer pair (Table S6). The amplified fragment was cloned into pGEM T-easy vector (Promega, Madison, WI) and sequenced. The GhRDL1 insert was subcloned into BamHI and NotI-digested 35SpBARN plant expression vector and named 35S:GhRDL1. For GFP and YFP fusion constructs, the cDNA of GhRDL1 and gDNA of GhMYB2 were amplified using primer pairs (Table S6) and cloned into pCGTBG (DQ370423.1) and pEarleyGate101, respectively.
Agrobacterium tumefaciens strain GV3101was used for floral dip transformation in A. thaliana [53]. pBARN and pCsGFPBT (Genbank DQ370426, 35S:GFP) vectors alone were transformed as controls. The transgenic plants were named 35S:GhRDL1, 35S:GFP:GhRDL1, 35S:GhMYB2:YFP, and 35S:pBARN, respectively. The transformed seedlings were genotyped using the primer pairs provided in Table S6.
Hygromycin (25 ml/L) and BASTA (40 ml/L) were used for selecting transgenic plants expressing pCGTBG_GhRDL1 (35S:GFP:GhRDL1) and pEarleyGate101_GhMYB2 (35S:GhMYB2:YFP), respectively.
Co-transformation using pCGTBG_GhRDL1 and pEarleyGate101_GhMYB2 was used to generate transgenic plants expressing both GFP:GhRDL1 and GhMYB2:YFP. The Agrobacterium GV3101 cells containing pCGTBG_GhRDL1 and pEarleyGate101_GhMYB2, respectively, were grown in 250 ml liquid media until OD600>1.8. The cells were harvested by centrifugation and resuspended with 500 ml sucrose buffer containing a few drops of silwet L77. A mixture containing 250 ml each of GV3101_pCGTBG_GhRDL1 and GV3101_pEarleyGate101_GhMYB2 culture was used for transformation. T0 seeds were germinated on media plate containing 25 ml/L Hygromycin and 40 ml/L BASTA. A total of 30–40 positive transgenic lines were used for further analysis.
Microscopy and histology
Scanning electronic microscopy (SEM) was performed using a modified protocol [54], [55]. The samples were scanned and analyzed using a Zeiss Supra 40 VP SEM with an accelerating voltage of 5 kV and a working distance of 39 mm. Images were scanned and stored as TIFF files.
The fluorescent samples were examined using a Leica SP2 AOBS Confocal microscope. The PALM laser-capture system, SEM, and confocal microscope are located in the Microscopy and Imaging Facility at The University of Texas at Austin.
RNA in situ hybridization
A GhRDL1 cDNAs fragment was amplified by PCR using the primer pair (GhRDL1-probe_F: CCAAACTAGGGAAAGTTGAT; GhRDL1-probe_R: TTACTTAGGGACCCAAACAA) and cloned into pGEM-T (Promega). Digoxigenin-labeled sense and antisense probes were synthesized with T7 or SP6 RNA polymerase (Roche, Indianapolis, IN, USA). G. hirsutum L. cv. TM-1 ovules were collected at 0 DPA and fixed in formalin/acetic acid/ethanol (1∶1∶18). Paraffin-embedded ovules were sectioned to 8 µm thickness for RNA in situ hybridization and detection using a published protocol [12].
Supporting Information
List of differentially expressed genes.
(XLS)
Number of differentially expressed genes.
(DOC)
Wilcoxon rank sum test of differentially expressed genes.
(DOC)
A subset of top differentially expressed genes in the epidermis or fibers.
(DOC)
A list of representative genes in each cluster.
(DOC)
Primer sequences used for RT-PCR and quantitative RT-PCR analyses.
(DOC)
Microarray experimental design and application of laser capture microdissection (LCM). (A) Development of cotton fiber. (B) Application of LCM in fibers (2 DPA, upper panel) and inner integuments of ovules (2 DPA, lower panel). The captured cells were removed (right photos in each panel). (C) Microarray experimental design. Arrows denote dye swaps used in each hybridization (red: Cy5, green: Cy3).
