Abstract
Osteoclasts are multinucleated cells that are responsible for resorption of bone, and increased activity of these cells is associated with several common bone diseases, including postmenopausal osteoporosis. Upon adhesion to bone, osteoclasts become polarized and reorganise their cytoskeleton and membrane to form unique domains including the sealing zone (SZ), which is a dense ring of F-actin-rich podosomes delimiting the ruffled border (RB), where protons and proteases are secreted to demineralise and degrade the bone matrix, respectively. These processes are dependent on the activity of small GTPases. Rho GTPases are well known to control the organization of F-actin and adhesion structures of different cell types, affecting subsequently their migration. In osteoclasts, RhoA, Rac, Cdc42, RhoU and also Arf6 regulate podosome assembly and their organization into the SZ. By contrast, the formation of the RB involves vesicular trafficking pathways that are regulated by the Rab family of GTPases, in particular lysosomal Rab7. Finally, osteoclast survival is dependent on the activity of Ras GTPases. The correct function of almost all these GTPases is absolutely dependent on post-translational prenylation, which enables them to localize to specific target membranes. Bisphosphonate drugs, which are widely used in the treatment of bone diseases such as osteoporosis, act by preventing the prenylation of small GTPases, resulting in the loss of the SZ and RB and therefore inhibition of osteoclast activity, as well as inducing osteoclast apoptosis. In this review we summarize current understanding of the role of specific prenylated small GTPases in osteoclast polarization, function and survival.
Key words: osteoclast, bone, Rab, Rho, Rac, Ras, Arf, bisphosphonate, prenylation
Introduction
Osteoclasts are large, multinucleated, motile cells that are formed by the fusion of hematopoietic cells of the monocyte/macrophage lineage.1,2 They are the only cells in the body that are able to degrade (resorb) extracellular bone matrix, a process that is required for bone morphogenesis during development, for the continual repair of microdamage in the skeleton and for adaptation of bone to mechanical load.3,4
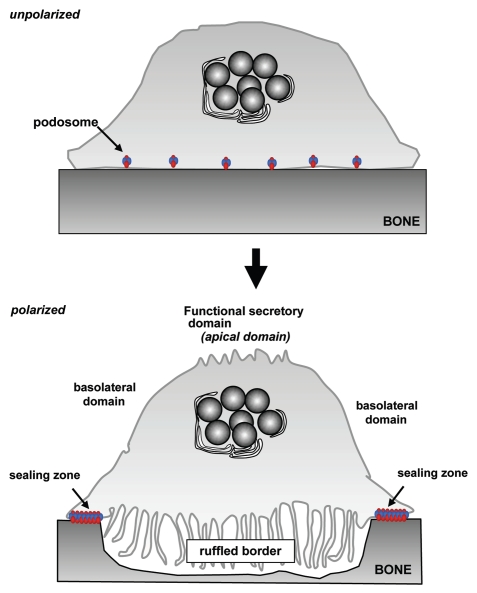
These cells represent a fascinating model of cell polarization because, when activated to resorb, osteoclasts reorganise their membrane into four distinct and unique domains, namely the sealing zone (SZ), the ruffled border (RB), the basolateral domain (BD) and the functional secretory domain (FSD) (Fig. 1). The SZ, where the osteoclast attaches tightly to the bone matrix, is formed by densely packed specific actin-rich adhesion structures called podosomes. These consist of a core of densely packed actin filaments and F-actin-associated proteins such as cortactin, WASp and Arp2/3, surrounded by integrins and attachment-related proteins such as vinculin and talin.5,6 Compared with focal adhesions, these structures are highly dynamic and have a lifespan of only 2–12 min.7,8 This tight attachment of the osteoclast to the extracellular matrix effectively seals off a compartment beneath the cell where bone degradation occurs (the resorption lacuna).9 Subsequently, polarized vesicular trafficking pathways result in the development of the membrane enclosed by the SZ into a highly convoluted ruffled border, where protons and proteases are secreted to demineralise and degrade the bone matrix, respectively.10–12 The resulting degradation products are then endocytosed at the RB, transported through the osteoclast by transcytosis, and finally released at the opposite side of the cell at the FSD.
Figure 1.
Alterations in membrane domains during osteoclast activation. Unpolarized, inactive osteoclasts present dispersed podosomes. During osteoclast activation, these podosomes coalesce into a peripheral belt and subsequently into distinct “actin ring” that forms the sealing zone where the osteoclast adheres tightly to the bone surface. Following this, trafficking of late endosomes/lysosomes toward the bone surface result in formation of the ruffled border, the resorptive organelle of the osteoclast. Finally, a membrane domain known as the functional secretory domain forms at the top of the cell, to which transcytotic vesicles formed at the ruffled border are targetted.
Small GTPases of the Ras superfamily, including the Ras, Rho, Arf, Ran and Rab subfamilies are key regulators of diverse cellular events, including cell division, vesicle transport, nuclear assembly and control of the cytoskeleton. In this review, their roles in osteoclast function will be highlighted, in particular the regulation of podosomes, vesicular trafficking and cell survival.
Pharmacological Evidence for the Importance of Small GTPases in Osteoclast Function
In general, small GTPases localize to specific membrane compartments. This property is dependent on post-translational prenylation, which involves the attachment of a hydrophobic isoprenoid lipid group (either a 15-carbon farnesyl or 20-carbon geranylgeranyl moiety) to a conserved cysteine residue contained within characteristic prenylation motifs in the C-terminus of most small GTPases. This modification is performed by one of three protein prenyl transferase enzymes (farnesyl transferase, geranylgeranyl transferase (GGTase) I or Rab GGTase), specificity being determined by the prenylation motif in the protein substrate.13 Most small GTPases, including the Rab subfamily and most of the Rho subfamily, are modified with geranylgeranyl groups; GTPases modified by farnesylation include most of the Ras proteins and some Rho family proteins.
The fundamental importance of small GTPases in osteoclast biology became apparent a decade ago, following our discovery that nitrogen-containing bisphosphonate (N-BP) drugs inhibit bone resorption by blocking protein prenylation.14,15 These “blockbuster” drugs are non-hydrolysable analogs of pyrophosphate that target rapidly to the skeleton and are used worldwide as the frontline treatment for common diseases associated with increased bone resorption, such as post-menopausal osteoporosis, cancer-associated bone disease and Paget's disease.16 N-BP drugs, such as risedronate, alendronate and zoledronate, are very effective inhibitors of bone resorption because they are internalised selectively into osteoclasts during the resorption of drug-coated bone.17,18 Once internalised, N-BPs disrupt osteoclast polarization (by preventing formation of the actin ring, disrupting normal vesicular trafficking and formation of the RB) and also cause osteoclast apoptosis.18 These effects of N-BPs are now known to be the result of disruption of protein prenylation, since these drugs mimic the structure of isoprenoid lipids such as geranyl diphosphate (GPP) and thereby potently inhibit farnesyl diphosphate (FPP) synthase, a key enzyme in the mevalonate pathway of isoprenoid and cholesterol biosynthesis.19–21 The exact mechanism of inhibition of FPP synthase has recently been clarified from the X-ray crystal structure of the enzyme and involves the “slow-tight” binding of the drug to one of the two isoprenoid lipid substrate pockets, resulting in conformational changes in the enzyme.22–24 Some N-BPs are also weak inhibitors of other enzymes in the mevalonate pathway, such as geranylgeranyl diphosphate (GGPP) synthase, squalene synthase and IPP isomerase.18
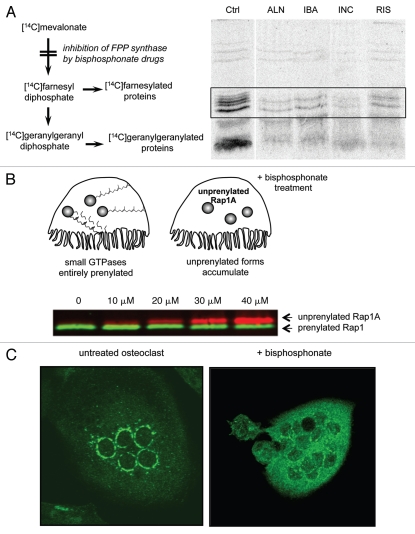
Inhibition of FPP synthase activity in osteoclasts results in the depletion of FPP and GGPP that are necessary for the prenylation of small GTPases. As a result, small GTPases accumulate in the unprenylated form and fail to localize to membrane compartments (Fig. 2).25 This effect can be easily measured in cultured cells by monitoring the metabolic incorporation of [14C] mevalonate into prenylated proteins (Fig. 2A),14,18,26 by protein gel blot analysis of cell lysates using an antibody that specifically hybridizes to the unprenylated form of the small GTPase Rap1A (Fig. 2B),27 or by visualizing the subcellular localization of small GTPases such as Rab6 (Fig. 2C).25
Figure 2.
Bisphosphonate drugs inhibit the prenylation of small GTPases. (A) Inhibition of protein prenylation by nitrogen-containing bisphosphonates can be demonstrated in vitro by culturing cells with [14C]mevalonate, which becomes incorporated into 14C-labeled, prenylated proteins. Radiolabelled, prenylated proteins (21–26 kD, boxed region) can then be detected by autoradiography following electrophoretic separation. Treatment with the bisphosphonate drugs alendronate (ALN), ibandronate (IBA), incadronate (INC) and risedronate (RIS) clearly inhibits prenylation compared with control (Ctrl) cells. Reproduced from Luckman et al.14 with permission of the American Society for Bone and Mineral Research. (B) Inhibition of protein prenylation by bisphosphonate drugs results in the accumulation of the unprenylated form of small GTPases in osteoclasts. The accumulation of unprenylated Rap1A (red) can be determined by protein gel blotting, for example after treatment of cultured cells with ≥10 µM zoledronate (image kindly provided by Gemma Shay). The unprenylated form (red) is of higher molecular mass than the prenylated form (green) due to lack of cleavage of the terminal tripepetide. (C) Inhibition of protein prenylation by bisphosphonate drugs alters the subcellular distribution of small GTPases such as Rab6. Multinucleated osteoclasts were immunostained for Rab6, which localizes to the perinuclear golgi in the untreated osteoclast (left) but has a cytosolic distribution in the osteoclast treated for 48 h with the bisphosphonate risedronate (right).
