Abstract
We recently identified the activity-inducible protein kinase Plk2 as a novel overseer of the balance between Ras and Rap small GTPases. Plk2 achieves a profound level of regulatory control by interacting with and phosphorylating at least four Ras and Rap guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). Combined, these actions result in synergistic suppression of Ras and hyperstimulation of Rap signaling. Perturbation of Plk2 function abolished homeostatic adaptation of synapses to enhanced activity and impaired behavioral adaptation in various learning tasks, indicating that this regulation was critical for maintaining appropriate Ras/Rap levels. These studies provide insights into the highly cooperative nature of Ras and Rap regulation in neurons. However, different GEF and GAP substrates of Plk2 also controlled specific aspects of dendritic spine morphology, illustrating the ability of individual GAPs/GEFs to assemble microdomains of Ras and Rap signaling that respond to different stimuli and couple to distinct output pathways.
Key words: homeostatic synaptic plasticity, Ras, Rap, Plk2, SNK, SPAR, SynGAP, RasGRF1, PDZGEF1, dendritic spines, AMPA receptors, GAP, GEF
Introduction
In philosophy, the golden mean represents the ideal middle path that lies between the extremes of deficiency and excess. In neurons, homeostatic mechanisms similarly seek to maintain a balanced level of activity regardless of the external stimuli that might be buffeting the cell at any given moment. In this way, neurons strive to avoid extremes of complete silence or overexcitation, which can degrade information processing capacity or worse, lead to synaptic loss and neurodegeneration. Thus, homeostatic plasticity represents a compensatory, negative feedback restraining influence, a counterpoint to destabilizing positive feedback mechanisms such as long-term potentiation and depression (LTP and LTD).1 These associative or Hebbian, forms of plasticity are frequently expressed by changes in AMPA subtype of glutamate receptor (AMPAR) content at many excitatory synapses, as well as by morphological remodeling of dendritic spines, small protrusions that serve as the primary postsynaptic sites of excitatory synapses in the CNS. However, unlike their much better-studied Hebbian cousins, homeostatic plasticity mechanisms are just beginning to be elucidated.2
Ras and Rap, closely related members of the Ras superfamily of small GTPases, are molecular signaling switches important in many forms of neuronal plasticity, memory formation and neurological disease,3,4 and are good candidates for mediating homeostatic responses. Nevertheless, the precise roles of Ras and Rap are not entirely clear, as there are a number of family members (in mammals, the major isoforms are H-, K- and N-Ras; and Rap1A/B and Rap2A/B) with overlapping and distinct roles, each embedded in complex signaling networks. Some general properties may be cautiously drawn, however. Accumulating evidence indicates that the functions of Ras and Rap are frequently antagonistic-indeed, Rap was originally characterized as a Ras suppressor.5,6 In terms of synaptic plasticity, Ras often favors postsynaptic strengthening or growth; its activity is required for and stimulated by LTP.7,8 Ras also drives synaptic delivery of AMPARs8–11 as well as production of dendritic protrusions/spines.12,13 In contrast, Rap typically favors postsynaptic weakening and is implicated in LTD or depotentiation, processes involving AMPAR internalization and loss of dendritic spines.8–10,14–17 However, this picture is somewhat muddled by observations that Rap1 mutant mice also display deficits in LTP,18,19 and LTP can be increased by H-Ras ablation or decreased by H-Ras overexpression.20,21
Possible explanations for some of these discrepancies are differences in the exact experimental paradigm used, memory task involved or brain area examined.4 Additionally, distinct pools of Ras or Rap have been shown to operate at particular subcellular loci.22 Such highly localized regulation may be critically dependent on the numerous guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) that catalyze Ras/Rap activation and inactivation, respectively, but that also likely function as scaffold proteins to target the GTPases to specific subcellular sites, thereby mediating responses to a variety of external stimuli and coupling signaling to divergent output pathways.23
This complex, site-specific regulation highlights a potential complication of many approaches in the literature that utilize Ras/Rap overexpression or knockout strategies, which are somewhat blunt instruments involving dramatic and cell-wide alterations in activity. Evidence suggests that the precise level of Ras and Rap must be finely controlled, with both increases and decreases causing impairment in learning and memory.3,24 Moreover, the balance between Ras and Rap activities also seems to be a critical parameter, not merely their individual levels.25 Thus, as discussed in further detail in the next section, our recent study26 has sought to analyze Ras and Rap regulation in a more surgical manner via manipulation of specific Ras and Rap GAPs/GEFs. This approach has also allowed us to ask whether coordinated Ras/Rap signaling is important for one form of homeostatic plasticity, adaptation to chronic synaptic overexcitation.