(TIF)
qRT-PCR validation of six differentially expressed genes detected by microarrays. Gene expression was analyzed in ten tissues, namely, leaves (L), petals (P), protodermal cells (−2E), fiber cell initials (0E, 0 DPA and 2E, 2 DPA), fibers (7F, 7 DPA), ovules at −2 DPA (−2O), 0 DPA (0O), 2 DPA (2O), and 7 DPA (7O), respectively. (A) Ring zinc finger protein (AI730621). (B) Fiber protein 37 (TC62849). (C) Transcription factor (TC74600). (D) GhMYB112b (TC75739). (E) GhRDL (AY072821). (F) ABC transporter (TC75912).
(TIF)
Sequence alignment of REPOSNSE TO DESSICATION 22 (RD22) and RD22-like ( RDL ) genes. GenBank accession numbers are AAL67991 (Gossypium hirsutum RDL1, GhRDL1), AAT66912 (Gossypium arboreum RDL1, GaRDL), and Q08298 (Arabidopsis thaliana RD22, AtRD22).
(DOC)
Effects of trichome regulatory genes on mucilage formation and columella cell morphology. Col-0: A. thaliana Col-0 shows relatively smooth seed surface. Hair-like structure was observed in try, etc, 35S:GFP:GhRDL1 and 35S:GFP:GhRDL1x35S:AtGL2 seeds. The seed surface was rough, but no obvious hair-like structure was observed in gl1 and gl3/egl3 seeds. The seed surface of gl2 seed was extremely smooth, suggesting loss of mucilage and columella cells.
(TIF)
Acknowledgments
We thank Xiao-Ya Chen for insightful discussions, Alan Lloyd for critical readings and suggestions to improve the manuscript, and the staff members in the Microscopy and Imaging Facility at The University of Texas at Austin for assistance in confocal and electron microscope imaging and laser capture microdissection.
Footnotes
Competing Interests: Part of the research is funded by Cotton Incorporated, a not-for-profit organization of cotton producers and users. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: The work was supported by the grants from the National Science Foundation Plant Genome Research Program (DBI1025947) and Cotton Incorporated (07-161). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lee JJ, Woodward AW, Chen ZJ. Gene expression changes and early events in cotton fibre development. Ann Bot (Lond) 2007;100:1391–1401. doi: 10.1093/aob/mcm232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szymanski DB, Lloyd AM, Marks MD. Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends Plant Sci. 2000;5:214–219. doi: 10.1016/s1360-1385(00)01597-1. [DOI] [PubMed] [Google Scholar]
- 3.Hülskamp M. Plant trichomes: a model for cell differentiation. Nat Rev Mol Cell Biol. 2004;5:471–480. doi: 10.1038/nrm1404. [DOI] [PubMed] [Google Scholar]
- 4.Pesch M, Hulskamp M. One, two, three…models for trichome patterning in Arabidopsis? Curr Opin Plant Biol. 2009;12:587–592. doi: 10.1016/j.pbi.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Esch JJ, Chen MA, Hillestad M, Marks MD. Comparison of TRY and the closely related At1g01380 gene in controlling Arabidopsis trichome patterning. Plant J. 2004;40:860–869. doi: 10.1111/j.1365-313X.2004.02259.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishida T, Kurata T, Okada K, Wada T. A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol. 2008;59:365–386. doi: 10.1146/annurev.arplant.59.032607.092949. [DOI] [PubMed] [Google Scholar]
- 7.Rerie WG, Feldmann KA, Marks MD. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev. 1994;8:1388–1399. doi: 10.1101/gad.8.12.1388. [DOI] [PubMed] [Google Scholar]
- 8.Li SF, Milliken ON, Pham H, Seyit R, Napoli R, et al. The Arabidopsis MYB5 transcription factor regulates mucilage synthesis, seed coat development, and trichome morphogenesis. Plant Cell. 2009;21:72–89. doi: 10.1105/tpc.108.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Western TL, Young DS, Dean GH, Tan WL, Samuels AL, et al. MUCILAGE-MODIFIED4 encodes a putative pectin biosynthetic enzyme developmentally regulated by APETALA2, TRANSPARENT TESTA GLABRA1, and GLABRA2 in the Arabidopsis seed coat. Plant Physiol. 2004;134:296–306. doi: 10.1104/pp.103.035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development. 2003;130:4859–4869. doi: 10.1242/dev.00681. [DOI] [PubMed] [Google Scholar]