Since prenylated small GTPases act as molecular switches, their activity must be tightly controlled. Recent studies suggest that some unprenylated small GTPases such as Rac that accumulate in the cytosol after exposure of cells to N-BPs, are predominantly their active GTP-bound state (presumably due to lack of interaction with regulatory GAP proteins), causing inappropriate activation of downstream signaling kinases such as p38.28 Inhibition of the mevalonate pathway by N-BPs therefore appears to disrupt osteoclast function either by causing loss of prenylated proteins (and loss of downstream signaling) and/or accumulation of unprenylated proteins (and therefore inappropriate activation of downstream signaling pathways or sequestration of effectors).18 These effects on small GTPase signaling result in disruption of cytoskeletal organization and vesicular trafficking in osteoclasts, causing loss of the SZ and RB, and in osteoclast apoptosis. In particular, geranylgeranylated, rather than farnesylated, small GTPases are essential for osteoclast function and survival, since geranylgeraniol (a cell-permeable form of GGPP) overcomes the inhibition of osteoclast formation and bone resorption by N-BPs.15,29 Furthermore, small GTPases geranylgeranylated by GGTase I (such as Rho, Rac and Cdc42) are crucial for osteoclast function (see below), since an inhibitor of GGTase I (GGTI-298) closely mimics the effects of N-BPs on osteoclast polarization and survival.30 We also identified the first-known specific inhibitors of Rab GGTase (RGGT), which closely resemble the structure of N-BPs but contain a carboxylate moiety instead of one of the phosphonate groups.25,31 These phosphonocarboxylate (PC) compounds appear to inhibit RGGT by preventing the second geranylgeranylation step of Rab proteins that are normally di-geranylgeranylated, perhaps by binding to a GG-cysteine recognition site on RGGT that may be required to align non-geranylgeranylated Rabs for the second step of lipid modification.32 These agents specifically disrupt the prenylation and localization of Rab GTPases in osteoclasts and also inhibit bone resorption, by disrupting vesicular trafficking.25,31
The naturally occurring mouse strain known as gunmetal is an interesting in vivo model of defective Rab prenylation. This defect is characterized by a 75% reduction of RGGT activity due an autosomal recessive mutation in the gene encoding the α-chain of this enzyme.33 We have shown that macrophages and osteoclasts from these mice are more sensitive to the effects of PCs, thereby confirming that these drugs act through inhibition of RGGT.34 Moreover, although gunmetal mice do not exhibit an overt bone phenotype, osteoclasts generated in vitro from these mice exhibit a reduced resorptive capacity, despite being able to rearrange their cytoskeleton into F-actin rings.35 This phenotype closely mimics the effects of pharmacological inhibition of RGGT on osteoclasts31 and confirms that Rab prenylation is essential for osteoclast activity; however the identity of the underprenylated Rab GTPases that contribute to the resorptive defect remains unclear.
Regulation of Cytoskeletal Arrangement and Osteoclast Polarity by Small GTPases
Osteoclasts possess a unique podosomal organization that depends on the substrate to which they adhere. Adhesion onto glass coverslips causes the formation of podosome clusters which subsequently assemble into small rings of podosomes and eventually into a podosome belt around the periphery of the cell, in which individual podosomes can clearly be distinguished.7 In contrast, in osteoclasts seeded onto bone or bone-like mineralised substrates, the podosomes become tightly packed into a dense F-actin ring, also referred to as the sealing zone (SZ). Although individual podosomes cannot be distinguished in the SZ by classical fluorescence microscopy, high-resolution scanning electron microscopy combined with fluorescence microscopy has demonstrated that this structure is composed of a dense network of podosome cores interconnected by radial actin fibers, called the F-actin cloud.6,9 The difference in podosome organization on these substrates suggests that, while adhesion promotes podosome formation, other extracellular signals regulate the organization of the podosomes. Osteoclast attachment to the bone surface is mainly dependent on integrins, particularly the vitronectin receptor (αvβ3), one of the major integrins in osteoclasts,36,37 which is more abundant on these cells than any other cell type.38,39 The deletion of the β3 subunit in mice has dramatic effects on osteoclast spreading and cytoskeletal organization, resulting in a progressive osteopetrosis, indicating a reduction in osteoclast activity.37 Upon ligand binding, αvβ3 integrins recruit numerous proteins, including the non-receptor tyrosine kinases Pyk2, Src and Syk as well as the RING finger-containing ubiquitin ligase c-Cbl,40–42 which transduce signals to regulate both podosome assembly/disassembly and podosome organization. Osteoclasts from src, pyk2 or syk knockout mice are unable to form a proper sealing zone, demonstrating that these three tyrosine kinases all play an important role in podosome organization in osteoclasts.42–44 In contrast, the deficiency of c-Cbl ubiquitin ligase does not affect formation of the sealing zone, but the migration of osteoclasts is impaired both in vitro and in vivo, suggesting that Cbl might be involved in podosome turnover.41,45 The number of podosomes and their lifespan are reduced in src−/− osteoclasts suggesting that Src also plays a critical role in podosome formation and stability.46 Moreover, the rate of flux of actin monomers into the podosomes of Src or Pyk2 deficient mice is decreased, indicating that Src and Pyk2 kinases regulate actin polymerization.44,46
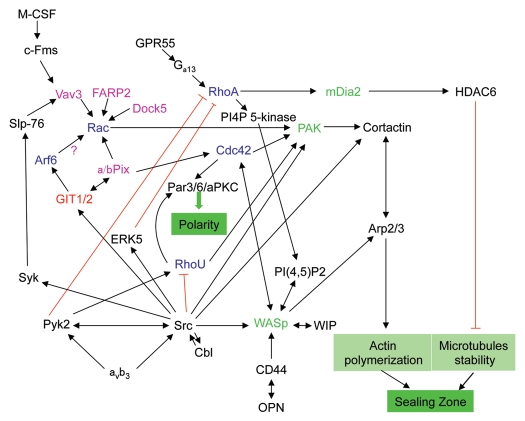
Given their role in regulation of the cytoskeleton, it is not surprising that Rho family GTPases are proving essential for the formation and organization of podosomes in osteoclasts and for polarization of these cells (Fig. 3). The Rho family of GTPases is composed of 20 members and transcript profiling analyses have revealed the presence of 18 Rho GTPases in osteoclasts.47 However, so far, only four members of the family have been identified to play a role in the regulation of cytoskeletal organization and polarization of osteoclasts: RhoA, Rac1, Cdc42 and RhoU.48 Recently, another family, the ARF GTPases, which are known to regulate membrane trafficking and actin dynamics,49 has been implicated in the regulation of the osteoclast cytoskeleton and the formation of the sealing zone.50
Figure 3.
Role of small GTPases in signaling pathways regulating the polarization of osteoclasts. Upon adhesion of osteoclasts to the bone matrix, engagement of the vitronectin receptor (αvβ3) and CD44 activates several downstream signaling pathways to promote the formation of the sealing zone and osteoclast polarization. These pathways involve a variety of small GTPases (blue), GAPs (red), GEFs (pink) and GTPase effectors (green) and can also be activated via other receptors such as the M-CSF receptor and the G protein-coupled receptor GPR55.
Rho
Rho was the first GTPase to be studied in osteoclasts. Inhibition of RhoA, RhoB and RhoC using the Clostridium botulinum C3 exoenzyme disrupted SZ formation, leading to inactivation of osteoclasts.51 Indeed, when osteoclasts are cultured on bone, C3 exoenzyme induces osteoclast spreading and the reorganisation of podosomes from a SZ into a belt at the periphery of the cell, suggesting that RhoA activity is required for SZ formation, but not for actin belt formation.52 Consistent with this, we recently showed that cannabinoid agonists of the G-protein coupled receptor GPR55 stimulate osteoclast polarization and bone resorption, which is associated with activation of RhoA, probably via Gα13.53 Moreover, microinjection of constitutively activate RhoA into osteoclasts cultured on glass coverslips did not trigger the formation of a sealing zone but caused disruption of the podosome belt and the creation of podosome clusters, suggesting that RhoA activation alone is not sufficient to induce SZ formation and that this requires additional signals from the bone matrix or more complex regulation of RhoA activity.54–56 Indeed, several observations confirm the importance of regulating RhoA activity in order to induce podosome and sealing zone formation. On the one hand, active RhoA induces the synthesis by phosphatidylinositol-4-phosphate-5-kinase (PI4P-5 kinase) of phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2), which subsequently interacts with WASp to promote sealing zone formation.57 On the other hand, in Src-transformed cells, ERK5 limits Rho activation by upregulating the expression of RhoGAP7 and thereby induces podosome formation.58 Moreover, in osteoclasts, activated Rho induces HDAC6-mediated deacetylation of microtubules through its effector mDia2 leading to disruption of the SZ.56 Pyk2-deficient osteoclasts, which have a decrease in microtubule acetylation and SZ formation, have increased Rho activity suggesting that Pyk2 downregulates Rho activity to allow the stabilization of the microtubules and SZ.44 This demonstrates that a tight regulation of RhoA activity is essential to osteoclast polarization.