Plk2 Acts as a “Master Override” Over Ras and Rap Signaling
Polo-like kinase 2 (Plk2, also called serum inducible kinase (SNK)) is a member of the polo-like family of serine/threonine kinases.27–29 Plk2 contains an N-terminal kinase domain and a conserved C-terminal polo box domain (PBD) characteristic of polo family kinases that binds to substrates and targets the kinase to specific subcellular loci.30 Plk2 expression is inducible by strong synaptic activity,31 one of only a few kinases known to be regulated in this manner. Following induction, Plk2 protein is distributed in a striking proximal-to-distal gradient in dendrites of hippocampal neurons, and appears to “erase” excitatory synapses and dendritic spines in its path.32 These observations and subsequent studies revealed that Plk2 acts to homeostatically depress the synaptic overactivity responsible for its induction,33,34 much like an emergency braking system that kicks in on a hurtling runaway train once it exceeds a set speed limit.
How is Plk2 so effective at causing synapse weakening and loss? We found that a major downstream function of Plk2 is to orchestrate Ras and Rap signaling26—not by targeting just one regulator, but four of them. Interaction and kinase assays demonstrated that Plk2 directly binds to and phosphorylates both a GAP and a GEF for Rap (the proteins SPAR and PDZGEF1, respectively), as well as a GAP and a GEF for Ras (SynGAP and RasGRF1). Each of these substrates is expressed at synapses and, with the exception of PDZGEF1, was already known to play important roles in synaptic plasticity or learning and memory (see Fig. 1). Interestingly, phosphorylation by Plk2 caused diametrically opposing effects on Ras vs. Rap signaling. Plk2 promoted ubiquitin-mediated proteasomal degradation of RasGRF1 and SPAR, while stimulating the enzymatic activities of SynGAP and PDZGEF1 (Fig. 1), all of which act to the same purpose: to suppress Ras and augment Rap.
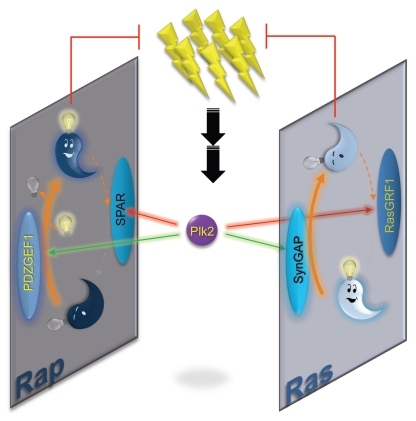
Figure 1.
Coordinated regulation of Ras/Rap GAPs and GEFs by Plk2. Schematic model for Plk2 function in Ras/Rap signaling. Strong synaptic activation (lightning bolts) induces commensurate levels of Plk2 gene expression. Plk2 targets multiple GAPs and GEFs that regulate Ras/Rap cycling between active, GTP bound (awake and glowing, light bulb on) and inactive, GDP bound (asleep and dull, light bulb off) states. RasGRF1/CDC25Mm is a neuron-specific Ras GEF involved in long-term memory consolidation.42,43 PDZGEF1 (also called RapGEF2, nRapGEP, CNrasGEF or RA-GEF) is a Rap GEF found at synapses.44,45 SynGAP is a Ras GAP that is an abundant component of the postsynaptic density.46,47 SPAR is a Rap GAP that is highly enriched in dendritic spines in association with actin cytoskeleton.15 Plk2 antagonizes SPAR and RasGRF1, while promoting PDZFEG1 and SynGAP activity, leading to synergistic inactivation of Ras and hyperactivation of Rap. As a result, AMPA receptors are removed from the neuronal surface and dendritic spines undergo shrinkage or elimination, helping to counteract the initial overexcitation.
