Abstract
The engulfment of apoptotic cell corpses is an evolutionary conserved process used by multicellular systems to remove cells with inappropriate potential (e.g., self-reactive T-cells, potentially cancerous cells). Neighboring or specialized phagocytic cells remove cell corpses through distinct steps: they first recognize the cell on the verge of death, then reorchestrate their cellular architecture toward it, actively contribute to cell killing, and eventually engulf the corpse. Thus engulfment signaling must be tightly controlled to maintain tissue homeostasis. Signaling cascades mediating cell corpse clearance likely converge at the level of the small GTPase CED-10 (Rac1); given this key position, CED-10 must be subject to a tight regulatory mechanism to prevent inappropriate phagocytic events. Here, we discuss recent work characterizing srgp-1 (nematode ortholog of mammalian srGAP), a candidate GTPase activating protein (GAP) for CED-10 involved in cell corpse clearance and “sick” cell killing in C. elegans. We additionally discuss several possible determinants of SRGP-1 function, contributing to either SRGP-1 localization and/or activation. We also survey other potential candidate GTPases that might contribute to cell corpse clearance in C. elegans, and eventually recapitulate the role of engulfment during cell killing.
Key words: apoptosis, phagocytosis, cell corpse clearance, engulfment regulator, cytoskeletal rearrangement, cell killing, cell competition, Rac regulator
Introduction
Programmed cell death (apoptosis), in addition to ‘defensive’ roles in removal of infected, mutated or damaged cells, also acts as a counterbalance to proliferation: excess cells are produced and superfluous cells removed, which ensures appropriate cell numbers during developmental morphogenesis and homeostasis in proliferating tissues.1 Once a single cell is induced to die by apoptosis (either through an innate suicide program or an external stimulus), neighboring or specialized phagocytic cells recognize, internalize and degrade the cell corpse. The immediate clearance of apoptotic cell corpses prevents inflammation and autoimmune disease, as dead cells are not able to release harmful intracellular contents into the surrounding tissue.2,3
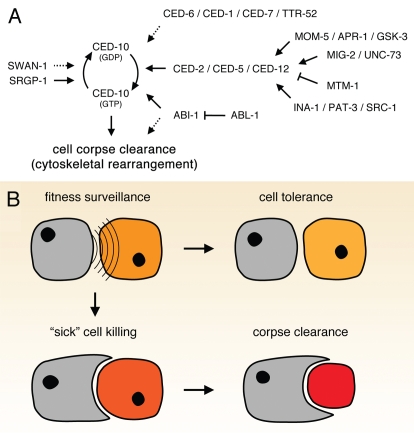
The nematode Caenorhabditis elegans (C. elegans) is a powerful genetic model organism for the study of programmed cell death and cell corpse clearance. To date, greater than 30 genes involved in the induction of cell death and corpse clearance (e.g., recognition, internalization and degradation of the corpse) have been described. Loss-of-function (lf) alleles of engulfment genes that mediate cell corpse clearance result in the accumulation of persistent cell corpses in the soma and/or in the hermaphrodite gonad. By contrast, mutations in genes that negatively regulate cell corpse engulfment result in reduced cell corpse numbers due to more efficient clearance. The interplay of genetics, biochemistry and cell biology led to the identification of at least two evolutionary conserved and partially redundant signaling cascades [comprised of ced-1 (mammalian mEGF10), ced-6 (GULP), ced-7 (ABCA1/ABCA7) and ced-2 (CrkII), ced-5 (Dock180), ced-12 (ELMO)] that likely converge at the level of the small GTPase CED-10 (Rac1), a key regulator of cell corpse internalization (Fig. 1A).4–6 Importantly, it appears that ‘quality’ or amplitude of CED-10 activation is important during engulfment, as overexpression of CED-10 can rescue mutants in individual engulfment pathways. Consequently, CED-10 cycling between GTP-bound (‘on’) and GDP-bound (‘off’) states is tightly regulated (Fig. 1A): GTP loading is promoted by guanine nucleotide exchange factors (GEFs), whereas GTP hydrolysis is facilitated by GTPase activating proteins (GAPs). Regulation of CED-10 activation during corpse removal by the bipartite CED-10 GEF complex CED-5 (Dock180) and CED-12 (Elmo) has been the subject of intense study,7–9 however, GAPs involved in corpse clearance have only recently been identified.