Rac
As in other cell types, Rac1 mediates lamellipodia formation and cell spreading in osteoclasts, for example in response to M-CSF, which promotes osteoclast spreading and motility.59,60 Its role in podosome organization is less well understood. Ory et al. reported that Rac has opposite effects to Rho in multinucleated giant cells, since expression of constitutively active Rac promoted lamellipodia formation and cell spreading whereas expression of dominant negative Rac caused cell retraction and podosome disorganisation.54,60 Razzouk et al. took an alternative approach involving the use of neutralising antibodies to Rac1 and Rac2, to demonstrate that inhibition of Rac activity disrupted actin rings, reduced resorptive activity and caused retraction of rat osteoclasts.61 More recently, osteoclasts from rac2−/− mice have been shown to have defects in SZ formation and M-CSF-induced migration in vitro, leading to a decrease in osteoclast activity both in vitro and in vivo,62 therefore confirming the important role played by Rac proteins in podosome and sealing zone formation.
Relatively little is known about the regulation of Rac and Rho by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) in osteoclasts. There are over 70 RhoGEFs in mammals, belonging to two different groups: the Dbl-homology domain containing proteins and the CZH proteins.63,64 Transcript profiling analyses have revealed the expression of 42 RhoGEFs in osteoclasts including Vav3, FARP2, Dock5 and αPIX.47 Currently, there is evidence that several of these that specifically regulate Rac activity play a role in osteoclasts.
All three Vav proteins are expressed by osteoclasts but so far only Vav3 has been shown to play a crucial role in osteoclasts,65 mediating M-CSF-induced cytoskeletal rearrangement and osteoclast spreading via association with Rac1.60 Deficiency of Vav3 in mice leads to osteopetrosis due to a defect in bone resorbing osteoclasts, which exhibit defective actin rings and loss of polarization.65 FARP2 is a Dbl family GEF specific for Rac1 that is important for cytoskeletal organization.66 Its expression is upregulated during osteoclastogenesis and it localizes to the actin core of podosomes.67 Overexpression of a dominant negative form of FARP2 lacking the RacGEF domain results in the disorganisation of the actin belt and in a lack of bone resorption.
Dock5, a Rac1 GEF of the CZH family, has been also recently been implicated in SZ formation.68 Expression of Dock5 is upregulated during RANKL-induced osteoclastogenesis.47 Osteoclasts from Dock5 knockout mice have decreased Rac1 activity and a disrupted actin cytoskeleton,68 further indicating that Rac1-regulated pathways play an important role in formation of the SZ.
ARF6
Recently, ARF6, which is known to regulate the plasma membrane localization of Rac1,69,70 has been identified to be necessary for sealing zone formation.50,69 GIT proteins (GIT1 and GIT2), which are ARF-GAPs, are targets of Src kinase and are localized to the sealing zone in polarized osteoclasts.50,71 GIT2 has been shown to downregulate ARF activity and possibly Rac1 recruitment at the SZ, thereby maintaining osteoclast polarity.50 Moreover, osteoclasts from GIT1 knockout mice have a disrupted actin cytoskeleton, confirming the importance of GIT proteins in the formation of the SZ.50 GIT proteins can interact with α and βPIX, exchange factors for Rac1 and Cdc42 that surround the actin core of podosomes in macrophages where they control podosome formation72 and coordinate Rho and ARF-induced cytoskeletal rearrangements and focal adhesion turnover.73,74 PIX proteins are also involved in the formation of podosome-like F-actin columns in phorbol ester treated vascular smooth muscle cells.75 However, αPIX was found to be unnecessary for the ARF6/GIT2-mediated regulation of SZ formation, suggesting that another GEF is involved in the coordination of ARF6 and Rac1/Cdc42 signaling pathways in osteoclasts.50 The ARF6/Rac1 pathway may also play a role in lysosomal trafficking (see section on Rab7 effectors).
Cdc42
Cdc42 is an important regulator of actin polymerization through its direct binding to Wiscott-Aldrich Syndrome proteins (WASp and N-WASP), which induces a conformational change to allow the binding of WASp to the Arp2/3 complex, thereby promoting actin nucleation.76,77 Microinjection of activated Cdc42 into osteoclasts leads to disruption of podosome organization, but following subsequent treatment with osteopontin, there is an increase in the interaction between WASp and Arp2/3 promoting actin polymerization, suggesting that Cdc42 alone cannot activate WASp in osteoclasts.57 Cdc42 can also regulate polarity of epithelial cells by interacting with the Par polarity complex containing Par-3, Par-6 and atypical PKC.78 The expression of these proteins in osteoclasts suggests that the association of Cdc42 and Par-6 could control osteoclast polarization.79 Although the depletion of Cdc42 in osteoclasts did not affect the formation of actin rings, when cell polarization was disrupted by serum starvation the recovery of actin rings was delayed, indicating that Cdc42 plays an important but non-essential role in establishing osteoclast polarization.
Osteoclasts from WASp knockout mice exhibit a reduction in podosome formation and therefore are unable to polarize.80 Moreover, the phosphorylation of WASp at Tyr-291 is regulated by several kinases, including Src, which increases Arp2/3-mediated actin polymerization and SZ formation.81,82 WASp also interacts with WIP (WASp-interacting protein), which induces its localization to podosomes and protects against calpain-dependent degradation.83 Deficiency of WIP in osteoclasts induces a reduction in WASp protein level and in the number of podosomes; both of these changes can be restored by the activation of CD44 receptors, confirming the critical role of WASp in osteoclast polarization.84
The other group of Rho family effector molecules implicated in podosome formation is the p21-activated kinases (PAK) family, which contains six members that can interact with both Cdc42 and Rac to mediate cytoskeleton rearrangement.85 Expression of dominant negative PAK4 mutants resulted in reduced size and number of podosomes in macrophages.72 Moreover, expression of a kinase-active PAK1 mutant induced the formation of podosome-like F-actin columns in vascular smooth muscle cells.75 Cortactin, an F-actin binding protein that interacts with the Arp2/3 complex to stimulate actin nucleation and to regulate podosome formation,86,87 can be phosphorylated by PAK thereby affecting its binding to actin in vascular smooth muscle cells.88 In osteoclasts, neutralizing antibodies to PAK1 disrupted actin rings, similar to the effects of neutralising anti-Rac antibodies, suggesting that PAK proteins promote not only podosome formation but also mediate Rac-induced sealing zone formation.61
RhoU
The most recent member of the Rho GTPase family implicated in the regulation of osteoclast cytoskeleton is RhoU, also known as Wrch1 (Wnt1-Responsive Cdc42 Homolog 1). RhoU shares significant sequence and functional similarity with Cdc42, but unlike Cdc42, RhoU has an extremely rapid, intrinsic guanine nucleotide exchange activity.89 Moreover, RhoU has putative Src homology 3 (SH3) domain-interacting motifs in its N-terminal region. In contrast to RhoA, Rac1 and Cdc42, RhoU expression is upregulated during osteoclast differentiation.47 RhoU colocalizes with vinculin around the actin cores in cells that form podosomes, i.e., in osteoclasts and in Hela cells transfected with a constitutively active form of Src.90 Studies in other cell types have indicated that RhoU is involved in cytoskeletal reorganisation and in cell polarity. In MDCK cells, the interaction of RhoU and Par-6 regulates the tight junction assembly necessary for epithelial cell polarization and for cystogenesis.91 Moreover, Src phosphorylation of RhoU at Tyr-254 is required for cystogenesis in 3D culture of MDCK cells.92 In H1299 cells, Src-mediated RhoU phosphorylation induces its subcellular relocalization and downregulates its binding to GTP, leading to a decrease in RhoU-induced PAK and Pyk2 autophosphorylation.92 However, in osteoclasts, overexpression of wild-type and constitutively active RhoU destabilizes the podosome belt and increases the formation of actin clusters but has no effect on sealing zone formation or in vitro bone resorption,93 suggesting that RhoU is not required for osteoclast polarization and its role in osteoclasts remains to be determined.
Regulation of Vesicular Trafficking in Osteoclasts by Small GTPases
Vesicular trafficking processes are essential in osteoclasts to establish and maintain cell polarization, release the mediators of bone resorption into the resorption lacuna, and to recycle receptors such as integrins that promote osteoclast motility and function. The crucial membrane domain for bone resorption, which is formed as a result of vesicular trafficking and fusion, is the RB, a highly convoluted membrane that forms only once osteoclasts have undergone cytoskeletal rearrangement to form the SZ.10–12 Unlike conventional plasma membranes, the RB expresses a number of proteins associated with the endosomal/lysosomal pathway, such as LAMP1, LAMP2, lgp110 and Rab7, defining the RB as a lysosomal membrane;12,94 markers of early endosomes, such as EEA1, are not found at the RB, but are restricted to vesicles within the cytoplasm of osteoclasts.95 The lysosomal nature of the RB is not surprising, as the degradation of the bone matrix requires acidification of the resorption lacuna and the release of proteases that degrade the organic matrix. This is mediated both by the fusion of lysosomes at the RB, which directly liberates acid into the resorption lacuna, and by the continued action of the V-ATPase that is inserted into the RB as a result of this trafficking pathway.96 The V-ATPase acidifies late endosomes and lysosomes by pumping protons generated by carbonic anhydrase II into the lumen of the vesicle.97 To maintain electroneutrality of the vesicles, chloride ions are transported into the lumen by the H+/Cl− antiporter ClC-7, which is highly expressed in osteoclasts and is also delivered to the RB by lysosomal trafficking.98 Secretion of proteases, in particular cathepsin K, via this pathway mediates the degradation of the organic matrix, which is predominantly type I collagen.99–101 This endocytic pathway that is responsible for the formation and maintenance of the RB originates from the basolateral membrane, since endosomal markers such as HRP and iron-loaded transferrin are internalised into endosomes from this membrane domain before delivery to the peripheral area of the RB close to, but distinct from, the sealing zone.102 Although podosomes and invadopodia are known to mediate matrix degradation via the release of metalloproteases such as MMP9 that can degrade collagen, it is cathepsin K delivered through the endocytic pathway that is critical for bone degradation,102 and the sealing zone itself is not the main site of bone resorption.
