Abstract
Rac GTPases promote formation of membrane ruffles, yet how key effectors of this small GTPase operate in response to intracellular signals is not well established. In our recent report, “Anchored PKA recruitment of active Rac,” we identify a cortical actin cytoskeletal signaling complex containing an A-Kinase Anchoring Protein (AKAP) and the IQGAP2 isoform. We show that dynamic assembly of this complex requires the combined action of calcium and cAMP signals. Furthermore, phosphorylation of IQGAP2 by the AKAP220-anchored PKA enhances Rac binding and membrane ruffling. We also discuss our recent findings and provide additional evidence that phosphorylation of IQGAP2 brings IQGAP2 to membrane ruffles.
Key words: AKAP220, IQGAP2, PKA, GTPase, Rac, actin, membrane ruffles
The Rho GTPases are master regulators of actin cytoskeletal dynamics. Accordingly, this branch of the small GTPase super-family synchronizes vital cellular processes that involve changes in cell shape, cell adhesion, polarity and migration.1 The three best known members of this family include Rac1, Cdc42 and RhoA, all of which can act as molecular switches that regulate actin dynamics in response to various extracellular and intracellular signals.2 They exist in an inactive, GDP-bound state until activated by GTP loading. While activation of Rac promotes membrane ruffling and lamellipodia, Cdc42 functions to support filopodium growth, and Rho induces the formation of stress fibers.2 Not surprisingly, both the nucleotide-bound state and GTPase activity of these proteins are carefully controlled. Two classes of effector proteins modulate these events. The Guanine-Nucleotide-Exchange Factors (GEFs) that promote activation by GTP loading, and the GTPase-Activating Proteins (GAPs) that accelerate inactivation by enhancing the rate of GTP hydrolysis.3 In addition, each GTPase has a complement of downstream effectors to propagate individual cellular responses.4
In our recent work “Anchored PKA recruitment of active Rac,” we look specifically at the interplay between Rac and IQGAP2 in response to a protein kinase-A (PKA) phosphorylation event. The IQGAPs are a conserved family of scaffolding proteins that are recognized as Rac and Cdc42 effector molecules.5 They are named for their IQ-domain repeats and observed homology to GAPs, although they do not possess GTPase activating activity.5 The IQGAP1 isoform is ubiquitously expressed, whereas the IQGAP2 isoform has a more restricted expression pattern.6 We show that IQGAP2 is regulated by an interaction with the A-kinase anchoring protein AKAP220. Phosphorylation of IQGAP2 via AKAP220-anchored PKA leads to enhanced Rac binding. Since AKAPs function to direct PKA toward specific substrates, we proposed that the formation of an IQGAP2/AKAP220/PKA ternary complex sharpens the response to cAMP. Although AKAP220 is constitutively bound to PKA, we show that the association of IQGAP2 occurs only in the presence of high intracellular calcium, such as that elicited by growth factor stimulation.7 Bringing PKA to this location provides an environment for anchored kinase to influence the association of active Rac with IQGAP2.
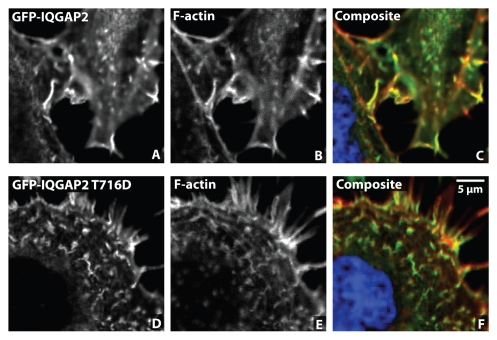
An important facet of our model is that formation of the AKAP220/IQGAP2 complex requires calcium. Additional data presented here in Figure 1 confirms the formation of the AKAP220/IQGAP2 complex can only be detected when experiments are performed in the presence of the calcium ionophore A23187. A secondary event is PKA phosphorylation at T716 of IQGAP2. This enhances binding to AKAP220 and ultimately allows the recruitment of active Rac to the complex. In the absence of PKA stimulation, formation of the complex can be partially recapitulated by the phosphomimetic IQGAP2 T716D (Fig. 1A, lane 7 and Fig. 1B). Furthermore, expression of this phosphomimetic protein was enriched in these regions of the cell and increased membrane ruffling (Fig. 2). It may also be possible that active Rac is recruited to ruffles by phosphorylated IQGAP2.