Figure 1.
Engulfment signaling mediates sick cell killing or tolerance. (A) C. elegans engulfment signaling pathways leading to the activation and subsequent inactivation of CED-10 (GTP- and GDP-bound, respectively). Solid and dashed arrows, characterized and unknown signaling events, respectively. (B) Sick cell killing vs. tolerance model. Cells containing the ability to engulf constantly survey their neighbors. Depending on the ‘fitness’ of a particular neighbor, the engulfing cell either tolerates, or—if necessary—actively kills and engulfs the unhealthy neighbor, thereby ensuring the fitness of the entire cell community or tissue.
SRGP-1 Negatively Regulates Cell Clearance
In contrast to the isolation of genes positively regulating corpse removal (where loss-of-function generates corpse persistence, which can be readily addressed by high-powered microscopy), the identification of genes negatively regulating phagocytosis has been hampered by the difficulty of screening for genes that subtly enhance an already efficient process. To simplify the search (which would otherwise require high-power DIC microscopy coupled with four-dimensional analyses), we and others developed a protocol for forward and reverse genetic screening using the vital dye Acridine Orange (AO).10,11 Briefly, internalized apoptotic germ cell corpses in acidic phagosomes are stained by AO (AO+ germ cell corpses) in wild-type worms. In worms defective in germ cell corpse internalization, the numbers of AO+ corpses are greatly reduced.6 Knock-down of a GAP involved in cell corpse clearance in strains with attenuated engulfment would thus result in increased GTP-bound CED-10 and a restoration of engulfment activity (and restoration of AO staining), which can readily be observed under a simple fluorescence dissecting scope. Using this experimental set up, we were able to isolate a single candidate, srgp-1 (srGAP), as a potential negative regulator of cell corpse clearance after screening all GAP domain containing proteins in the C. elegans genome.
A series of subsequent in-depth rescue experiments as well as genetic and microscopic analyses revealed a tissue specific function of srgp-1 in the engulfing cell. Detailed analyses of cell corpse clearance and distal tip cell (DTC) migration suggested that srgp-1 inhibits signaling that otherwise promotes remodeling of the cytoskeleton. Additionally, double- and triple-mutant analyses indicated that srgp-1 acts genetically on the small GTPase ced-10, where both engulfment signaling pathways ‘converge’.
These genetic analyses are consistent with the previously described role of srGAP proteins as GAPs for the Rho-family of GTPases;12 biochemical analyses revealed that SRGP-1 specifically interacts with the GTP-bound, but not GDP-bound, isoforms of CED-10, but not with other GTPases such as MIG-2(RhoG) or RHO-1(RhoA). Importantly, the GAP domain of SRGP-1 enhanced the intrinsic GTPase catalytic activity of mammalian Rac1 in vitro; mutation of a conserved arginine finger in the SRGP-1 GAP domain (R563),13 which results in a protein that can interact with Rac, but is catalytically dead, showed no enhanced GTPase activity. Consistent with these in vitro data, worms expressing SRGP-1(R563A) or a truncated protein completely lacking the GAP domain failed to rescue the srgp-1 suppressor phenotype. It is thus likely that worm SRGP-1 functions as a GAP for CED-10 (Rac1) in vivo.
The role of SRGP-1 during corpse removal also appears to be evolutionarily conserved. The overexpression of srGAP1 (or a dominant negative Rac1 mutant) could inhibit the engulfment activity of LR73 cells, and by contrast, a simultaneous siRNA-mediated knock-down of all 3 srGAP family members in NIH/3T3 fibroblasts resulted in a significant increase in engulfment activity. Thus, srGAP family members, particularly srGAP1, can also function to inhibit cell corpse clearance in mammals.