Rab proteins, the largest family of small GTPases, comprising more than 70 members in humans,103 are the master regulators of vesicular trafficking, playing a role in vesicle budding, transport and fusion with destination membranes. While many Rab proteins are likely to play similar fundamental roles in all cell types, the expression of some Rabs is restricted to specialized cell types, where they have a distinct function; for example, Rab17 is specifically expressed by polarized epithelial cells, where it mediates the apical polarized transport of recycling endosomes.104 Since osteoclasts exhibit unique vesicular trafficking pathways to maintain their polarization, it is likely that some Rab proteins play unique roles in osteoclast function (Fig. 4). One strong candidate that is known to have restricted tissue distribution is Rab27a, since this is involved in trafficking of “secretory lysosomes” in other cell types such as melanocytes and cytotoxic T cells. However, Rab27a is not expressed in osteoclasts, despite being present in precursor cells, while the ashen mouse that is deficient in Rab27a does not have an overt bone phenotype (Coxon FP and Seabra MC, unpublished data). Intriguingly though, Rab27a and b isoforms are expressed in bone-forming osteoblasts, where they play a role in the trafficking of RANK ligand-expressing vesicles to the plasma membrane.105 This is therefore likely to impact on osteoclast activity in vivo, since RANK ligand is essential for the differentiation and function of osteoclasts.
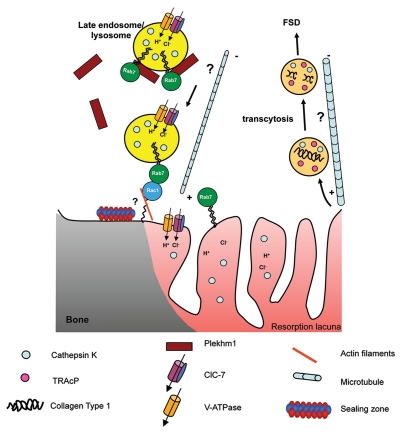
Figure 4.
Hypothetical model for the transport of late endosomes/lysosomes to the ruffled border in osteoclasts. Rab7 is localized to late endosomes/lysosomes and to the peripheral area of the ruffled border (close to the sealing zone) in osteoclasts and regulates the trafficking of these vesicles toward the plus end of microtubules. The other mediators of this process remain unknown, although the Rab7-binding protein Plekhm1, which is recruited to late endosomes/lysosomes by Rab7, is likely to be involved, since osteoclasts from osteopetrosis patients with mutations in this protein have defective ruffled borders. One possibility is that Plekhm1 may bridge Rab7 and a kinesin motor to enable trafficking to occur on microtubules. Rab7 has been shown to interact directly with Rac1 close to the sealing zone, and it has been postulated that this interaction may mediate the transfer of the late endosomes/lysosomes from the microtubule network to the cortical actin network prior to fusion with the ruffled border acceptor membrane and the release of cathepsin K and acid at the periphery of the RB into the resorption lacuna. This process also serves to insert the V-ATPase, ClC-7 and Rab7 into the ruffled border membrane. The V-ATPase itself may also have a role in this process, since subunits of the pump have been shown to bind to actin microfilaments. Transcytotic vesicles, which are involved in the further degradation and removal of collagen fragments and are trafficked on microtubules to the functional secretory domain (FSD), originate from the central region of the ruffled border; as yet the Rab GTPases governing this pathway remain unknown.
While studies have identified the expression of Rab1B, Rab3A/B/C/D, Rab4B, Rab5C, Rab7, Rab9, Rab10, Rab11B and Rab35 in osteoclasts,95,106,107 little is known about the function of most of these Rabs in osteoclasts. Rab9 colocalizes with Rab7 to late endosomes, but not to the RB in resorbing osteoclast.95,106 Rab6 is also highly expressed at the protein level and displays a perinuclear Golgi localization, but its exact role remains undefined.32,108 Rab11 is localized in perinuclear recycling compartments in osteoclasts where it may be involved in ruffled border membrane turnover, promoting osteoclast migration and resorption.95 However, a definitive role in vesicular trafficking to the RB of osteoclasts has been ascribed to only Rab7 and Rab3D thus far.
Rab7
Rab7 mediates heterotypic fusion between early endosomes and late endosomes, and between late endosomes and lysosomes.109,110 It also regulates the retrograde trafficking of late endosomes along microtubules toward the microtubule organizing center through interactions with the effector protein RILP and the motor protein dynein.111 Given the importance of this trafficking pathway in RB formation, Rab7 may therefore be involved in RB formation by controlling both the fusion of late/mature endosomes with each other and with the RB,12 and/or the trafficking of these vesicles on microtubules. In support of this, Rab7 co-localizes with markers of the ruffled border and of late endosomes, but not with markers of early endosomes or the Golgi apparatus in osteoclasts.12,112 In addition, antisense knockdown studies have confirmed that Rab7 is essential for RB formation and resorption by osteoclasts, and for trafficking of the V-ATPase to the RB.112 While transferrin uptake into early endosomes is normal in Rab7-depleted cells, trafficking to the RB is disrupted, providing further evidence that Rab7 regulates the latter stages of the endosomal pathway from the basolateral membrane to the RB.112
Rab7 Effectors
There is currently no evidence for a role of the established Rab7 effectors, such as RILP, ORP1L, Rabring7, hVPS34 or FYCO-1 in osteoclast function. However, it has been shown that Rab7 interacts with the small GTPase Rac1 in osteoclasts in a GTP-dependent manner.113 As outlined above, Rac1 plays an important role in formation of the sealing zone and both Rac1 and Rab7 colocalize at the fusion zone of the RB, just inside the sealing zone. It has been hypothesized that this association may be required for the fusion of late endosomes at the RB, by controlling the switch of late endosomes from microtubule-based transport to the cortical actin network (Fig. 4). In this respect, an analogy can be drawn with the transport of melanosomes in melanocytes, which require the interaction of Rab27a with melanophilin to switch from microtubule to actin-based transport in peripheral dendrites.114 However, Arf6-activated Rac1 has been shown recently to recruit an effector Armus, a member of the TBC/RabGAP family, which leads to Rab7 inactivation and promotes E-cadherin lysosomal degradation, suggesting another possible function for the Rac1/Rab7 complex.115 Moreover, there is also evidence that the V-ATPase on the late-endosomes/lysosomes may be the mediator of their tethering at the cortical actin network by directly binding to F-actin.116,117
Interestingly, the protein Plekhm1 has been identified as another putative effector of Rab7 in osteoclasts.118 Mutations in Plekhm1 are a cause of the bone disease osteopetrosis, in which the osteoclasts are dysfunctional due to defective RB formation, suggesting that Plekhm1 is involved in Rab7-dependent endosomal/lysosomal transport to the RB.118 Plekhm1 is a large, cytosolic protein that also shows some localization on endosomal/lysosomal vesicles, to which it is recruited to by GTP-bound Rab7.118 The osteopetrosis-causing mutation results in the introduction of a premature stop codon, and therefore a truncated form of Plekhm1 that fails to localize to endosomes/lysosomes, suggesting that transport of lysosomes to the RB in osteoclasts depends on the association of Plekhm1 with Rab7. The physical interaction between Plekhm1 and Rab7 has recently been confirmed, although intriguingly in this study the authors suggest that Plekhm1 negatively regulates the endocytic pathway in Hela cells.119 Interestingly, another study reported a point mutation (R714C) in Plekhm1 mutation that has no effect on Plekhm1 localization, but causes generalized osteopenia (low bone mass), suggesting that this may be a gain-of-function mutation.120 Taken together, we propose a model (Fig. 4) in which Plekhm1 may link Rab7 to microtubule-based transport of the late endosomes/lysosomes toward the RB (for example through interacting with a kinesin motor), where an interaction between Rab7 and Rac1 may enable the transfer from the microtubules to the cortical actin network.
