Abstract
α1-Antitrypsin (A1AT) is a polyvalent, acute-phase reactant with an extensive range of biological functions that go beyond those usually linked to its antiprotease (serpin) activities. Genetic mutations cause a systemic deficiency of A1AT, leading to liver and pulmonary diseases, including emphysema and chronic bronchitis. The pathogenesis of emphysema, which involves the destruction of small airway structures and alveolar units, is triggered by cigarette smoke and pollutants. The tissue damage caused by these agents is further potentiated by the mutual interactions between apoptosis, oxidative stress, and protease/antiprotease imbalance. These processes lead to the activation of endogenous mediators of tissue destruction, including the lipid ceramide, extracellular matrix proteins, and abnormal inflammatory cell signaling. In this review, we propose that A1AT has a range of actions that are not restricted to protease inhibition but rather extend to mitigate a range of these pathological processes involved in the development of emphysema. We discuss the evidence indicating that A1AT blocks apoptosis by binding and inhibiting active caspase-3 and modulates a broad range of inflammatory responses induced by neutrophils and by lipopolyssacharide and tumor necrosis factor-α signaling.
Keywords: COPD, apoptosis, inflammation, proteinase inhibitor, serpin
α1-Antitrypsin (A1AT), an acute-phase protein, is the prototypic member of the serpin super family and a major inhibitor of serine proteases such as neutrophil elastase and proteinase-3 (1). Present in large concentrations in the blood, A1AT is thought to play an important role in limiting host tissue injury by proteases at sites of inflammation. The clinical importance of A1AT is highlighted in individuals with inherited A1AT deficiency who exhibit an increased susceptibility to chronic inflammatory conditions, including chronic obstructive lung disease (COPD), liver diseases, and, occasionally, systemic vasculitis and necrotizing panniculitis (2). Recent findings highlight the potential inhibitory actions of A1AT in several proteolytic pathways, which also indicate that it has broader antiprotease activities than previously anticipated. A1AT may also exhibit antiinflammatory activities independent of its protease inhibitor function, which suggests the existence of receptor-mediated A1AT intracellular internalization and provides further evidence of a broad protective role of A1AT in lung and systemic organ injury (3, 4).
These actions have been uncovered by studies that addressed the underlying mechanisms of A1AT deficiency. A1AT deficiency most often occurs due to mutation of glutamic acid to lysine at position 342 (also known as the PiZ mutant of A1AT). The severe deficiency of A1AT (PiZZ) is associated with spontaneous A1AT polymerization, retained A1AT in the cytoplasm of liver cells resulting in marked reduction of serum levels of the inhibitor, and increased risk to develop COPD and liver cirrhosis (2). Population studies suggest a minimum plasma threshold of 11 μmol/L, below which there is a risk of developing COPD prematurely; pulmonary symptoms usually occur in the third to fifth decade of life, and the disease may be fatal by age 60. The disease is markedly accelerated by cigarette smoking; smokers with A1AT deficiency typically live 10 to 20 years less than do those who are life-long nonsmokers.
COPD involves the remodeling of the large airway segments and destruction of small airway units and alveolar structures. A1AT deficiency underlies approximately 5% of all cases of COPD (5) and has therefore provided compelling evidence in favor of the protease/antiprotease imbalance (6) hypothesis of COPD. However, several studies in the past 10 years have revealed that the pathogenesis of COPD, particularly of emphysema, involves several additional pathobiological processes that interact with the pulmonary inflammation and protease/antiprotease triggered by cigarette smoke. The increasingly evident complexity of the pathogenesis of COPD (7, 8), including cases due to A1AT deficiency, explains the lack of successful therapeutic approaches aimed at controlling inflammation and excessive extracellular matrix proteolyses and may reveal potential new targets for modulation of disease activity and therapy.
There is growing evidence that disruption of the cell signaling that underlies the structural and molecular maintenance of alveolar structure may play an important role in emphysema (9). Paradigmatic of the so-called hypothesis of the “lung maintenance program” was the demonstration of the role of vascular endothelial growth factor (VEGF) in the preservation of alveolar structure and how its disruption triggers alveolar enlargement in rodents (10, 11); subsequent studies validated these experimental observations in diseased human lungs and rat models involving chronic cigarette smoke exposure (12). In fact, VEGF is one of a host of molecules linked to structural lung preservation and, once depleted, is causative of emphysematous lung destruction (13, 14). The VEGF paradigm is linked mechanistically alveolar cell apoptosis, alveolar destruction, and emphysema (15), validating the early hypothesis of Aoshiba and Nogai (16) and parallel findings in diseased human lung (17, 18).