A role for SRGP-1 in “Sick Cell” Killing and Competetion
Engulfment signaling in C. elegans is required not only for the clearance of apoptotic cells, but also for the killing of cells brought to the verge of death: in animals with reduced caspase activity (reduction-of-function ced-3(rf) mutants), significantly more cells survive in engulfment mutant backgrounds as compared with wild type,14,15 suggesting that phagocytic processes must promote cell killing under these conditions. Indeed, studies on cell competition in Drosophila have identified engulfment genes as key promoters of cell competition,16 confirming the importance of these pathways. Intriguingly, significantly fewer cells survived when engulfment signaling was over-activated, as in srgp-1 mutants. Similar results were observed in mutants with neurotoxic or cytotoxic cell death,17,18 where fewer cells survived in srgp-1 mutants. Thus, engulfment signaling does more than remove apoptotic debris, it can also promote the killing of unhealthy or less ‘fit’ cells within a tissue.
SRGP-1 Localization and Activation during Corpse Clearance
These observations raise a number of important questions: What determines the localization and subsequently the activation of SRGP-1 which leads to the inhibition of both the engulfment signaling cascade and, more importantly, the killing of unhealthy cells? Does re-localization rely on an upstream signaling cascade, for example a transmembrane receptor that is involved in the recruitment of SRGP-1? What determines the SRGP-1 GAP activity? Is the GAP activation subjected to an intramolecular conformational change? Are there other SRGP-1 GTPase targets that could be involved in engulfment signaling? There are a variety of possibilities that could regulate SRGP-1 localization and activity, and there are potential other candidate GTPase targets for SRGP-1, and we will discuss a few of them below.
Mammalian srGAP family members are required for neuronal migration; the secreted extracellular ‘avoidance’ cue Slit activates an intracellular signal transduction pathway through the Robo receptor and srGAP1,12,19 with Slit-bound Robo inducing srGAP1 activity and reducing actin polymerization via Cdc42 in this context. Two pieces of evidence suggest Slit/Robo is not an upstream signaling module in cell corpse clearance in the nematode: First, neither Slit nor Robo mutants (worm proteins SLT-1 and SAX-3, respectively) showed an effect on cell clearance (our unpublished observations). Second, SRGP-1 lacks the SH3 domain important for Robo binding,20 suggesting that the role of srGAP1 in Slit/Robo signaling may not be conserved in the nematode. These observations suggest that another signaling cascade is likely involved in recruiting SRGP-1 to membranes.
How could SRGP-1 membrane recruitment occur, independent of any transmembrane receptor? Our in vivo structure/function analyses revealed that SRGP-1 proteins lacking the BAR domain (ΔBAR) failed to rescue the srgp-1 suppressor phenotype. Several familiy members of BAR domain protein structures have been solved and their membrane curvature binding abilities characterized.21 In the context of cell corpse clearance, the SRGP-1 BAR domain might directly sense membrane invaginations; these could serve as “late-stage-features” of cell clearance. Consistent with this idea, SRGP-1 would be recruited to areas of membrane curvature during late stages of engulfment, limiting active Rac to the ‘leading edge’ of the phagocytic cup. SRGP-1 might also bind—as an alternative—to an unknown player or a bridging molecule which would specify binding to a particular phospholipid; the coordinated membrane localization might then specifically target GAP activity to specific domains on the membrane.
Another open question remains: what leads to the activation of the GAP activity, once SRGP-1 is at its correct location? To our initial surprise, SRGP-1 proteins lacking the C-terminus (ΔC-term) completely failed to rescue the engulfment phenotype of srgp-1 mutants. We subsequently observed in yeast 2-hybrid assays that the C-terminus is necessary for SRGP-1 homo-dimerization (our unpublished results). Interestingly, in another signaling context, it has been proposed that the C-terminal domain of SRGP-1 can act as an auto-inhibitory switch,20 where the C-terminal half of SRGP-1 directly binds to the N-terminal half. Consistent with the above observations, it is thus tempting to speculate that SRGP-1 recruitment to membrane invaginations leads to an intramolecular conformational change exposing the GAP domain and facilitating Rac-GTP hydrolysis. However, this hypothesis remains extremely speculative.
Other SRGP-1 GTPase Targets
Are there SRGP-1 targets other than CED-10(Rac1) during cell corpse clearance in C. elegans? Our in vitro binding assays revealed a specific interaction of the SRGP-1 GAP domain with CED-10, but neither with RHO-1 nor with MIG-2. That excludes those two candidate GTPases as targets for SRGP-1. This is also supported by our epistatic analyses, where srgp-1 acts downstream of the ced-5-ced-12 GEF complex, whereas mig-2 acts upstream of it.