This coordinated response overseeing multiple regulators can lead to quite dramatic alterations in the relative levels of Ras and Rap signaling, depending on the amount of active Plk2 protein present. With Plk2 overexpression, Ras undergoes nearly complete inactivation, while Rap is elevated by a commensurate degree, at least by the somewhat crude measures of biochemical active GTPase pulldown assays. On the other end of the spectrum, interference with Plk2 kinase activity in vivo led to nearly undetectable levels of active Rap in the forebrain, with Ras concomitantly hyperactive. Thus, degree of Plk2 induction following synaptic activation may serve as a homeostatic sensor to dictate Ras and Rap balance under wide variations of chronic activity states. Synergistic targeting of multiple GAPs/GEFs by Plk2 is likely a key factor in permitting such drastic swings in Ras/Rap balance.
These data showing reciprocal and bidirectional regulation of Ras and Rap also reinforce the notion that Ras and Rap are indeed functionally antagonistic, with Ras favoring synapse strengthening and Rap favoring weakening, and that changes in their activity need to be coupled for maximum efficacy, at least in homeostatic processes. Perturbation of this balance by transgenic forebrain expression of a dominant-negative Plk2 mutant (kinase dead) in mice led to deficits in hippocampal-dependent spatial memory tasks as well as inappropriately persistent fear responses following contextual and cued fear conditioning, emphasizing the importance of Ras and Rap balance in varied forms of learning and memory.
However, a deeper analysis suggests another reason Plk2 may target multiple GAPs and GEFs, beyond the requirements of simple efficacy. RNA interference knockdown and overexpression experiments revealed that each Plk2 substrate actually affects relatively specific aspects of dendritic spine morphology. The GEFs RasGRF1 and PDZGEF1 preferentially determine spine density, positively and negatively, while the GAPs SPAR and SynGAP exercise bidirectional control primarily over a different parameter—spine head size (Fig. 2). The effects of SPAR and SynGAP on spine size are consistent with previous reports that increased SPAR levels causes spine head expansion,15 akin to the loss of SynGAP.35 These results suggest that dedicated and specialized Ras and Rap signaling cascades emanate from each GAP/GEF, presenting a clear rationale for Plk2 to target each pathway.
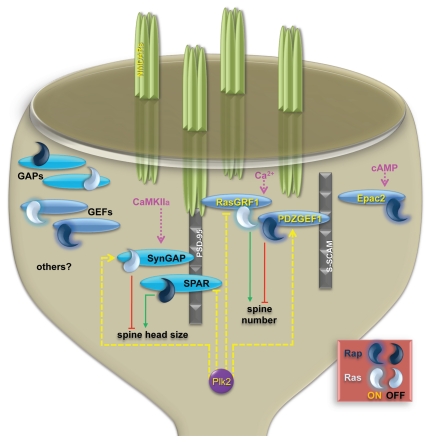
Figure 2.
Microdomains of Ras/Rap signaling mediated by GAPs/GEFs. A variety of synaptically localized GAPs (shown in teal) and GEFs (periwinkle) maintain Ras and Rap in the OFF and ON states, respectively. RasGRF1 interacts with the NR2B subunit of NMDA receptors (NMDARs).48 PDZGEF1 associates with S-SCAM, a postsynaptic scaffolding protein.49 SynGAP binds to the PDZ domains of scaffold protein PSD-95.46,47 SPAR is also associated with PSD-95 via a guanylate kinase domain interaction.15 Microdomains of Ras and Rap scaffolded by different GAPs/GEFs mediate specific aspects of dendritic spine morphogenesis. Under appropriate stimuli, homeostatic plasticity driven by Plk2 (dashed yellow lines) coordinately regulate GAPs/GEFs. Acute plasticity mechanisms (pink) may engage similar sets of proteins as homeostatic ones but differ in time course as well as type of stimulus. Other GAPs/GEFs not studied here may form additional microdomains that control different facets of plasticity and respond to distinct patterns of synaptic activity.