The understanding of the role of disrupted alveolar maintenance and alveolar cell apoptosis in the pathogenesis of emphysema has further evolved with the demonstration of the experimental link between alveolar cell apoptosis, extracellular matrix proteases (19), and oxidative stress (20). When pathologically activated, these processes are mutually interactive and promote feed-forward acceleration of alveolar injury, leading to emphysema development. The importance of the interplay between alveolar cell apoptosis and cigarette smoke–induced alveolar destruction was further strengthened by the findings of increased susceptibility to alveolar cell apoptosis, inflammation, and alveolar enlargement in nuclear factor E2–related factor 2–null mice (21) and the protection afforded by 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole, an NRF-2 activator that correlated with decreased alveolar cell apoptosis (but not inflammation) in cigarette smoke-exposed mice (22).
More recently, alveolar cell senescence has also been linked to emphysematous lung destruction (23–25). The initial insights of the role(s) of aging in COPD have originated from observations showing that cigarette smoke triggers cellular senescence in vitro (26, 27), human emphysematous lungs express markers of senescence (28), and peripheral blood mononuclear cells of COPD patients have decreased expression of telomerase (29, 30). Indeed, COPD lungs have evidence of increased expression of oxidative stress (31, 32), often associated with DNA damage and development of senescence in cell culture systems (33) and in vivo (34).
This changing pathogenetic landscape linked to COPD begs the reassessment of the concepts of inflammation and protease/antiprotease imbalance. Because this “classic” paradigm cannot explain the aggregate of these new data, a more fitting and all-inclusive model is warranted. Rather than being a uniform process throughout the course of cigarette smoke exposure, it is becoming apparent that the inflammation in COPD may be due to an autoimmune component that arises from the extensive damage imposed by chronic exposure of the lung to cigarette smoke (35); this hypothesis has been recently updated with additional experimental evidence (36). The “autoimmune” hypothesis would therefore explain the progression of the disease and persistence of inflammation despite cigarette smoke cessation (37).
In light of these data and recent conceptual breakthroughs, the authors postulate that lung injury by cigarette smoke, including that caused in patients deficient in A1AT, involves “endogenous” molecular processes of destruction that overcome the lungs' ability to repair and maintain its structure. This paradigm is also supported by the following solid experimental evidence: (1) Extracellular matrix degradation products elicit enhanced inflammation (38, 39) and sensitize immune cells (40); (2) the engagement of apoptosis and oxidative stress activate the proapoptotic lipid signaling molecule ceramide (41, 42), causing it to further stimulate its own synthesis; and (3) RNA viral products sensitize and accelerate cigarette smoke–induced alveolar cell apoptosis, inflammation, and alveolar enlargement (43).
It is our goal to develop the concept that A1AT acts at multiple biological levels in addition to its serpin function, most notably in controlling several of these “endogenous” mediators of alveolar injury due to cigarette smoke. We underscore the antiapoptotic and antiinflammatory properties of A1AT. A1AT is synthesized and released by numerous cells, including lung airway cells, liver cells (which are the source of the majority of A1AT in the circulation), monocytes, alveolar macrophages, corneal and intestinal epithelial, and cancer cells. Its high abundance in the serum (in excess of 1.34 mg/ml) and potential efficacy in neutralizing extracellular proteases when in excess of 800 μg/ml provide support for A1AT extracellular actions while potentially overshadowing important intracellular functions of A1AT that are reliant on cell access and receptor-mediated internalization. This paradigm serves as a framework for future investigation in lung disease associated with A1AT deficiency because it raises important questions related to the most fundamental properties of this acute phase reactant.
A1AT ACTS AS APOPTOSIS INHIBITOR
The hypothesis that A1AT acts as a potential apoptosis inhibitor originated from the observation of Shapiro and colleagues that A1AT protects against replication of HIV-1 in infected lymphocytes (44) and the documentation that HIV-1 causes lymphocyte apoptosis (45). Alveolar cell apoptosis had been documented in human diseased lungs with COPD and in an experimental model of rodent emphysema caused by VEGF-receptor blockade. In support of an antiapoptotic role for A1AT were the related findings that the serpin α-1 antichymotrypsin and the acute-phase reactant α-2 macrogolublin protected against serum withdrawal–induced vascular smooth muscle cell death (46) and that A1AT inhibited cell death and inflammation against ischemia-reperfusion–induced cell injury (47).