Rab7 is also necessary for the fusion of lysosomes with autophagosomes to form autolysosomes and enable degradation of the material within the autophagosome.121 In addition, the transport of autolysosomes along microtubules is promoted by the interaction of Rab7 with its effector FYCO1.122 Interestingly, it has recently been suggested that the RB also exhibits characteristics of autophagosomal membranes, such as expression of the protein Atg8/LC3, which participates in the recruitment of material to be degraded by autophagy as well as fusion of autophagosomes with lysosomes.123,124 This raises the intriguing possibility that the fusion of lysosomes with the RB could be mediated via LC3, and/or that autophagosomes are trafficked to the RB in osteoclasts. A recent paper indicates that LC3 also plays a role in phagosomes in macrophages, supporting the idea that LC3 localization and function may not be restricted to autophagosomes.125
Rab3D
Rab3 proteins are involved in regulated exocytosis and are therefore mostly expressed in cells with high secretory requirements, particularly neurons and neuroendocrine cells.126,127 Of the Rab3 isoforms, Rab3D is highly expressed in osteoclasts both at the mRNA and protein levels while expression of Rab3A, Rab3B and Rab3C is much lower.95,107 In addition, Rab3D is essential for osteoclast function, since Rab3D-deficient mice exhibit an osteosclerotic (high bone mass) phenotype due to defective resorption by osteoclasts, which can be replicated by overexpression of a dominant negative form of Rab3D in osteoclasts in vitro.107 Moreover, osteoclasts from the Rab3D-deficient mice exhibit an abnormal RB, suggesting a defect in vesicular trafficking to this domain. However, Rab3D does not appear to be involved in the lysosomal trafficking pathway, since it localizes to a subpopulation of post-TGN vesicles in osteoclasts. Rab3D may therefore regulate the biogenesis of a secretory compartment from the TGN, although it is unclear whether this compartment represents a completely distinct vesicular population from the Rab7-regulated endocytic pathway or whether these vesicles merge with the endocytic pathway in order to reach the RB. Rab3D may regulate the trafficking of these putative secretory vesicles by recruiting the dynein motor component Tctex-1, with which it binds in a GTP-dependent manner.128
Transcytosis
A high concentration of degraded collagen fragments, calcium and phosphate are created by the degradation of the bone matrix, which need to be removed from the resorption lacuna so that they can ultimately be excreted in the urine. However, the SZ is impermeable to molecules larger than 10 kDa suggesting the existence of a cellular route to clear the lacuna of these products.128 Indeed, bone degradation products can be detected within intracellular vesicles, at a specific domain of the basal membrane and in the extracellular space close to this membrane,130,131 suggesting that these degradation products are trafficked though the osteoclast by transcytosis.
This process may play a role in balancing the membrane domains in osteoclasts, as well as enabling further degradation of matrix fragments through the action of tartrate-resistant acid phosphatase (TRAcP).132 The central region of the basolateral membrane domain, to where the transcytotic vesicles are trafficked, has therefore become known as the functional secretory domain (FSD), and has characteristics of the apical membrane in epithelial cells.10,94 Consistent with the transcytosis model, the central area of the RB exhibits extensive endocytic activity and vesicle coat proteins such as clathrin and AP-2 are restricted to this domain, together with the GTPase dynamin,102,133 which is crucial for separating the budding vesicles from the membrane. Similarly, to compensate for the membrane loss during the matrix uptake process, recent evidence suggests that there is an additional ‘reverse’ transcytotic pathway that delivers membrane from the FSD to the RB.134
Despite the elucidation of these unique trafficking pathways in osteoclasts, the Rab GTPases that regulate them remain completely unknown. In epithelial cells, Rab17 is involved in transcytosis of the polymeric immunoglobulin receptor from the basolateral to the apical domain,135 while a more recent report indicates that the Rab11 effector Rab11-FIP5 also plays a role in this process.136 However, it is difficult to extrapolate these findings to osteoclasts, given the unique nature of the membranes between which transcytosis occurs.
Small GTPases and Osteoclast Survival
Small GTPases play an important role in osteoclast survival, exemplified by the ability of bisphosphonate drugs to induce osteoclast apoptosis as a result of inhibiting the prenylation of these proteins.137 Ras GTPases are key regulators of cell survival and are activated by numerous cell surface receptors to induce key signal transduction pathways, including ERK, MAP kinase and PI3-kinase.138 When activated, Ras recruits Raf-1, a serine/threonine kinase that subsequently phosphorylates and activates MEK (MAPK/Erk kinase), that, in turn, phosphorylates and activates Erk1 and Erk2, which are required for cell survival.36,138 Another Ras effector is phosphatidylinositol-3-kinase (PI3K), which activates the pro-survival serine/threonine kinase Akt/PKB.139 Overexpression of constitutively active Ras in murine osteoclasts promotes cell survival, which was blocked by inhibition of PI3K or Raf-1, demonstrating the involvement of both Ras-induced pathways to osteoclast survival.140 Confirming the implication of the Raf-1-dependent pathway, a constitutively active form of MEK greatly increases osteoclast survival, without affecting bone-resorbing activity.141
PAK1 has recently been described to activate expression of survivin downstream of Raf-1 in a MEK-independent manner, promoting osteoclast survival.142 Mutations in the nf1 gene, coding for the Ras GAP Neurofibromin, leads to neurofibromatosis type 1, a disease characterized by neurofibroma tumors, skin pigmentation defects, osseous malformations, learning defects and predisposition to selected malignancies.143 Nf1 patients have bone loss due to defects in both osteoblasts and osteoclasts.143 Osteoclasts from these patients survive longer in the absence of serum than control osteoclasts confirming Ras as an essential regulator of osteoclast survival.144
Although Ras plays a role in osteoclast survival, its effect on osteoclast activity remains unclear. Indirectly interfering with the function of most forms of Ras by inhibiting its prenylation (using the specific inhibitor of farnesyl transferase, FTI-277), had no effect on the cytoskeleton or activity of rabbit osteoclasts.30 In contrast, dominant negative Ras expression in avian osteoclasts increased lysosomal enzyme secretion, suggesting that Ras may negatively regulate osteoclast activity.145 Moreover, murine osteoclasts from Nf1/− mice have increased Ras activity, actin ring formation and resorption.146,147 This effect is mediated by Rac, as the absence of Rac abolishes the Nf1-induced osteoclast polarization and activity.147 The precise role of Ras GTPases in osteoclasts therefore remains to be fully clarified.
Little is known about the role of other small GTPases in osteoclast survival. However, the ability of the specific GGTase I inhibitor, GGTI-298, to potently induce apoptosis in osteoclasts suggests that the Rho family proteins may be involved in this process.30 Indeed, overexpression of a dominant negative form of Rac1 in mouse osteoclasts induces apoptosis and decreases bone resorption while the constitutively active Rac1 increases cell survival PI3K-dependent manner suggesting that Rac acts not only on osteoclast cytoskeleton but also promotes osteoclast survival.59
Conclusion
In conclusion, it is clear that osteoclast function, and hence bone resorption, is highly dependent on small GTPases of the Ras, Rho and Rab families, which all require post-translational prenylation for their correct localization and function. Inhibition of prenylation of small GTPases is the major mechanism of action of the drugs that are most widely used to inhibit bone resorption in common metabolic bone diseases. Studies into the role of specific small GTPases has revealed further insights into the unique cytoskeletal arrangement and vesicular trafficking routes in resorbing osteoclasts. However, further such studies are required to fully understand how these pathways are regulated in this fascinating cell type.
Acknowledgments
The authors' current research on small GTPases in osteoclasts is funded by program grant 17285 and project grant 19379 from Arthritis Research UK.
Abbreviations
- ARF
ADP-ribosylation factor
- BD
basolateral domain
- CZH
CDM-zizimin homology
- ERK
extracellular regulated kinases
- FPP
farnesyl diphosphate
- FSD
functional secretory domain
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- GGPP
geranylgeranyl diphosphate
- GGTase
geranylgeranyl transferase
- GPP
geranyl diphosphate
- HDAC
histone deacetylase
- HRP
horseradish peroxidase
- IPP
isopentenyl diphosphate
- MMP
matrix metalloprotease
- M-CSF
macrophage-colony stimulating factor
- MEK
MAPK/Erk kinase
- N-BP
nitrogen-containing bisphosphonate
- Nf1
neurofibromin
- PAK
p21-activated kinase
- PI3K
phosphatidylinositol-3-kinase
- PI4P-5 kinase
phosphatidylinositol-4-phosphate-5-kinase
- PI(4,5) P2
phosphatidylinositol-4,5-bisphosphate
- PKC
protein kinase C
- RANK
receptor activator of NFkappaB
- RB
ruffled border
- RGGT
Rab geranylgeranyl transferase
- SZ
sealing zone
- TGN
trans-Golgi network
- TRAcP
tartrate-resistant acid phosphatase
- V-ATPase
vacuolar-type H+-ATPase
- WASp
wiscott-aldrich syndrome proteins
- WIP
WASp-interacting protein
References