More broadly, a similar concept could apply generally to other synaptic GAPs and GEFs, including RasGRF2, PDZGEF2, Rap1GAP, Epac2 and additional ones that we did not examine directly. In this model, each regulator nucleates a unique and separate pool of Ras or Rap, potentially governing quite specific aspects of synaptic change. This arrangement also could determine which of the several Ras and Rap family members are activated in any given circumstance or even influence the selectivity of regulators for Ras vs. Rap. For instance, although our data are more consistent with SynGAP acting against Ras, it has also been shown that SynGAP can function as a Rap GAP.36,37 It is possible that localized calcium transients with appropriate spatiotemporal dynamics could activate the C2 domain of SynGAP,37 resulting in a switch from Ras to Rap inhibition in particular microdomains.
The advantage of such a system as outlined above would be maximal flexibility of possible responses to environmental cues. Thus, while Plk2 targets multiple GAPs/GEFs, perhaps due to the urgency of its mission to dampen neuronal activity, other pathways need not do so. One could envision that invoking different combinations of GAPs/GEFs could yield a wide and finely tunable array of plasticity outcomes. These diverse, microdomain-specific roles illustrate the difficulty in any attempt to generalize the duties of Ras and Rap, as turning the same small GTPase on (or off) could have many different biological meanings depending on the context of its activation. A complex phenomenon such as LTP or homeostatic synaptic plasticity may involve summing the effects of a multitude of separate Ras and Rap loci, each performing its own defined task but acting in concert. In this regard, relatively specialized GAP and GEF functions may be exploited therapeutically to allow selective modulation of desired signaling paths with fewer deleterious side effects than global perturbation of Ras or Rap signaling.
The function of Ras/Rap in the service of both acute forms of plasticity such as LTP and LTD and homeostatic plasticity as described in our work, brings up the additional question of how these two classes of plasticity can engage the same molecular components but in response to vastly different forms of stimulation. We suggest that a core system of basic synaptic Ras/Rap machinery exists that is essential for modulating synaptic strength, and can be recruited in different situations by a variety of stimuli acting on the same or overlapping GAPs and GEFs (Fig. 2). For example, acute stimuli could regulate Ras and Rap in Plk2-independent ways via CaMKII phosphorylation of SynGAP,38 calcium-dependent activation of RasGRF1,39 or cAMP-dependent activation of other GEFs not studied here, such as Epac2.40 Multiple modes of activating and suppressing GAPs/GEFs explains how they can function in response to seemingly opposing activity paradigms but produce similar effects on synaptic strength. It is worth noting that although homeostatic proteins like Plk2 may not be essential components of this core synaptic machinery, understanding how they interface with this system has been very useful in identifying its critical control points.
Conclusions and Perspectives
Our study has uncovered a novel homeostatic Ras and Rap regulatory network governed by the activity-dependent kinase Plk2. Characterization of this system has shed light on physiological mechanisms that simultaneously regulate Ras and Rap activity, as well as the roles of GAPs and GEFs in acting as scaffolds to organize specific outputs even while affecting the same set of small GTPases. Several outstanding questions remain, including identification of the molecular connections linking Ras/Rap to AMPAR trafficking or spine morphogenesis, and how other small GTPases previously implicated in synaptic plasticity such as Rac, Rho or Rab might be involved. Clearly, Plk2 controls multiple pathways beyond small GTPases,41 and understanding the interplay of these pathways with each other and with other known homeostatic proteins such as Arc will be of importance going forward. Continuing to elucidate the functions of Plk2 in synaptic plasticity will be sure to provide additional insights into achieving and maintaining optimal synaptic balance.
Acknowledgments
The authors thank Aaron Rozeboom for critical comments on the manuscript. This work was supported by NIH grant R01NS048085 (D.T.S.P.).
Extra View to: Lee KJ, Lee Y, Rozeboom AR, Lee J-Y, Udagawa N, Hoe H-S, Pak DTS. Requirement for Plk2 in orchestrated Ras and Rap signaling, homeostatic structural plasticity and memory. Neuron. 2011;69:957–973. doi: 10.1016/j.neuron.2011.02.004.