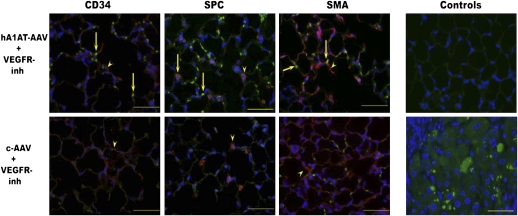
Based on the evidence outlined above, we aimed to show that: (1) Human A1AT supplementation protects against emphysema, alveolar cell apoptosis, and oxidative stress caused by VEGF receptor inhibition or by direct intratracheal instillation of active caspase in mice and rats; (2) A1AT is internalized and inhibits endothelial cell apoptosis in vitro; (3) A1AT specifically interacts with and inhibits active caspase-3 active in a cell-free system; and (4) A1AT is internalized in cells via potential cell surface endocytotic processes. Despite minor increases of total serum levels of A1AT in mice transduced with human A1AT (as determined by weekly ELISA measurements for up to 4 wk), we noted clear evidence of intracellular accumulation of human A1AT in alveolar endothelial and epithelial cells (Figure 1), suggesting preferential uptake of the transduced protein in rodent alveolar septal cells in vivo (48). A1AT supplementation given systemically via adeno-associated virus transduction in the muscle or locally in the lung via intratracheal instillation afforded protection against alveolar cell apoptosis, oxidative stress, and alveolar enlargement caused by VEGF receptor inhibition (48) or alveolar enlargement caused intratracheal instillation of active caspase-3 (49). A similar protective role was also observed in vitro against staurosporine-induced endothelial cell death (49). Because these models are largely independent of inflammation, the protective effects could be assigned to the inhibition of alveolar cell apoptosis by A1AT.
Figure 1.
Expression of human α1-antitrypsin (A1AT) in mouse lungs, which were transduced with adenoassociated virus-human A1AT or adenoassociated virus control via muscular route. Human A1AT (green signal) colocalized with the endothelial cell marker CD34 (red signal), type II cell marker prosurfactant protein C (SPC, red signal), and interstitial fibroblasts (smooth muscle cell actin [SMA], red signal). Mice were treated with the vascular endothelial growth factor–receptor blocker SU5416, which promotes apoptosis-dependent emphysema phenotype. Negative controls consisted of a mouse lung section stained with goat serum (upper right panel), and positive control consisted of A1AT liver with the characteristic globular A1AT cellular deposits (lower right panel). Arrows highlight colocalization signals between human A1AT and cell-specific marker (yellow signal). (Reprinted by permission from Reference 48).
To provide additional data on the mechanisms underlying the protective effects of A1AT, we documented that A1AT directly bound active caspase-3 and inhibited its activity with approximately half of the specific inhibitory activity than observed with its natural target, neutrophil elastase (49). Extensive experimental manipulations supported the specificity and accuracy of these findings because A1AT does not have the Bir2 domain required for specific inhibition of caspases, as observed with XIAP. Heat-induced polymerization, enzymatic cleavage of the neutrophil elastase binding site, and the C36 fragment of A1AT had decreased (with polymers) or abrogated (cleaved A1AT) anti–caspase-3 activity (49). Similar activities against active caspase-3 were not observed at equimolar levels of α-2 macrogolulin and serum albumin; moreover, human A1AT did not inhibit caspase-8 in cell-free assays with purified proteins.
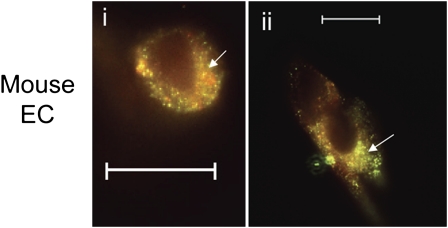
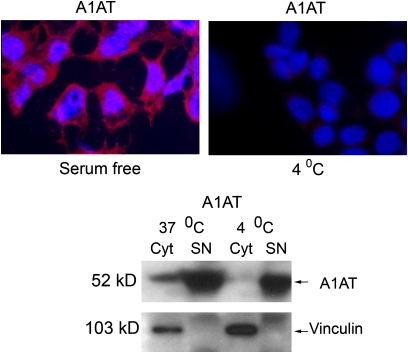
Perhaps the most striking findings were the areas of discrete colocalization of fluorochrome-labeled A1AT and active caspase-3 in cultured endothelial cells (49, 50) (Figure 2); these findings suggest that A1AT can bind to potential cell surface receptors or molecules, can be internalized, and can be localized within the cytosolic compartment. There is documentation of cell surface receptors that can localize and act in the nucleus (51) and of cytoplasmic localization of engulfed molecules, possibly mediated by the karyopherin carrier nuclear import system (52). Further strengthening the specificity of these findings and underscoring the potential importance of A1AT internalization by cells that do not produce the serpin, we have recently documented that A1AT internalization is inhibited at 4°C, implicating potential cell surface receptors in this process, and the central role of clathrin-coated vesicles as the internalization was inhibited by chlorpromazine (50) (Figure 3).