- 11.Haughn G, Chaudhury A. Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci. 2005;10:472–477. doi: 10.1016/j.tplants.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Wang JW, Yu N, Li CH, Luo B, et al. Control of plant trichome development by a cotton fiber MYB gene. Plant Cell. 2004;16:2323–2334. doi: 10.1105/tpc.104.024844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan XY, Li QJ, Shan CM, Wang S, Mao YB, et al. The HD-Zip IV gene GaHOX1 from cotton is a functional homologue of the Arabidopsis GLABRA2. Physiol Plant. 2008;134:174–182. doi: 10.1111/j.1399-3054.2008.01115.x. [DOI] [PubMed] [Google Scholar]
- 14.Humphries JA, Walker AR, Timmis JN, Orford SJ. Two WD-repeat genes from cotton are functional homologues of the Arabidopsis thaliana TRANSPARENT TESTA GLABRA1 (TTG1) gene. Plant Mol Biol. 2005;57:67–81. doi: 10.1007/s11103-004-6768-1. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Machado AC, White RG, Llewellyn DJ, Dennis ES. Expression profiling identifies genes expressed early during lint fibre initiation in cotton. Plant Cell Physiol. 2006;47:107–127. doi: 10.1093/pcp/pci228. [DOI] [PubMed] [Google Scholar]
- 16.Lee JJ, Hassan OS, Gao W, Wei NE, Kohel RJ, et al. Developmental and gene expression analyses of a cotton naked seed mutant. Planta. 2005:1–15. doi: 10.1007/s00425-005-0098-7. [DOI] [PubMed] [Google Scholar]
- 17.Loguercio LL, Zhang JQ, Wilkins TA. Differential regulation of six novel MYB-domian genes defines two distinct expression patterns in allotetraploid cotton (Gossypium hirsutum L.). Mol Gen Genet. 1999;261:660–671. doi: 10.1007/s004380050009. [DOI] [PubMed] [Google Scholar]
- 18.Taliercio EW, Boykin D. Analysis of gene expression in cotton fiber initials. BMC Plant Biol. 2007;7:22. doi: 10.1186/1471-2229-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado A, Wu Y, Yang Y, Llewellyn DJ, Dennis ES. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. Plant J. 2009;59:52–62. doi: 10.1111/j.1365-313X.2009.03847.x. [DOI] [PubMed] [Google Scholar]
- 20.Udall JA, Flagel LE, Cheung F, Woodward AW, Hovav R, et al. Spotted cotton oligonucleotide microarrays for gene expression analysis. BMC Genomics. 2007;8:81. doi: 10.1186/1471-2164-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang SS, Cheung F, Lee JJ, Ha M, Wei NE, et al. Accumulation of genome-specific transcripts, transcription factors and phytohormonal regulators during early stages of fiber cell development in allotetraploid cotton. Plant J. 2006;47:761–775. doi: 10.1111/j.1365-313X.2006.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herwig R, Poustka AJ, Muller C, Bull C, Lehrach H, et al. Large-scale clustering of cDNA-fingerprinting data. Genome Res. 1999;9:1093–1105. doi: 10.1101/gr.9.11.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi YH, Zhu SW, Mao XZ, Feng JX, Qin YM, et al. Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. Plant Cell. 2006;18:651–664. doi: 10.1105/tpc.105.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruan YL, Llewellyn DJ, Furbank RT. Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. Plant Cell. 2003;15:952–964. doi: 10.1105/tpc.010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji S, Lu Y, Li J, Wei G, Liang X, et al. A beta-tubulin-like cDNA expressed specifically in elongating cotton fibers induces longitudinal growth of fission yeast. Biochem Biophys Res Commun. 2002;296:1245–1250. doi: 10.1016/s0006-291x(02)02069-7. [DOI] [PubMed] [Google Scholar]
- 26.Meinert MC, Delmer DP. Changes in Biochemical Composition of the Cell Wall of the Cotton Fiber During Development. Plant Physiol. 1977;59:1088–1097. doi: 10.1104/pp.59.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi-Shinozaki K, Shinozaki K. The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of rd22, a gene responsive to dehydration stress in Arabidopsis thaliana. Mol Gen Genet. 1993;238:17–25. doi: 10.1007/BF00279525. [DOI] [PubMed] [Google Scholar]