- 1.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 4.Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113:377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 5.Spinardi L, Marchisio PC. Podosomes as smart regulators of cellular adhesion. Eur J Cell Biol. 2006;85:191–194. doi: 10.1016/j.ejcb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: Specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block MR, Badowski C, Millon-Fremillon A, Bouvard D, Bouin AP, Faurobert E, et al. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Luxenburg C, Geblinger D, Klein E, Anderson K, Hanein D, Geiger B, et al. The architecture of the adhesive apparatus of cultured osteoclasts: From podosome formation to sealing zone assembly. PLoS One. 2007;2:179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulari M, Vaaraniemi J, Vaananen HK. Intracellular membrane trafficking in bone resorbing osteoclasts. Microsc Res Tech. 2003;61:496–503. doi: 10.1002/jemt.10371. [DOI] [PubMed] [Google Scholar]
- 11.Lakkakorpi PT, Vaananen HK. Cytoskeletal changes in osteoclasts during the resorption cycle. Microsc Res Tech. 1996;33:171–181. doi: 10.1002/(SICI)1097-0029(19960201)33:2<171::AID-JEMT7>3.0.CO;2-W. DOI: 2-W. [DOI] [PubMed] [Google Scholar]
- 12.Palokangas H, Mulari M, Vaananen HK. Endocytic pathway from the basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. J Cell Sci. 1997;110:1767–1780. doi: 10.1242/jcs.110.15.1767. [DOI] [PubMed] [Google Scholar]
- 13.Zhang FL, Casey PJ. Protein prenylation: Molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 14.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 15.Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, et al. Alendronate mechanism of action: Geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption and kinase activation in vitro. Proc Natl Acad Sci USA. 1999;96:133–138. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 17.Coxon FP, Thompson K, Roelofs AJ, Ebetino FH, Rogers MJ. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone. 2008;42:848–860. doi: 10.1016/j.bone.2007.12.225. [DOI] [PubMed] [Google Scholar]
- 18.Rogers MJ, Crockett JC, Coxon FP, Monkkonen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2010 doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 19.van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. 1999;264:108–111. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- 20.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- 21.Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9:2643–2658. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- 22.Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, et al. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci USA. 2006;103:7829–7834. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rondeau JM, Bitsch F, Bourgier E, Geiser M, Hemmig R, Kroemer M, et al. Structural basis for the exceptional in vivo efficacy of bisphosphonate drugs. ChemMedChem. 2006;1:267–273. doi: 10.1002/cmdc.200500059. [DOI] [PubMed] [Google Scholar]
- 24.Dunford JE, Kwaasi AA, Rogers MJ, Barnett BL, Ebetino FH, Russell RG, et al. Structure-activity relationships among the nitrogen containing bisphosphonates in clinical use and other analogues: Time-dependent inhibition of human farnesyl pyrophosphate synthase. J Med Chem. 2008;51:2187–2195. doi: 10.1021/jm7015733. [DOI] [PubMed] [Google Scholar]
- 25.Coxon FP, Ebetino FH, Mules EH, Seabra MC, McKenna CE, Rogers MJ. Phosphonocarboxylate inhibitors of rab geranylgeranyl transferase disrupt the prenylation and membrane localization of rab proteins in osteoclasts in vitro and in vivo. Bone. 2005;37:349–358. doi: 10.1016/j.bone.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Luckman SP, Coxon FP, Ebetino FH, Russell RG, Rogers MJ. Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: Evidence from structure-activity relationships in J774 macrophages. J Bone Miner Res. 1998;13:1668–1678. doi: 10.1359/jbmr.1998.13.11.1668. [DOI] [PubMed] [Google Scholar]
- 27.Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: Current status. Clin Cancer Res. 2006;12:6222–6230. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 28.Dunford JE, Rogers MJ, Ebetino FH, Phipps RJ, Coxon FP. Inhibition of protein prenylation by bisphosphonates causes sustained activation of rac, Cdc42 and rho GTPases. J Bone Miner Res. 2006;21:684–694. doi: 10.1359/jbmr.060118. [DOI] [PubMed] [Google Scholar]
- 29.van beek E, Lowik C, van der Pluijm G, Papapoulos S. The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: A clue to the mechanism of action of nitrogen-containing bisphosphonates. J Bone Miner Res. 1999;14:722–729. doi: 10.1359/jbmr.1999.14.5.722. [DOI] [PubMed] [Google Scholar]
- 30.Coxon FP, Helfrich MH, Van't Hof R, Sebti S, Ralston SH, Hamilton A, et al. Protein geranylgeranylation is required for osteoclast formation, function and survival: Inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15:1467–1476. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- 31.Coxon FP, Helfrich MH, Larijani B, Muzylak M, Dunford JE, Marshall D, et al. Identification of a novel phosphonocarboxylate inhibitor of rab geranylgeranyl transferase that specifically prevents rab prenylation in osteoclasts and macrophages. J Biol Chem. 2001;276:48213–48222. doi: 10.1074/jbc.M106473200. [DOI] [PubMed] [Google Scholar]
- 32.Baron RA, Tavare R, Figueiredo AC, Blazewska KM, Kashemirov BA, McKenna CE, et al. Phosphonocarboxylates inhibit the second geranyl-geranyl addition by rab geranylgeranyl transferase. J Biol Chem. 2009;284:6861–6868. doi: 10.1074/jbc.M806952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Detter JC, Zhang Q, Mules EH, Novak EK, Mishra VS, Li W, et al. Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces rab prenylation and platelet synthesis. Proc Natl Acad Sci USA. 2000;97:4144–4149. doi: 10.1073/pnas.080517697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coxon FP, Taylor A, Stewart CA, Baron R, Seabra MC, Ebetino FH, et al. The gunmetal mouse reveals rab geranylgeranyl transferase to be the major molecular target of phosphonocarboxylate analogues of bisphosphonates. Bone. 2011 doi: 10.1016/j.bone.2011.03.686. [DOI] [PubMed] [Google Scholar]
- 35.Taylor A, Mules EH, Seabra MC, Helfrich MH, Rogers MJ, Coxon FP. Impaired prenylation of Rab GTPases in the gunmetal mouse causes defects in bone cell function. Small GTPases. 2011;2:131–142. doi: 10.4161/sgtp.2.3.16488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura I, Pilkington MF, Lakkakorpi PT, Lipfert L, Sims SM, Dixon SJ, et al. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J Cell Sci. 1999;112:3985–3993. doi: 10.1242/jcs.112.22.3985. [DOI] [PubMed] [Google Scholar]
- 37.McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clover J, Dodds RA, Gowen M. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 1992;103:267–271. doi: 10.1242/jcs.103.1.267. [DOI] [PubMed] [Google Scholar]
- 39.Shinar DM, Schmidt A, Halperin D, Rodan GA, Weinreb M. Expression of alphav and beta3 integrin subunits in rat osteoclasts in situ. J Bone Miner Res. 1993;8:403–414. doi: 10.1002/jbmr.5650080404. [DOI] [PubMed] [Google Scholar]
- 40.Duong LT, Lakkakorpi PT, Nakamura I, Machwate M, Nagy RM, Rodan GA. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin and phosphorylated by src kinase. J Clin Invest. 1998;102:881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanjay A, Houghton A, Neff L, DiDomenico E, Bardelay C, Antoine E, et al. Cbl associates with Pyk2 and src to regulate src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion and osteoclast motility. J Cell Biol. 2001;152:181–195. doi: 10.1083/jcb.152.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VL, Shattil SJ, et al. Syk, c-src, the alphavbeta3 integrin and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazaki T, Sanjay A, Neff L, Tanaka S, Horne WC, Baron R. Src kinase activity is essential for osteoclast function. J Biol Chem. 2004;279:17660–17666. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- 44.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiusaroli R, Sanjay A, Henriksen K, Engsig MT, Horne WC, Gu H, et al. Deletion of the gene encoding c-cbl alters the ability of osteoclasts to migrate, delaying resorption and ossification of cartilage during the development of long bones. Dev Biol. 2003;261:537–547. doi: 10.1016/s0012-1606(03)00299-9. [DOI] [PubMed] [Google Scholar]
- 46.Destaing O, Sanjay A, Itzstein C, Horne WC, Toomre D, De Camilli P, et al. The tyrosine kinase activity of c-src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell. 2008;19:394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brazier H, Stephens S, Ory S, Fort P, Morrison N, Blangy A. Expression profile of RhoGTPases and RhoGEFs during RANKL-stimulated osteoclastogenesis: Identification of essential genes in osteoclasts. J Bone Miner Res. 2006;21:1387–1398. doi: 10.1359/jbmr.060613. [DOI] [PubMed] [Google Scholar]
- 48.Ory S, Brazier H, Pawlak G, Blangy A. Rho GTPases in osteoclasts: Orchestrators of podosome arrangement. Eur J Cell Biol. 2008;87:469–477. doi: 10.1016/j.ejcb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Myers KR, Casanova JE. Regulation of actin cytoskeleton dynamics by arf-family GTPases. Trends Cell Biol. 2008;18:184–192. doi: 10.1016/j.tcb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heckel T, Czupalla C, Expirto Santo AI, Anitei M, Arantzazu Sanchez-Fernandez M, Mosch K, et al. Src-dependent repression of ARF6 is required to maintain podosome-rich sealing zones in bone-digesting osteoclasts. Proc Natl Acad Sci USA. 2009;106:1451–1456. doi: 10.1073/pnas.0804464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang D, Udagawa N, Nakamura I, Murakami H, Saito S, Yamasaki K, et al. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J Cell Sci. 1995;108:2285–2292. doi: 10.1242/jcs.108.6.2285. [DOI] [PubMed] [Google Scholar]