Figure 2.
Colocalization of human A1AT labeled with DyLight 547 (green signal) and active caspase 3 (red signal) in cultured mouse lung endothelial cells (panels I and ii). Superimposed fluorescence signals (yellow signal) are highlighted by arrows. EC = endothelial cells. (Reprinted by permission from Reference 49).
Figure 3.
Evidence of receptor mediated internalization of labeled human A1AT by primary cultures of lung endothelial cells. Human A1AT (labeled in red) is internalized in serum-free conditions at 37°C; internalization is abolished at 4°C. The immunofluorescence data are confirmed by Western blot for human A1AT and vinculin (loading control) in cytoplasmic (cyt) and supernatant (SN) protein fractions of cells cultured at 37°C and 4°C culture conditions. (Reprinted by permission from Reference 50).
These data highlight the importance of A1AT in the intracellular environment, reaching specialized cytosolic regions involved in the preservation of cellular integrity. This process may also mediate the protective effects of A1AT in islet cell rejection (53). The modification of apoptosis responses will have an impact on subsequent inflammatory events because an enhanced cell death with decreased apoptotic cell clearance (efferocytosis) (54) amplifies inflammatory stimuli and may create favorable exposure of autoantigens, leading to autoimmunity. In turn, the cellular internalization of wild-type A1AT may be altered by posttranslational modifications induced, for example, by oxidative stress, which may modulate the serpin's access to putative cellular receptors.
A1AT as a Modulator of Inflammation and Extracellular Matrix Proteolysis
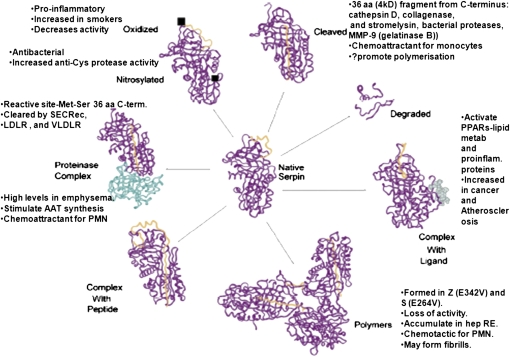
Many of the relevant antiinflammatory functions of A1AT are lost due to A1AT deficiency (Figure 4), primarily resulting from levels of elastase that exceed the concentration of its inhibitor. Indeed, A1AT deficiency–related emphysema is characterized by increased levels of free elastase and a high neutrophil burden (55). Neutrophils from subjects with ZZ A1AT deficiency show enhanced responses to delayed hypersensitivity (56), and PMNs of infants and children with ZZ A1AT deficiency show defective chemotactic migration (57); moreover, PiZZ sera generate a higher quantity of chemotactic factor(s). Consistent with these results, A1AT treatment abolished cigarette smoke–induced increase in neutrophils in the bronchoalveolar lavage in mice (58), whereas patients with cystic fibrosis (CF) who received inhaled A1AT had decreased numbers of induced sputum neutrophils (59). Accordingly, our laboratory reported that A1AT augmentation therapy is associated with a reduction in the release of the proinflammatory cytokine IL-8 by neutrophils (60). Although no direct studies have been performed to elucidate the mechanisms behind these differences between MM and ZZ neutrophils, physiological levels of purified A1AT inhibit PMN chemokinesis by about 35% and chemotaxis by 10% (61), whereas the polymerized form of A1AT, which is generated as a result of the ZZ mutation, has potent neutrophil chemotactic effects (62). These properties of the A1AT conformers may at least partly explain why neutrophils isolated from PiZZ A1AT deficiency differ from those isolated from subjects with normal PiMM A1AT.
Figure 4.
Summary of known functions that may affect inflammation of the different forms of A1AT. aa = aminoacid; PMN = neutrophils; PPAR = peroxisome protein activated receptor; RE = endoplasmic reticulum). (Adapted from Reference 4 with permission).