Figure 1.
IQGAP2 binding to AKAP220 is enhanced in response to increased intercellular calcium and cAMP. (A) Immunoprecipitation of either GFP control, lane 1; wildtype GFPIQGAP2 (WT), lanes 2, 3, 5, 6; or the phosphomimetic GFP-IQGAP-T716D mutant (T716D), lanes 4, 7; and detection of co-precipitating AKAP220 from COS cells. Lanes 1–4, low intracellular calcium, buffer included cheleators (EDTA, EGTA, 1 mM each) for 20 min. Lanes 5–7, high intracellular calcium, cells were simultaneously treated with 5 µM A23187 for 20 min. Lanes 3 and 6, elevated intracellular cAMP, cells were stimulated with 20 µM forskolin (Fsk) and 75 µM IBMX for 20 min. Co-purifying AKAP220 (top part) and total lysate AKAP220 (middle part) was assessed by immunoblot. Immunoprecipitated GFP control, WT and T716D GFP-IQGAP2 protein verified using an antibody recognizing the GFP tag. (B) AKAP220 co-precipitated with IQGAP2 proteins. Amalgamated densitometry data from four experiments, arbitrary units (AU), mean ± SEM.
Figure 2.
Phosphomimetic IQGAP2 is recruited to membrane ruffles. (A–C) Detection of GFP tagged IQGAP2 in cultures of HEK 293 cells. F-actin was stained with fluorescently conjugated phalloidin. (C) Composite image showing GFP-IQGAP2 (green) and F-actin (red). Nuclei were identified with DRAQ5 stain (blue). (D–F) Expression of GFP-IQGAP2 T716D phosphomimetic mutant. (F) Composite image showing GFP-IQGAP2 T716D (green) and F-actin (red). Membrane ruffles are evident in this cell.
Since Cdc42 binding to IQGAP is unaffected under all conditions examined so far, we can conclude that recruitment of Rac is a principle binding event that promotes formation of membrane ruffles. A role for AKAP220 in this process is solely as an organizational platform to orient anchored PKA in a manner that favors the phosphorylation of IQGAP2 on T716. This molecular event facilitates the relay of information via recruitment of active Rac to the actin cytoskeleton. A coincident factor in this process is the fluctuation of intracellular calcium levels. Our current findings have been consolidated into a hypothetical model which is presented in Figure 3. Under resting conditions, intracellular calcium levels are kept low, which prevents IQGAP2 association with AKAP220 (Fig. 3A). Upon growth factor stimulation calcium levels sharply rise and IQGAP2 is able to bind the anchoring protein (Fig. 3B). If cAMP levels are elevated concomitantly the anchored PKA preferentially phosphorylates IQGAP2 (Fig. 3B). Following a sustained growth factor stimulus, intracellular calcium levels decline to an intermediate concentration. By lowering the affinity of IQGAP for AKAP220, a dynamic state of rapid release and binding of IQGAP2 to AKAP220 could ensue. As a result, AKAP220 is able to process multiple molecules of IQGAP, thus favoring IQGAP2 phosphorylation and amplifying the signal (Fig. 3C). This succession of events augments binding of Rac to IQGAP and actin remodeling (Fig. 3C). These events are also likely to be affected by our finding that binding of IQGAP2 to AKAP220 is augmented by cAMP (Fig. 1). Additional studies are needed to examine the dynamics of this interaction in response to a physiological ligand.
Figure 3.