- 52.Saltel F, Destaing O, Bard F, Eichert D, Jurdic P. Apatite-mediated actin dynamics in resorbing osteoclasts. Mol Biol Cell. 2004;15:5231–5241. doi: 10.1091/mbc.E04-06-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whyte LS, Ryberg E, Sims NA, Ridge SA, Mackie K, Greasley PJ, et al. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci USA. 2009;106:16511–16516. doi: 10.1073/pnas.0902743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ory S, Munari-Silem Y, Fort P, Jurdic P. Rho and rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J Cell Sci. 2000;113:1177–1188. doi: 10.1242/jcs.113.7.1177. [DOI] [PubMed] [Google Scholar]
- 55.Chellaiah MA, Soga N, Swanson S, McAllister S, Alvarez U, Wang D, et al. Rho-A is critical for osteoclast podosome organization, motility and bone resorption. J Biol Chem. 2000;275:11993–12002. doi: 10.1074/jbc.275.16.11993. [DOI] [PubMed] [Google Scholar]
- 56.Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, et al. A novel rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci. 2005;118:2901–2911. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- 57.Chellaiah MA. Regulation of actin ring formation by rho GTPases in osteoclasts. J Biol Chem. 2005;280:32930–32943. doi: 10.1074/jbc.M500154200. [DOI] [PubMed] [Google Scholar]
- 58.Schramp M, Ying O, Kim TY, Martin GS. ERK5 promotes src-induced podosome formation by limiting rho activation. J Cell Biol. 2008;181:1195–1210. doi: 10.1083/jcb.200801078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fukuda A, Hikita A, Wakeyama H, Akiyama T, Oda H, Nakamura K, et al. Regulation of osteoclast apoptosis and motility by small GTPase binding protein Rac1. J Bone Miner Res. 2005;20:2245–2253. doi: 10.1359/JBMR.050816. [DOI] [PubMed] [Google Scholar]
- 60.Sakai H, Chen Y, Itokawa T, Yu KP, Zhu ML, Insogna K. Activated c-fms recruits vav and rac during CSF-1-induced cytoskeletal remodeling and spreading in osteoclasts. Bone. 2006;39:1290–1301. doi: 10.1016/j.bone.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Razzouk S, Lieberherr M, Cournot G. Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur J Cell Biol. 1999;78:249–255. doi: 10.1016/S0171-9335(99)80058-2. [DOI] [PubMed] [Google Scholar]
- 62.Itokowa T, Zhu ML, Troiano N, Bian J, Kawano T, Insogna K. Osteoclasts lacking Rac2 have defective chemotaxis and resorptive activity. Calcif Tissue Int. 2010 doi: 10.1007/s00223-010-9435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossman KL, Der CJ, Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 64.Meller N, Merlot S, Guda C. CZH proteins: A new family of rho-GEFs. J Cell Sci. 2005;118:4937–4946. doi: 10.1242/jcs.02671. [DOI] [PubMed] [Google Scholar]
- 65.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, et al. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 66.Kubo T, Yamashita T, Yamaguchi A, Sumimoto H, Hosokawa K, Tohyama M. A novel FERM domain including guanine nucleotide exchange factor is involved in rac signaling and regulates neurite remodeling. J Neurosci. 2002;22:8504–8513. doi: 10.1523/JNEUROSCI.22-19-08504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takegahara N, Kang S, Nojima S, Takamatsu H, Okuno T, Kikutani H, et al. Integral roles of a guanine nucleotide exchange factor, FARP2, in osteoclast podosome rearrangements. FASEB J. 2010;24:4782–4792. doi: 10.1096/fj.10-158212. [DOI] [PubMed] [Google Scholar]
- 68.Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, et al. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J Bone Miner Res. 2010 doi: 10.1002/jbmr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- 70.Tushir JS, D'Souza-Schorey C. ARF6-dependent activation of ERK and Rac1 modulates epithelial tubule development. EMBO J. 2007;26:1806–1819. doi: 10.1038/sj.emboj.7601644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menon P, Yin G, Smolock EM, Zuscik MJ, Yan C, Berk BC. GPCR kinase 2 interacting protein 1 (GIT1) regulates osteoclast function and bone mass. J Cell Physiol. 2010;225:777–785. doi: 10.1002/jcp.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gringel A, Walz D, Rosenberger G, Minden A, Kutsche K, Kopp P, et al. PAK4 and alphaPIX determine podosome size and number in macrophages through localized actin regulation. J Cell Physiol. 2006;209:568–579. doi: 10.1002/jcp.20777. [DOI] [PubMed] [Google Scholar]
- 73.Frank SR, Hansen SH. The PIX-GIT complex: A G protein signaling cassette in control of cell shape. Semin Cell Dev Biol. 2008;19:234–244. doi: 10.1016/j.semcdb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenberger G, Kutsche K. AlphaPIX and betaPIX and their role in focal adhesion formation. Eur J Cell Biol. 2006;85:265–274. doi: 10.1016/j.ejcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Webb BA, Eves R, Crawley SW, Zhou S, Cote GP, Mak AS. PAK1 induces podosome formation in A7r5 vascular smooth muscle cells in a PAK-interacting exchange factor-dependent manner. Am J Physiol Cell Physiol. 2005;289:898–907. doi: 10.1152/ajpcell.00095.2005. [DOI] [PubMed] [Google Scholar]
- 76.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 77.Monypenny J, Chou HC, Banon-Rodriguez I, Thrasher AJ, Anton IM, Jones GE, et al. Role of WASP in cell polarity and podosome dynamics of myeloid cells. Eur J Cell Biol. 2010 doi: 10.1016/j.ejcb.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 79.Ito Y, Teitelbaum SL, Zou W, Zheng Y, Johnson JF, Chappel J, et al. Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J Clin Invest. 2010;120:1981–1993. doi: 10.1172/JCI39650; 10.1172/JCI39650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calle Y, Jones GE, Jagger C, Fuller K, Blundell MP, Chow J, et al. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood. 2004;103:3552–3561. doi: 10.1182/blood-2003-04-1259. [DOI] [PubMed] [Google Scholar]
- 81.Chellaiah MA, Kuppuswamy D, Lasky L, Linder S. Phosphorylation of a wiscott-aldrich syndrome protein-associated signal complex is critical in osteoclast bone resorption. J Biol Chem. 2007;282:10104–10116. doi: 10.1074/jbc.M608957200. [DOI] [PubMed] [Google Scholar]
- 82.Ma T, Samanna V, Chellaiah MA. Dramatic inhibition of osteoclast sealing ring formation and bone resorption in vitro by a WASP-peptide containing pTyr294 amino acid. J Mol Signal. 2008;3:4. doi: 10.1186/1750-2187-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou HC, Anton IM, Holt MR, Curcio C, Lanzardo S, Worth A, et al. WIP regulates the stability and localization of WASP to podosomes in migrating dendritic cells. Curr Biol. 2006;16:2337–2344. doi: 10.1016/j.cub.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chabadel A, Banon-Rodriguez I, Cluet D, Rudkin BB, Wehrle-Haller B, Genot E, et al. CD44 and beta3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol Biol Cell. 2007;18:4899–4910. doi: 10.1091/mbc.E07-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szczepanowska J. Involvement of Rac/Cdc42/PAK pathway in cytoskeletal rearrangements. Acta Biochim Pol. 2009;56:225–234. [PubMed] [Google Scholar]
- 86.Webb BA, Eves R, Mak AS. Cortactin regulates podosome formation: Roles of the protein interaction domains. Exp Cell Res. 2006;312:760–769. doi: 10.1016/j.yexcr.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 87.Luxenburg C, Parsons JT, Addadi L, Geiger B. Involvement of the src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J Cell Sci. 2006;119:4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- 88.Webb BA, Zhou S, Eves R, Shen L, Jia L, Mak AS. Phosphorylation of cortactin by p21-activated kinase. Arch Biochem Biophys. 2006;456:183–193. doi: 10.1016/j.abb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 89.Saras J, Wollberg P, Aspenstrom P. Wrch1 is a GTPase-deficient Cdc42-like protein with unusual binding characteristics and cellular effects. Exp Cell Res. 2004;299:356–369. doi: 10.1016/j.yexcr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 90.Ory S, Brazier H, Blangy A. Identification of a bipartite focal adhesion localization signal in RhoU/Wrch-1, a rho family GTPase that regulates cell adhesion and migration. Biol Cell. 2007;99:701–716. doi: 10.1042/BC20070058. [DOI] [PubMed] [Google Scholar]
- 91.Brady DC, Alan JK, Madigan JP, Fanning AS, Cox AD. The transforming rho family GTPase wrch-1 disrupts epithelial cell tight junctions and epithelial morphogenesis. Mol Cell Biol. 2009;29:1035–1049. doi: 10.1128/MCB.00336-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alan JK, Berzat AC, Dewar BJ, Graves LM, Cox AD. Regulation of the rho family small GTPase wrch-1/RhoU by C-terminal tyrosine phosphorylation requires src. Mol Cell Biol. 2010;30:4324–4338. doi: 10.1128/MCB.01646-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brazier H, Pawlak G, Vives V, Blangy A. The rho GTPase Wrch1 regulates osteoclast precursor adhesion and migration. Int J Biochem Cell Biol. 2009;41:1391–1401. doi: 10.1016/j.biocel.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Salo J, Metsikko K, Palokangas H, Lehenkari P, Vaananen HK. Bone-resorbing osteoclasts reveal a dynamic division of basal plasma membrane into two different domains. J Cell Sci. 1996;109:301–307. doi: 10.1242/jcs.109.2.301. [DOI] [PubMed] [Google Scholar]
- 95.Zhao H, Ettala O, Vaananen HK. Intracellular membrane trafficking pathways in bone-resorbing osteoclasts revealed by cloning and subcellular localization studies of small GTP-binding rab proteins. Biochem Biophys Res Commun. 2002;293:1060–1065. doi: 10.1016/S0006-291X(02)00326-1. [DOI] [PubMed] [Google Scholar]
- 96.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 97.Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, et al. From lysosomes to the plasma membrane: Localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem. 2003;278:22023–22030. doi: 10.1074/jbc.M302436200. [DOI] [PubMed] [Google Scholar]