Increased airway elastase levels due to A1AT deficiency impair pulmonary bacterial killing of neutrophils via cleavage of the cell surface chemokine receptor CXCR1. Airway neutrophils from patients with COPD, bronchiectasis, or CF showed reduced surface expression of CXCR1 (IL-8RA), which directly correlated with levels of airway free elastase and with reduced bacterial killing capacity relative to control subjects (63). In addition, proteolytically cleaved fragments of CXCR1 can stimulate IL-8 release via a mechanism involving Toll-like receptor–2 (64). Taken together, the lack of A1AT and increased levels of free elastase would exacerbate bacteria-induced pulmonary neutrophilia, oxidative burst responses, and defective bacterial killing. Moreover, in vitro data have demonstrated that A1AT can promote fibroblast proliferation and procollagen synthesis (65) via classic mitogen-type interactions with cell-surface receptors and activation of tyrosine kinase and mitogen-activated protein kinase pathways. Therefore, in A1AT deficiency, excessive proteolysis of the interstitium may also be coupled with loss of repair functions to worsen tissue damage.
Notwithstanding the abundant correlative evidence of A1AT deficiency and increased elastase burden, recent studies support even broader antiinflammatory roles for exogenous A1AT. For example, in vitro A1AT abrogates bacterial endotoxin LPS-induced chemokine release from monocytes (tumor necrosis factor [TNF]-α and IL-1β) and neutrophils (IL-8), enhances IL-10 production, and regulates CD14 and toll-like receptor–4 expression (66, 67). At least two key antiinflammatory activities of A1AT—inhibition of endotoxin-stimulated TNF-α and enhancement of IL-10 in human monocytes/macrophages—are mediated by an elevation of cAMP and activation of cAMP-dependent protein kinase A (67). We also demonstrated that A1AT induces a broad antiinflammatory profile on gene expression in primary human lung microvascular endothelial cells, including the suppression of self-induced TNF-α expression (68). Animal studies provide further evidence of antiinflammatory effects achieved by using A1AT therapy, which inhibited TNF-α or LPS-induced lethality (69–71) and prolonged islet graft survival and normoglycemia in transplanted allogeneic diabetic mice (72). A1AT also increases IL-1 receptor antagonist levels (73), which plays a key role in controlling the susceptibility to LPS and TNF-α–induced shock (74). In addition to the effect of A1AT replacement therapy on reducing elastase activity, short-term A1AT treatment decreased the levels of leukotriene B4, a central mediator of pulmonary inflammation in patients with A1AT deficiency (75–77), which is found elevated during disease exacerbations (20). Furthermore, there is recent evidence that A1AT regulates matriptase, a cell surface serine protease involved in the activation of epithelial sodium channels. Our report that A1AT inactivates the catalytic domain of matriptase in vitro and inhibits epithelial sodium transport in vitro and in vivo (67, 78) may provide a novel pharmacologic target for improving mucociliary clearance in COPD and CF.
Churg and colleagues documented that A1AT inhibits proinflammatory activation of alveolar macrophages by neutralizing proteases of the coagulation cascade, particularly thrombin and plasmin, thereby inhibiting the activation of the protease-activated receptors by cigarette smoke or thrombin (3, 79). These findings further highlighted the multitude of regulatory functions exerted by A1AT, which might affect the activation of endogenous pathways of alveolar destruction found in emphysema.
Notwithstanding the insights gained on the pleiotropic functions of A1AT, the pathogenesis of the pulmonary and liver disease caused by the PiZZ mutant remains unclear (Figure 4). The PiZZ mutant form of A1AT has been linked to increased inflammation by means of polymer formation (80). Recent in vitro work with the airway epithelial cell line 16HBE14o has indicated that overexpression of the PiZZ mutant of A1AT inhibits apoptosis to a larger degree than that observed with overexpressed wild-type A1AT (81). Overproduction of PiZZ A1AT leads to endoplasmic reticulum stress, which may engage apoptosis if there is progressive accumulation of misfolded proteins, such as polymerized PiZZ A1AT; an antiapoptotic effect of the mutant protein may therefore offset death signals in specific lung cells. It remains to be demonstrated whether the PiZ mutant can access the intracytoplasmic environment from the extracellular space and whether it interacts and inhibits active caspase-3 to the same extent as seen with the wild-type (MM) A1AT in peripheral versus central airway cells.
CONCLUSIONS
These data support the multitude of important biological functions exerted by A1AT, which in aggregate are in line with our hypothesis that A1AT modulates several pathogenetic processes underlying lung and systemic diseases. These processes modulate inflammation, tissue and cell injury, and repair. A1AT might therefore represent an important damper of innate immune responses and an integrator of antiapoptotic processes and control of inflammation that protects the lung and possibly other organs.