References
- 1.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/S0959-4388(00)00091-X. [DOI] [PubMed] [Google Scholar]
- 2.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stornetta RL, Zhu JJ. Ras and rap signaling in synaptic plasticity and mental disorders. Neuroscientist. 2011;17:54–78. doi: 10.1177/1073858410365562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye X, Carew TJ. Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron. 2010;68:340–361. doi: 10.1016/j.neuron.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook SJ, Rubinfeld B, Albert I, McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993;12:3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 7.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/S0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- 9.Kielland A, Bochorishvili G, Corson J, Zhang L, Rosin DL, Heggelund P, et al. Activity patterns govern synapse-specific AMPA receptor trafficking between deliverable and synaptic pools. Neuron. 2009;62:84–101. doi: 10.1016/j.neuron.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack SG, Stornetta RL, Zhu JJ. Synaptic AMPA receptor exchange maintains bidirectional plasticity. Neuron. 2006;50:75–88. doi: 10.1016/j.neuron.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, et al. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arendt T, Gartner U, Seeger G, Barmashenko G, Palm K, Mittmann T, et al. Neuronal activation of Ras regulates synaptic connectivity. Eur J Neurosci. 2004;19:2953–2966. doi: 10.1111/j.0953-816X.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 13.Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- 14.Fu Z, Lee SH, Simonetta A, Hansen J, Sheng M, Pak DT. Differential roles of Rap1 and Rap2 small GTPases in neurite retraction and synapse elimination in hippocampal spiny neurons. J Neurochem. 2007;100:118–131. doi: 10.1111/j.1471-4159.2006.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pak DT, Yang S, Rudolph-Correia S, Kim E, Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31:289–303. doi: 10.1016/S0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- 16.Ryu J, Futai K, Feliu M, Weinberg R, Sheng M. Constitutively active Rap2 transgenic mice display fewer dendritic spines, reduced extracellular signal-regulated kinase signaling, enhanced long-term depression and impaired spatial learning and fear extinction. J Neurosci. 2008;28:8178–8188. doi: 10.1523/JNEUROSCI.1944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 18.Morozov A, Muzzio IA, Bourtchouladze R, Van-Strien N, Lapidus K, Yin D, et al. Rap1 couples cAMP signaling to a distinct pool of p42/44MAPK regulating excitability, synaptic plasticity, learning and memory. Neuron. 2003;39:309–325. doi: 10.1016/S0896-6273(03)00404-5. [DOI] [PubMed] [Google Scholar]
- 19.Pan BX, Vautier F, Ito W, Bolshakov VY, Morozov A. Enhanced cortico-amygdala efficacy and suppressed fear in absence of Rap1. J Neurosci. 2008;28:2089–2098. doi: 10.1523/JNEUROSCI.5156-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, et al. Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci. 2000;20:2504–2511. doi: 10.1523/JNEUROSCI.20-07-02504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton C, Yaka R, Dinh S, Ron D. H-Ras modulates N-methyl-D-aspartate receptor function via inhibition of Src tyrosine kinase activity. J Biol Chem. 2003;278:23823–23829. doi: 10.1074/jbc.M302389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochizuki N, Yamashita S, Kurokawa K, Ohba Y, Nagai T, Miyawaki A, et al. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature. 2001;411:1065–1068. doi: 10.1038/35082594. [DOI] [PubMed] [Google Scholar]
- 23.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 25.Ye X, Shobe JL, Sharma SK, Marina A, Carew TJ. Small G proteins exhibit pattern sensitivity in MAPK activation during the induction of memory and synaptic facilitation in Aplysia. Proc Natl Acad Sci USA. 2008;105:20511–20516. doi: 10.1073/pnas.0808110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KJ, Lee Y, Rozeboom A, Lee JY, Udagawa N, Hoe HS, et al. Requirement for plk2 in orchestrated ras and rap signaling, homeostatic structural plasticity and memory. Neuron. 2011;69:957–973. doi: 10.1016/j.neuron.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donohue PJ, Alberts GF, Guo Y, Winkles JA. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem. 1995;270:10351–10357. doi: 10.1074/jbc.270.17.10351. [DOI] [PubMed] [Google Scholar]