Slit/Robo signaling results in an srGAP1-dependent inactivation of Cdc42 in mammalian systems;12 however, distinct activities for Cdc42 and Rac1 in the context of actin- and membranes-rearrangements have been described.22 Nevertheless, both GTPases share several effector proteins,23 thus it would not be surprising for these GTPases to share siminar regulatory machineries. Interestingly, animals carrying a cdc-42(gk388) null allele do not show any defects in cell corpse clearance (our unpublished observations): Homozygous cdc-42 animals arrest as L3/L4 larvae, which could be due to maternal contribution of cdc-42(+) mRNA provided by heterozygous mothers; such a compensatory effect could thereby mask an engulfment defect. Further studies of cdc-42 function might provide insights into the cellular and molecular role of this GTPase in cell corpse clearance in C. elegans.
Engulfment and Cell Killing
The decision to commit any kind of death program is not based on the dying cell alone; rather, it comprises both cell intrinsic and extrinsic events that coordinately regulate cell fate, as suggested by our findings. Therefore, we speculate that upon intrinsic induction of a particular death program within a suboptimal cell, the surrounding tissue, consisting of fit and healthy neighboring cells, might support the unhealthy cell's commitment to a terminal fate, which leads to its eventual demise/clearance. The probability of such a scenario is elevated in srgp-1 mutants: once a ‘sick cell’ sends out weak ‘eat-me’ signal(s), sustained activation of engulfment signaling (as in srgp-1 mutants) is thought to contribute to the killing of such a cell on the verge of death. The strength of activation appears to partially depend on SRGP-1 activity, as it modulates the “ON/OFF” balance of the small GTPase CED-10(Rac).
Such a mechanism for cell killing appears very similar to that observed during cell competition in Drosophila melanogaster,24 where cells compete for ‘survival signals’. Consistent with that idea, Li and Baker recently described the involvement of several engulfment genes in cell competition in this model.16 Phagocytic potential might thus serve as a quality control mechanism that surveys the fitness of neighboring cells, and if necessary, induces their elimination (Fig. 1B). Indeed, the discrimination of fit and healthy cells from those that are unfit is of key importance for the whole organism as well, as cell competition can also affect organismal fitness.
If this is the case, it raises an additional number of important questions: How would such a fitness sensing system work, and what are the molecular components required for the sensing/killing of sick cells on the verge of death? What molecules define whether a neighboring sick cell should be eliminated or tolerated? How can sick cells escape their fate and evade death and clearance?
A recent study described the effect of progranulin on changes in the kinetics of cell death in a neuronal context: normal levels of secreted Progranulin provide a neuron that undergoes a sublethal insult with adequate time to repair itself and survive. However, if insufficient Progranulin is present, the rate at which the very same injured neuron is recognized and/or engulfed by phagocytic cells is accelerated, and the damaged cell has less time to recover.25 Changing the regulation of programmed cell death kinetics represents a valid and potentially important hypothesis by which phagocytic cells may contribute to neuron loss and/or neurodegeneration.
A shift in the dynamic equilibrium toward survival or death in individual cells, as triggered by engulfment activity, could therefore explain a cumulative neuronal loss. A defense strategy to kill/remove or to promote survival of any sick cells within a community has to be tightly regulated. Either the impairment or the over-activation of such a strategy might lead to a handicap for the whole organism, implying important and yet unstudied mechanisms regulating cell clearance. Further, this might represent a druggable target by which cells on the verge of death might be saved, for example, during ischemic events in the heart and brain, which, in combination with other interventional strategies, may positively affect clinical outcome.
Acknowledgments
This work was supported by grants from the European Union (FP5 project APOCLEAR) to L.J.N. and the American Cancer Society and the American Heart Association to J.M.K.
Abbreviations
- AO
Acridine Orange
- ced
cell death abnormal
- DTC
distal tip cell
- GAP
GTPase-activating proteins
- GEF
guanine nucleotide exchange factors
- RNAi
RNA interference
- srgp
Slit-Robo Rho GTPase activating protein
Extra View to: Neukomm LJ, Frei AP, Cabello J, Kinchen JM, Zaidel-Bar R, Ma Z, et al. Loss of the RhoGAP SRGP-1 promotes the clearance of dead and injured cells in Caenorhabditis elegans. Nat Cell Biol. 2011;13:79–862011. doi: 10.1038/ncb2138.