It is unclear whether the manifestations of A1AT deficiency are related to loss of these protective actions of wild-type A1AT and whether they can be recovered if sufficient PiZZ mutant reaches the circulation (i.e., if the MM and ZZ mutants are equally protective). Alternatively, the pathogenesis of A1AT deficiency may involve independent pathogenetic actions of the PiZZ mutant, including the potential for extracellular polymerization, which can trigger inflammation. Moreover, the PiZZ mutant may not be internalized as efficiently as the wild-type serpin and is therefore incapable of modulating intracellular processes. The fate of the intravascular and extravascular (i.e., pericellular) pools of A1AT appear to be critical to its broad biological functions. The continued investigation of these questions will broaden our understanding of the pathogenesis of A1AT deficiency, improve our definition of disease susceptibility, and direct us to potential therapies.
Supported by grant HL66554 and Alpha 1 Foundation grant-in-aid (R.M.T.), VA Merit Award (I.P.), and NIH PO1 1P50 HL084945 (R.T. and I.P.).
Author Disclosure: R.M.T. received grant support from the NHLBI (more than $100,001). S.M.J. received grant support from Talecris Biotherapeitics GmbH, Germany (more than $100,001). I.P. received grant support from Quark Bio ($10,001–$50,000), the NIH, VA (more than $100,001), and the AHA ($10,001–$50,000).
References
- 1.Lomas DA. The selective advantage of α1-antitrypsin deficiency. Am J Respir Crit Care Med 2006;173:1072–1077. [DOI] [PubMed] [Google Scholar]
- 2.Stoller JK, Aboussouan LS. α1-Antitrypsin deficiency. Lancet 2005;2005:2225–2236. [DOI] [PubMed] [Google Scholar]
- 3.Tuder RM, Petrache I. Molecular multitasking in the airspace: α1-antitrypsin takes on thrombin and plasmin. Am J Respir Cell Mol Biol 2007;37:130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janciauskiene S. Conformational properties of serine proteinase inhibitors (serpins) confer multiple pathophysiological roles. Biochim Biophys Acta 2001;1535:221–235. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson S. Studies in α1-atitrypsin. Acta Med Scand 1965;177:1–85. [PubMed] [Google Scholar]
- 6.Shapiro SD. The pathogenesis of emphysema: the elastase:antielastase hypothesis 30 years later. Proc Assoc Am Physicians 1995;107:346–352. [PubMed] [Google Scholar]
- 7.Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. Cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc 2006;3:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuder RM, Yoshida T, Fijalkowka I, Biswal S, Petrache I. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proc Am Thorac Soc 2006;3:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuder RM, Voelkel NF. Pathobiology of chronic bronchitis and emphysema. In N.F. Voelkel and W. McNee, editors. Chronic obstructive lung disease, 1st ed. 2001; Montreal, Quebec, Canada: B.C. Decker.
- 10.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman SH, Hirth P, Waltenberger J, Voelkel NF. Inhibition of vascular endothelial growth factor receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 2004;97:1559–1566. [DOI] [PubMed] [Google Scholar]
- 12.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006;290:L209–L221. [DOI] [PubMed] [Google Scholar]
- 13.Tuder RM, McGrath S, Neptune E. The pathobiological mechanisms of emphysema models: what do they have in common? Pulm Pharmacol Ther 2003;16:67–78. [DOI] [PubMed] [Google Scholar]
- 14.Le A, Zielinski R, He C, Crow MT, Biswal S, Tuder RM, Becker PM. Pulmonary epithelial neuropilin-1 deletion enhances development of cigarette smoke-induced emphysema. Am J Respir Crit Care Med 2009;180:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and emphysema: the missing link. Am J Respir Cell Mol Biol 2003;28:551–554. [DOI] [PubMed] [Google Scholar]
- 16.Aoshiba K, Nagai A. Apoptosis in chronic obstructive pulmonary disease. Nippon Rinsho 1999;57:1972–1975. [PubMed] [Google Scholar]
- 17.Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 2000;117:684–694. [DOI] [PubMed] [Google Scholar]
- 18.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 19.Zheng T, Kang MJ, Crothers K, Zhu Z, Liu W, Lee CG, Rabach LA, Chapman HA, Homer RJ, Aldous D, et al. Role of cathepsin S-dependent epithelial cell apoptosis in IFN-γ-induced alveolar remodeling and pulmonary emphysema. J Immunol 2005;174:8106–8115. [DOI] [PubMed] [Google Scholar]
- 20.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97. [DOI] [PubMed] [Google Scholar]