- 28.Batchelor AK, Boutilier K, Miller SS, Hattori J, Bowman LA, et al. SCB1, a BURP-domain protein gene, from developing soybean seed coats. Planta. 2002;215:523–532. doi: 10.1007/s00425-002-0798-1. [DOI] [PubMed] [Google Scholar]
- 29.Li CH, Zhu YQ, Meng YL, Wang JW, Xu KX, et al. Isolation of genes preferentially expressed in cotton fibers by cDNA filter arrays and RT-PCR. Plant Sci. 2002;163:1113–1120. [Google Scholar]
- 30.Basra A, Malik CP. Development of the cotton fiber. Int Rev Cytol. 1984;89:65–113. [Google Scholar]
- 31.Kirik V, Simon M, Huelskamp M, Schiefelbein J. The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev Biol. 2004;268:506–513. doi: 10.1016/j.ydbio.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Szymanski DB, Marks MD. GLABROUS1 overexpression and TRIPTYCHON alter the cell cycle and trichome cell fate in Arabidopsis. Plant Cell. 1998;10:2047–2062. doi: 10.1105/tpc.10.12.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, et al. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. Embo J. 2002;21:5036–5046. doi: 10.1093/emboj/cdf524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hülskamp M, Misra S, Jurgens G. Genetic dissection of trichome cell development in Arabidopsis. Cell. 1994;76:555–566. doi: 10.1016/0092-8674(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 35.Payne T, Clement J, Arnold D, Lloyd A. Heterologous myb genes distinct from GL1 enhance trichome production when overexpressed in Nicotiana tabacum. Development. 1999;126:671–682. doi: 10.1242/dev.126.4.671. [DOI] [PubMed] [Google Scholar]
- 36.Zhao M, Morohashi K, Hatlestad G, Grotewold E, Lloyd A. The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development. 2008;135:1991–1999. doi: 10.1242/dev.016873. [DOI] [PubMed] [Google Scholar]
- 37.Pu L, Li Q, Fan X, Yang W, Xue Y. The R2R3 MYB transcription factor GhMYB109 is required for cotton fiber development. Genetics. 2008;180:811–820. doi: 10.1534/genetics.108.093070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casson S, Spencer M, Walker K, Lindsey K. Laser capture microdissection for the analysis of gene expression during embryogenesis of Arabidopsis. Plant J. 2005;42:111–123. doi: 10.1111/j.1365-313X.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 39.Cai S, Lashbrook CC. Laser capture microdissection of plant cells from tape-transferred paraffin sections promotes recovery of structurally intact RNA for global gene profiling. Plant J. 2006;48:628–637. doi: 10.1111/j.1365-313X.2006.02886.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Llewellyn DJ, White R, Ruggiero K, Al-Ghazi Y, et al. Laser capture microdissection and cDNA microarrays used to generate gene expression profiles of the rapidly expanding fibre initial cells on the surface of cotton ovules. Planta. 2007;226:1475–1490. doi: 10.1007/s00425-007-0580-5. [DOI] [PubMed] [Google Scholar]
- 41.Jakoby MJ, Falkenhan D, Mader MT, Brininstool G, Wischnitzki E, et al. Transcriptional profiling of mature Arabidopsis trichomes reveals that NOECK encodes the MIXTA-like transcriptional regulator MYB106. Plant Physiol. 2008;148:1583–1602. doi: 10.1104/pp.108.126979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beasley CA. In vitro culture of fertilized cotton ovules. BioScience. 1971;21:906–907. [Google Scholar]
- 43.Luschnig C. Auxin transport: ABC proteins join the club. Trends Plant Sci. 2002;7:329–332. doi: 10.1016/s1360-1385(02)02292-6. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Steinebrunner I, Sun Y, Butterfield T, Torres J, et al. Apyrases (nucleoside triphosphate-diphosphohydrolases) play a key role in growth control in Arabidopsis. Plant Physiol. 2007;144:961–975. doi: 10.1104/pp.107.097568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark G, Torres J, Finlayson S, Guan X, Handley C, et al. Apyrase (nucleoside triphosphate-diphosphohydrolase) and extracellular nucleotides regulate cotton fiber elongation in cultured ovules. Plant Physiol. 