Model depicting dynamic binding of IQGAP2 to AKAP220 in response to a growth factor. (A) In resting cells, intracellular calcium levels are kept low. Under these conditions, IQGAP2 is unbound. (B) Stimulation with growth factors causes calcium levels to sharply increase allowing binding of IQGAP2 to AKAP220. A simultaneous rise in cAMP concentration activates PKA allowing this kinase to phosphorylate anchored IQGAP2 which leads to increased association with active Rac. (C) In the later phases of growth factor treatment, the concentration of calcium decreases to an intermediate level. We propose that this permits passage of many IQGAP2 molecules through the AKAP220 signaling complex. Coordination of these events by AKAP220 and IQGAP2 promotes actin remodeling and membrane ruffling.
This work extends previous findings implicating IQGAPs as effectors of the small GTPase Rac and expands our understanding of how AKAP220 participates in the regulation of cytoskeletal events. It may be noteworthy that IQGAPs and AKAP220 have been implicated in the development of certain malignancies.8,9 Additional investigation is needed to better understand the roles these proteins play in cancer biology and to elucidate the signaling pathways that particpate in these processes. Furthermore, this study offers new insight into how diverse second messengers like calcium and cAMP can be managed by anchoring and scaffolding proteins at discrete locations inside the cell. Additional anchoring proteins including WAVE1, AKAP-Lbc, and Gravin are also engaged in regulation of the actin cytoskeleton, thus underscoring utility of local signal integration in the regulation of related cellular processes.10–12
Acknowledgments
The authors would like to thank Lorene Langeberg for critical evaluation of this work. J.D.S. was supported in part by National Institutes of Health grant DK54441.
Extra View to: Logue JS, Whiting JL, Tunquist B, Langeberg LK, Scott JD. Anchored PKA recruitment of active Rac. J Biol Chem. 2011 doi: 10.1074/jbc.M111.232660jbc.M111.232660.. In press.
References
- 1.Madaule P, Axel R. A novel ras-related gene family. Cell. 1985;41:31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/S0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- 3.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–255. doi: 10.1042/0264-6021:3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 2006;16:242–249. doi: 10.016/j.tcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Watanabe T, Noritake J, Fukata M, Yoshimura T, Itoh N, et al. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci. 2007;120:567–577. doi: 10.1242/jcs.03356. [DOI] [PubMed] [Google Scholar]
- 7.Lester LB, Coghlan VM, Nauert B, Scott JD. Cloning and characterization of a novel A-kinase anchoring protein, AKAP220, association with testicular peroxisomes. J Biol Chem. 1996;271:9460–9465. doi: 10.1074/jbc.271.16.9460. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto N, Imoto I, Fukuda Y, Kurihara N, Kuroda S, Tanigami A, et al. IQGAP1, a negative regulator of cell-cell adhesion, is upregulated by gene amplification at 15q26 in gastric cancer cell lines HSC39 and 40A. J Hum Genet. 2001;46:21–25. doi: 10.1007/s100380170119. [DOI] [PubMed] [Google Scholar]
- 9.Garnis C, Rosin MP, Zhang L, Lam WL. Alteration of AKAP220, an upstream component of the Rb pathway, in oral carcinogenesis. Int J Cancer. 2005;116:813–819. doi: 10.1002/ijc.21065. [DOI] [PubMed] [Google Scholar]
- 10.Soderling SH, Binns KL, Wayman GA, Davee SM, Ong SH, Pawson T, et al. The WRP component of the WAVE-1 complex attenuates Rac-mediated signalling. Nat Cell Biol. 2002;4:970–975. doi: 10.1038/ncb886. [DOI] [PubMed] [Google Scholar]
- 11.Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Galpha 12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276:44247–42257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 12.Weiser DC, Pyati UJ, Kimelman D. Gravin regulates mesodermal cell behavior changes required for axis elongation during zebrafish gastrulation. Genes Dev. 2007;21:1559–1571. doi: 10.1101/gad.1535007. [DOI] [PMC free article] [PubMed] [Google Scholar]