- 21.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 2004;114:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA 2009;106:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuder RM. Aging and cigarette smoke: fueling the fire. Am J Respir Crit Care Med 2006;174:490–491. [DOI] [PubMed] [Google Scholar]
- 24.MacNee W, Tuder RM. New paradigms in the pathogenesis of chronic obstructive pulmonary disease I. Proc Am Thorac Soc 2009;6:527–531. [DOI] [PubMed] [Google Scholar]
- 25.Aoshiba K, Nagai A. Senescence hypothesis for the pathogenetic mechanism of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009;6:596–601. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Resp Cell Mol Biol 2004;31:643–649. [DOI] [PubMed] [Google Scholar]
- 27.Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol 2006;35:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in pulmonary emphysema patients. Am J Respir Crit Care Med 2006;174:886–893. [DOI] [PubMed] [Google Scholar]
- 29.Morla M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. Eur Respir J 2006;27:525–528. [DOI] [PubMed] [Google Scholar]
- 30.Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, Sarni M, Housset B, Weitzenblum E, Matrat M, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;179:566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman I, van Schadewijk AAM, Crowther AJL, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:490–495. [DOI] [PubMed] [Google Scholar]
- 32.Deslee G, Woods JC, Moore C, Conradi SH, Gierada DS, Atkinson JJ, Battaile JT, Liu L, Patterson GA, Adair-Kirk TL, et al. Oxidative damage to nucleic acids in severe emphysema. Chest 2009;135:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005;120:513–522. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007;317:803–806. [DOI] [PubMed] [Google Scholar]
- 35.Agusti A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax 2003;58:832–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 2009;360:2445–2454. [DOI] [PubMed] [Google Scholar]
- 37.Hogg JC. Why does airway inflammation persist after the smoking stops? Thorax 2006;61:96–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 2006;116:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 2006;12:317–323. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med 2007;13:567–569. [DOI] [PubMed] [Google Scholar]
- 41.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medler, T. R., D. N. Petrusca, P. J. Lee, W. C. Hubbard, E. V. Berdyshev, J. Skirball, K. Kamocki, E. Schuchman, R. M. Tuder, and I. Petrache. Apoptotic sphingolipid signaling by ceramides in lung endothelial cells. Am J Respir Cell Mol Biol 38:639–646. [DOI] [PMC free article] [PubMed]
- 43.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP and viruses-induced pulmonary innate immunity and remodeling responses. J Clin Invest 2008;118:2771–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro L, Pott GB, Ralston AH. α1-Antitrypsin inhibits human immunodeficiency virus type 1. FASEB J 2001;15:115–122. [DOI] [PubMed] [Google Scholar]
- 45.Terai C, Kornbluth RS, Pauza CD, Richman DD, Carson DA. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest 1991;87:1710–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikari Y, Mulvihill E, Schwartz SM. α1–Proteinase inhibitor, α1-antichymotrypsin, and α2-macroglolbulin are the antiapoptotic factors of vascular smooth muscle cells. J Biol Chem 2001;276:11798–11803. [DOI] [PubMed] [Google Scholar]
- 47.Daemen, M. A., V. H. Heemskerk, van't Veer C, Denecker G, Wolfs TG, Vandenabeele P, Buurman WA. Functional protection by acute phase proteins α1–acid glycoprotein and α1-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation 2000;102:1420–1426. [DOI] [PubMed] [Google Scholar]
- 48.Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, et al. A novel antiapoptotic role for α1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med 2006;173:1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, Petrache HI, Flotte T, Tuder RM. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol 2006;169:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohrab S, Petrusca DN, Lockett AD, Schweitzer KS, Rush NI, Gu Y, Kamocki K, Garrison J, Petrache I. Mechanism of α1-antitrypsin endocytosis by lung endothelium. FASEB J 2009;23:3149–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol 2001;3:802–808. [DOI] [PubMed] [Google Scholar]
- 52.Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell 2007;18:1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song S, Goudy K, Campbell-Thompson M, Wasserfall C, Scott-Jorgensen M, Wang J, Tang Q, Crawford JM, Ellis TM, Atkinson MA, et al. Recombinant adeno-associated virus-mediated α1- antitrypsin gene therapy prevents type I diabetes in NOD mice. Gene Ther 2004;11:181–186. [DOI] [PubMed] [Google Scholar]