References
- 1.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/S0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 2.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 3.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 5.Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol. 2000;2:131–136. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- 6.Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H, et al. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature. 2005;434:93–99. doi: 10.1038/nature03263. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Z, Caron E, Hartwieg E, Hall A, Horvitz HR. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell. 2001;1:477–489. doi: 10.1016/S1534-5807(01)00058-2. [DOI] [PubMed] [Google Scholar]
- 8.Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/S0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 9.Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–504. doi: 10.1038/32195. [DOI] [PubMed] [Google Scholar]
- 10.Lettre G, Kritikou EA, Jaeggi M, Calixto A, Fraser AG, Kamath RS, et al. Genome-wide RNAi identifies p53-dependent and -independent regulators of germ cell apoptosis in C. elegans. Cell Death Differ. 2004;11:1198–1203. doi: 10.1038/sj.cdd.4401488. [DOI] [PubMed] [Google Scholar]
- 11.Neukomm LJ, Frei AP, Cabello J, Kinchen JM, Zaidel-Bar R, Ma Z, et al. Loss of the RhoGAP SRGP-1 promotes the clearance of dead and injured cells in Caenorhabditis elegans. Nat Cell Biol. 2011;13:79–86. doi: 10.1038/ncb2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–221. doi: 10.1016/S0092-8674(01)00530-X. [DOI] [PubMed] [Google Scholar]
- 13.Barrett T, Xiao B, Dodson EJ, Dodson G, Ludbrook SB, Nurmahomed K, et al. The structure of the GTPase-activating domain from p50rhoGAP. Nature. 1997;385:458–461. doi: 10.1038/385458a0. [DOI] [PubMed] [Google Scholar]
- 14.Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in C. elegans. Nature. 2001;412:198–202. doi: 10.1038/35084096. [DOI] [PubMed] [Google Scholar]
- 15.Hoeppner DJ, Hengartner MO, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature. 2001;412:202–206. doi: 10.1038/35084103. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Baker NE. Engulfment is required for cell competition. Cell. 2007;129:1215–1225. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Bianchi L, Lee WH, Wang Y, Israel S, Driscoll M. Intersubunit interactions between mutant DEG/ENaCs induce synthetic neurotoxicity. Cell Death Differ. 2008;15:1794–1803. doi: 10.1038/cdd.2008.114. [DOI] [PubMed] [Google Scholar]
- 18.Galvin BD, Kim S, Horvitz HR. Caenorhabditis elegans genes required for the engulfment of apoptotic corpses function in the cytotoxic cell deaths induced by mutations in lin-24 and lin-33. Genetics. 2008;179:403–417. doi: 10.1534/genetics.108.087221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacon C, Endris V, Rappold G. Dynamic expression of the Slit-Robo GTPase activating protein genes during development of the murine nervous system. J Comp Neurol. 2009;513:224–236. doi: 10.1002/cne.21955. [DOI] [PubMed] [Google Scholar]
- 20.Zaidel-Bar R, Joyce MJ, Lynch AM, Witte K, Audhya A, Hardin J. The F-BAR domain of SRGP-1 facilitates cell-cell adhesion during C. elegans morphogenesis. J Cell Biol. 2010;191:761–769. doi: 10.1083/jcb.201005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suetsugu S, Toyooka K, Senju Y. Subcellular membrane curvature mediated by the BAR domain superfamily proteins. Semin Cell Dev Biol. 2010;21:340–349. doi: 10.1016/j.semcdb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Hoppe AD, Swanson JA. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell. 2004;15:3509–3519. doi: 10.1091/mbc.E03-11-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 24.Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/S0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- 25.Kao AW, Eisenhut RJ, Martens LH, Nakamura A, Huang A, Bagley JA, et al. A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc Natl Acad Sci USA. 2011;108:4441–4446. doi: 10.1073/pnas.1100650108. [DOI] [PMC free article] [PubMed] [Google Scholar]