- 98.Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 99.Zhao H, Vaananen HK. Pharmacological sequestration of intracellular cholesterol in late endosomes disrupts ruffled border formation in osteoclasts. J Bone Miner Res. 2006;21:456–465. doi: 10.1359/JBMR.051204. [DOI] [PubMed] [Google Scholar]
- 100.Zaidi M, Troen B, Moonga BS, Abe E. Cathepsin K, osteoclastic resorption and osteoporosis therapy. J Bone Miner Res. 2001;16:1747–1749. doi: 10.1359/jbmr.2001.16.10.1747. [DOI] [PubMed] [Google Scholar]
- 101.Everts V, Delaisse JM, Korper W, Niehof A, Vaes G, Beertsen W. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J Cell Physiol. 1992;150:221–231. doi: 10.1002/jcp.1041500202. [DOI] [PubMed] [Google Scholar]
- 102.Mulari MT, Zhao H, Lakkakorpi PT, Vaananen HK. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic. 2003;4:113–125. doi: 10.1034/j.1600-0854.2003.40206.x. [DOI] [PubMed] [Google Scholar]
- 103.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 104.Zacchi P, Stenmark H, Parton RG, Orioli D, Lim F, Giner A, et al. Rab17 regulates membrane trafficking through apical recycling endosomes in polarized epithelial cells. J Cell Biol. 1998;140:1039–1053. doi: 10.1083/jcb.140.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kariya Y, Honma M, Hanamura A, Aoki S, Ninomiya T, Nakamichi Y, et al. Rab27a and Rab27b are involved in stimulation-dependent RANKL release from secretory lysosomes in osteoblastic cells. J Bone Miner Res. 2010 doi: 10.1002/jbmr.268. [DOI] [PubMed] [Google Scholar]
- 106.Ha BG, Hong JM, Park JY, Ha MH, Kim TH, Cho JY, et al. Proteomic profile of osteoclast membrane proteins: Identification of Na+/H+ exchanger domain containing 2 and its role in osteoclast fusion. Proteomics. 2008;8:2625–2639. doi: 10.1002/pmic.200701192. [DOI] [PubMed] [Google Scholar]
- 107.Pavlos NJ, Xu J, Riedel D, Yeoh JS, Teitelbaum SL, Papadimitriou JM, et al. Rab3D regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol Cell Biol. 2005;25:5253–5269. doi: 10.1128/MCB.25.12.5253-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taylor A, Rogers MJ, Tosh D, Coxon FP. A novel method for efficient generation of transfected human osteoclasts. Calcif Tissue Int. 2007;80:132–136. doi: 10.1007/s00223-006-0245-6. [DOI] [PubMed] [Google Scholar]
- 109.Feng Y, Press B, Wandinger-Ness A. Rab 7: An important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: A key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 112.Zhao H, Laitala-Leinonen T, Parikka V, Vaananen HK. Downregulation of small GTPase Rab7 impairs osteoclast polarization and bone resorption. J Biol Chem. 2001;276:39295–39302. doi: 10.1074/jbc.M010999200. [DOI] [PubMed] [Google Scholar]
- 113.Sun Y, Buki KG, Ettala O, Vaaraniemi JP, Vaananen HK. Possible role of direct Rac1-Rab7 interaction in ruffled border formation of osteoclasts. J Biol Chem. 2005;280:32356–32361. doi: 10.1074/jbc.M414213200. [DOI] [PubMed] [Google Scholar]
- 114.Hume AN, Ushakov DS, Tarafder AK, Ferenczi MA, Seabra MC. Rab27a and MyoVa are the primary mlph interactors regulating melanosome transport in melanocytes. J Cell Sci. 2007;120:3111–3122. doi: 10.1242/jcs.010207. [DOI] [PubMed] [Google Scholar]
- 115.Frasa MA, Maximiano FC, Smolarczyk K, Francis RE, Betson ME, Lozano E, et al. Armus is a Rac1 effector that inactivates Rab7 and regulates E-cadherin degradation. Curr Biol. 2010;20:198–208. doi: 10.1016/j.cub.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 116.Nakamura I, Takahashi N, Udagawa N, Moriyama Y, Kurokawa T, Jimi E, et al. Lack of vacuolar proton ATPase association with the cytoskeleton in osteoclasts of osteosclerotic (oc/oc) mice. FEBS Lett. 1997;401:207–212. doi: 10.1016/s0014-5793(96)01454-8. [DOI] [PubMed] [Google Scholar]
- 117.Zuo J, Jiang J, Chen SH, Vergara S, Gong Y, Xue J, et al. Actin binding activity of subunit B of vacuolar H+-ATPase is involved in its targeting to ruffled membranes of osteoclasts. J Bone Miner Res. 2006;21:714–721. doi: 10.1359/jbmr.060201. [DOI] [PubMed] [Google Scholar]
- 118.Van Wesenbeeck L, Odgren PR, Coxon FP, Frattini A, Moens P, Perdu B, et al. Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J Clin Invest. 2007;117:919–930. doi: 10.1172/JCI30328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tabata K, Matsunaga K, Sakane A, Sasaki T, Noda T, Yoshimori T. Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain. Mol Biol Cell. 2010;21:4162–4172. doi: 10.1091/mbc.E10-06-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Del Fattore A, Fornari R, Van Wesenbeeck L, de Freitas F, Timmermans JP, Peruzzi B, et al. A new heterozygous mutation (R714C) of the osteopetrosis gene, pleckstrin homolog domain containing family M (with run domain) member 1 (PLEKHM1), impairs vesicular acidification and increases TRACP secretion in osteoclasts. J Bone Miner Res. 2008;23:380–391. doi: 10.1359/jbmr.071107. [DOI] [PubMed] [Google Scholar]
- 121.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 122.Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, et al. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol. 2010;188:253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noda T, Fujita N, Yoshimori T. The late stages of autophagy: How does the end begin? Cell Death Differ. 2009;16:984–990. doi: 10.1038/cdd.2009.54. [DOI] [PubMed] [Google Scholar]
- 125.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 126.Lledo PM, Johannes L, Vernier P, Zorec R, Darchen F, Vincent JD, et al. Rab3 proteins: Key players in the control of exocytosis. Trends Neurosci. 1994;17:426–432. doi: 10.1016/0166-2236(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 127.Millar AL, Pavios NJ, Xu J, Zheng MH. Rab3D: A regulator of exocytosis in non-neuronal cells. Histol Histopathol. 2002;17:929–936. doi: 10.14670/HH-17.929. [DOI] [PubMed] [Google Scholar]
- 128.Pavlos NJ, Xu J, Feng H, Ng P, Cheng T, Ang E, et al. Rab3D directs organelle transport by recruiting cytoplasmic dynein motor protein tctex-1 to secretory membranes of osteoclast. Bone. 2009;44:160. doi: 10.1016/j.bone.2009.01.354. [DOI] [Google Scholar]
- 129.Stenbeck G, Horton MA. A new specialized cell-matrix interaction in actively resorbing osteoclasts. J Cell Sci. 2000;113:1577–1587. doi: 10.1242/jcs.113.9.1577. [DOI] [PubMed] [Google Scholar]
- 130.Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 131.Nesbitt SA, Horton MA. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science. 1997;276:266–269. doi: 10.1126/science.276.5310.266. [DOI] [PubMed] [Google Scholar]
- 132.Vaaraniemi J, Halleen JM, Kaarlonen K, Ylipahkala H, Alatalo SL, Andersson G, et al. Intracellular machinery for matrix degradation in bone-resorbing osteoclasts. J Bone Miner Res. 2004;19:1432–1440. doi: 10.1359/JBMR.040603. [DOI] [PubMed] [Google Scholar]
- 133.Stenbeck G, Horton MA. Endocytic trafficking in actively resorbing osteoclasts. J Cell Sci. 2004;117:827–836. doi: 10.1242/jcs.00935. [DOI] [PubMed] [Google Scholar]
- 134.Mulari MT, Nars M, Laitala-Leinonen T, Kaisto T, Metsikko K, Sun Y, et al. Recombinant VSV G proteins reveal a novel raft-dependent endocytic pathway in resorbing osteoclasts. Exp Cell Res. 2008;314:1641–1651. doi: 10.1016/j.yexcr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 135.Hunziker W, Peters PJ. Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J Biol Chem. 1998;273:15734–15741. doi: 10.1074/jbc.273.25.15734. [DOI] [PubMed] [Google Scholar]
- 136.Su T, Bryant DM, Luton F, Verges M, Ulrich SM, Hansen KC, et al. A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2010;12:1143–1153. doi: 10.1038/ncb2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001;28:465–473. doi: 10.1016/s8756-3282(01)00412-4. [DOI] [PubMed] [Google Scholar]
- 138.Fehrenbacher N, Bar-Sagi D, Philips M. Ras/MAPK signaling from endomembranes. Mol Oncol. 2009;3:297–307. doi: 10.1016/j.molonc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: More than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 140.Bradley EW, Ruan MM, Vrable A, Oursler MJ. Pathway crosstalk between Ras/Raf and PI3K in promotion of M-CSF-induced MEK/ERK-mediated osteoclast survival. J Cell Biochem. 2008;104:1439–1451. doi: 10.1002/jcb.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A, et al. Reciprocal role of ERK and NFkappaB pathways in survival and activation of osteoclasts. J Cell Biol. 2000;148:333–342. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bradley EW, Ruan MM, Oursler MJ. PAK1 is a novel MEK-independent raf target controlling expression of the IAP survivin in M-CSF-mediated osteoclast survival. J Cell Physiol. 2008;217:752–758. doi: 10.1002/jcp.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schindeler A, Little DG. Recent insights into bone development, homeostasis and repair in type 1 neurofibromatosis (NF1) Bone. 2008;42:616–622. doi: 10.1016/j.bone.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 144.Heerva E, Alanne MH, Peltonen S, Kuorilehto T, Hentunen T, Vaananen K, et al. Osteoclasts in neurofibromatosis type 1 display enhanced resorption capacity, aberrant morphology and resistance to serum deprivation. Bone. 2010;47:583–590. doi: 10.1016/j.bone.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 145.Pascoe D, Oursler MJ. The src signaling pathway regulates osteoclast lysosomal enzyme secretion and is rapidly modulated by estrogen. J Bone Miner Res. 2001;16:1028–1036. doi: 10.1359/jbmr.2001.16.6.1028. [DOI] [PubMed] [Google Scholar]
- 146.Yang FC, Chen S, Robling AG, Yu X, Nebesio TD, Yan J, et al. Hyperactivation of p21ras and PI3K cooperate to alter murine and human neurofibromatosis type 1-haploinsufficient osteoclast functions. J Clin Invest. 2006;116:2880–2891. doi: 10.1172/JCI29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yan J, Chen S, Zhang Y, Li X, Li Y, Wu X, et al. Rac1 mediates the osteoclast gains-in-function induced by haploinsufficiency of Nf1. Hum Mol Genet. 2008;17:936–948. doi: 10.1093/hmg/ddm366. [DOI] [PubMed] [Google Scholar]