2010;152:1073–1083. doi: 10.1104/pp.109.147637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hovav R, Udall JA, Chaudhary B, Hovav E, Flagel L, et al. The Evolution of Spinnable Cotton Fiber Entailed Prolonged Development and a Novel Metabolism. PLoS Genet. 2008;4:e25. doi: 10.1371/journal.pgen.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marks MD, Wenger JP, Gilding E, Jilk R, Dixon RA. Transcriptome analysis of Arabidopsis wild-type and gl3-sst sim trichomes identifies four additional genes required for trichome development. Mol Plant. 2009;2:803–822. doi: 10.1093/mp/ssp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai X, Wang G, Yang DS, Tang Y, Broun P, et al. TrichOME: a comparative omics database for plant trichomes. Plant Physiol. 2010;152:44–54. doi: 10.1104/pp.109.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim HJ, Triplett BA. Cotton fiber growth in planta and in vitro: Models for plant cell elongation and cell wall biogenesis. Plant Physiol. 2001;127:1361–1366. [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Tian L, Lee HS, Wei NE, Jiang H, et al. Genomewide nonadditive gene regulation in Arabidopsis allotetraploids. Genetics. 2006;172:507–517. doi: 10.1534/genetics.105.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soci (Series B) 1995;57:289–300. [Google Scholar]
- 52.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bulletin. 1945;1:80–83. [Google Scholar]
- 53.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 54.Tian L, Wang J, Fong MP, Chen M, Cao H, et al. Genetic control of developmental changes induced by disruption of Arabidopsis histone deacetylase 1 (AtHD1) expression. Genetics. 2003;165:399–409. doi: 10.1093/genetics/165.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murai K, Takumi S, Koga H, Ogihara Y. Pistillody, homeotic transformation of stamens into pistil-like structures, caused by nuclear-cytoplasm interaction in wheat. Plant J. 2002;29:169–181. doi: 10.1046/j.0960-7412.2001.01203.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of differentially expressed genes.
(XLS)
Number of differentially expressed genes.
(DOC)
Wilcoxon rank sum test of differentially expressed genes.
(DOC)
A subset of top differentially expressed genes in the epidermis or fibers.
(DOC)
A list of representative genes in each cluster.
(DOC)
Primer sequences used for RT-PCR and quantitative RT-PCR analyses.
(DOC)
Microarray experimental design and application of laser capture microdissection (LCM). (A) Development of cotton fiber. (B) Application of LCM in fibers (2 DPA, upper panel) and inner integuments of ovules (2 DPA, lower panel). The captured cells were removed (right photos in each panel). (C) Microarray experimental design. Arrows denote dye swaps used in each hybridization (red: Cy5, green: Cy3).
(TIF)
qRT-PCR validation of six differentially expressed genes detected by microarrays. Gene expression was analyzed in ten tissues, namely, leaves (L), petals (P), protodermal cells (−2E), fiber cell initials (0E, 0 DPA and 2E, 2 DPA), fibers (7F, 7 DPA), ovules at −2 DPA (−2O), 0 DPA (0O), 2 DPA (2O), and 7 DPA (7O), respectively. (A) Ring zinc finger protein (AI730621). (B) Fiber protein 37 (TC62849). (C) Transcription factor (TC74600). (D) GhMYB112b (TC75739). (E) GhRDL (AY072821). (F) ABC transporter (TC75912).
(TIF)
Sequence alignment of REPOSNSE TO DESSICATION 22 (RD22) and RD22-like ( RDL ) genes. GenBank accession numbers are AAL67991 (Gossypium hirsutum RDL1, GhRDL1), AAT66912 (Gossypium arboreum RDL1, GaRDL), and Q08298 (Arabidopsis thaliana RD22, AtRD22).
(DOC)
Effects of trichome regulatory genes on mucilage formation and columella cell morphology. Col-0: A. thaliana Col-0 shows relatively smooth seed surface. Hair-like structure was observed in try, etc, 35S:GFP:GhRDL1 and 35S:GFP:GhRDL1x35S:AtGL2 seeds. The seed surface was rough, but no obvious hair-like structure was observed in gl1 and gl3/egl3 seeds. The seed surface of gl2 seed was extremely smooth, suggesting loss of mucilage and columella cells.
(TIF)