- 28.Seeburg DP, Pak D, Sheng M. Polo-like kinases in the nervous system. Oncogene. 2005;24:292–298. doi: 10.1038/sj.onc.1208277. [DOI] [PubMed] [Google Scholar]
- 29.Simmons DL, Neel BG, Stevens R, Evett G, Erikson RL. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992;12:4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowery DM, Lim D, Yaffe MB. Structure and function of Polo-like kinases. Oncogene. 2005;24:248–259. doi: 10.1038/sj.onc.1208280. [DOI] [PubMed] [Google Scholar]
- 31.Kauselmann G, Weiler M, Wulff P, Jessberger S, Konietzko U, Scafidi J, et al. The polo-like protein kinases Fnk and Snk associate with a Ca(2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 1999;18:5528–5539. doi: 10.1093/emboj/18.20.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pak DT, Sheng M. Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science. 2003;302:1368–1373. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- 33.Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeburg DP, Sheng M. Activity-induced Polo-like kinase 2 is required for homeostatic plasticity of hippocampal neurons during epileptiform activity. J Neurosci. 2008;28:6583–6591. doi: 10.1523/JNEUROSCI.1853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazquez LE, Chen HJ, Sokolova I, Knuesel I, Kennedy MB. SynGAP regulates spine formation. J Neurosci. 2004;24:8862–8872. doi: 10.1523/JNEUROSCI.3213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Pena V, Hothorn M, Eberth A, Kaschau N, Parret A, Gremer L, et al. The C2 domain of SynGAP is essential for stimulation of the Rap GTPase reaction. EMBO Rep. 2008;9:350–355. doi: 10.1038/embor.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh JS, Manzerra P, Kennedy MB. Regulation of the neuron-specific Ras GTPase-activating protein, synGAP, by Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:17980–17988. doi: 10.1074/jbc.M314109200. [DOI] [PubMed] [Google Scholar]
- 39.Farnsworth CL, Freshney NW, Rosen LB, Ghosh A, Greenberg ME, Feig LA. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 40.Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, et al. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci. 2009;12:1275–1284. doi: 10.1038/nn.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evers DM, Matta JA, Hoe HS, Zarkowsky D, Lee SH, Isaac JT, et al. Plk2 attachment to NSF induces homeostatic removal of GluA2 during chronic overexcitation. Nat Neurosci. 2010;13:1199–1207. doi: 10.1038/nn.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, et al. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 43.Shou C, Farnsworth CL, Neel BG, Feig LA. Molecular cloning of cDNAs encoding a guanine-nucleotide-releasing factor for Ras p21. Nature. 1992;358:351–354. doi: 10.1038/358351a0. [DOI] [PubMed] [Google Scholar]
- 44.de Rooij J, Boenink NM, van Triest M, Cool RH, Wittinghofer A, Bos JL. PDZ-GEF1, a guanine nucleotide exchange factor specific for Rap1 and Rap2. J Biol Chem. 1999;274:38125–38130. doi: 10.1074/jbc.274.53.38125. [DOI] [PubMed] [Google Scholar]
- 45.Liao Y, Kariya K, Hu CD, Shibatohge M, Goshima M, Okada T, et al. RA-GEF, a novel Rap1A guanine nucleotide exchange factor containing a Ras/Rap1A-associating domain, is conserved between nematode and humans. J Biol Chem. 1999;274:37815–37820. doi: 10.1074/jbc.274.53.37815. [DOI] [PubMed] [Google Scholar]
- 46.Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/S0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/S0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 48.Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, et al. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/S0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 49.Ohtsuka T, Hata Y, Ide N, Yasuda T, Inoue E, Inoue T, et al. nRap GEP: a novel neural GDP/GTP exchange protein for rap1 small G protein that interacts with synaptic scaffolding molecule (S-SCAM) Biochem Biophys Res Commun. 1999;265:38–44. doi: 10.1006/bbrc.1999.1619. [DOI] [PubMed] [Google Scholar]