- 54.Henson PM, Cosgrove GP, Vandivier RW. State of the art: apoptosis and cell homeostasis in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wencker M, Banik N, Buhl R, Seidel R, Konietzko N. Long-term treatment of α1-antitrypsin deficiency-related pulmonary emphysema with human alpha1-antitrypsin. Wissenschaftliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankungen (WATL)- α1–AT-study group. Eur Respir J 1998;11:428–433. [DOI] [PubMed] [Google Scholar]
- 56.Breit SN, Robinson JP, Luckhurst E, Clark P, Penny R. Immunoregulation by α1-antitrypsin. J Clin Lab Immunol 1982;7:127–131. [PubMed] [Google Scholar]
- 57.Khan AJ, Evans HE, Agbayani MM. Defective neutrophil chemotaxis in patients with α1-antitrypsin deficiency. Eur J Pediatr 1986;144:464–466. [DOI] [PubMed] [Google Scholar]
- 58.Churg A, Wang RD, Xie C, Wright JL. α1-Antitrypsin ameliorates cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 2003;168:199–207. [DOI] [PubMed] [Google Scholar]
- 59.Griese M, Latzin P, Kappler M, Weckerle K, Heinzlmaier T, Bernhardt T, Hartl D. α1-Antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur Respir J 2007;29:240–250. [DOI] [PubMed] [Google Scholar]
- 60.Sandstrom CS, Pitulainen E, Janciauskiene S. Augmentation therapy in emphysema patient with ZZ α1-antitrypsin deficiency. Respir Med CME 2008;1:153–157. [Google Scholar]
- 61.Breit SN, Robinson JP, Penny R. The effect of α1-antitrypsin on phagocyte function. J Clin Lab Immunol 1983;10:147–149. [PubMed] [Google Scholar]
- 62.Mulgrew AT, Taggart CC, Lawless MW, Greene CM, Brantly ML, O'Neill SJ, McElvaney NG. Z α1-antitrypsin polymerizes in the lung and acts as a neutrophil chemoattractant. Chest 2004;125:1952–1957. [DOI] [PubMed] [Google Scholar]
- 63.Hartl D, Latzin P, Hordijk P, Marcos V, Rudolph C, Woischnik M, Krauss-Etschmann S, Koller B, Reinhardt D, Roscher AA, et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med 2007;13:1423–1430. [DOI] [PubMed] [Google Scholar]
- 64.Pease JE, Sabroe I. The role of interleukin-8 and its receptors in inflammatory lung disease: implications for therapy. Am J Respir Med 2002;1:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dabbagh K, Laurent GJ, Shock A, Leoni P, Papakrivopoulou J, Chambers RC. α1-Antitrypsin stimulates fibroblast proliferation and procollagen production and activates classical MAP kinase signalling pathways. J Cell Physiol 2001;186:73–81. [DOI] [PubMed] [Google Scholar]
- 66.Janciauskiene S, Zelvyte I, Jansson L, Stevens T. Divergent effects of α1-antitrypsin on neutrophil activation, in vitro. Biochem Biophys Res Commun 2004;315:288–296. [DOI] [PubMed] [Google Scholar]
- 67.Janciauskiene SM, Nita IM, Stevens T. α1-Antitrypsin, old dog, new tricks: α1-antitrypsin exerts in vitro anti-inflammatory activity in human monocytes by elevating cAMP. J Biol Chem 2007;282:8573–8582. [DOI] [PubMed] [Google Scholar]
- 68.Subramaniyam D, Virtala R, Pawlowski K, Clausen IG, Warkentin S, Stevens T, Janciauskiene S. TNF-α-induced self expression in human lung endothelial cells is inhibited by native and oxidized α1-antitrypsin. Int J Biochem Cell Biol 2008;40:258–271. [DOI] [PubMed] [Google Scholar]
- 69.Libert C, Van MW, Brouckaert P, Fiers W. α1-Antitrypsin inhibits the lethal response to TNF in mice. J Immunol 1996;157:5126–5129. [PubMed] [Google Scholar]
- 70.Churg A, Dai J, Zay K, Karsan A, Hendricks R, Yee C, Martin R, MacKenzie R, Xie C, Zhang L, et al. α1-Antitrypsin and a broad spectrum metalloprotease inhibitor, RS113456, have similar acute anti-inflammatory effects. Lab Invest 2001;81:1119–1131. [DOI] [PubMed] [Google Scholar]
- 71.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 2006;173:1139–1144. [DOI] [PubMed] [Google Scholar]
- 72.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. α1-Antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA 2005;102:12153–12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med 1993;178:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol 1998;16:27–55. [DOI] [PubMed] [Google Scholar]
- 75.Hubbard RC, Fells G, Gadek J, Pacholok S, Humes J, Crystal RG. Neutrophil accumulation in the lung in α1-antitrypsin deficiency: spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest 1991;88:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with α1-antitrypsin deficiency (PiZ). Am J Respir Crit Care Med 1999;160:1968–1975. [DOI] [PubMed] [Google Scholar]
- 77.Lieberman J. Augmentation therapy reduces frequency of lung infections in antitrypsin deficiency: a new hypothesis with supporting data. Chest 2000;118:1480–1485. [DOI] [PubMed] [Google Scholar]
- 78.Lazrak A, Nita I, Subramaniyam D, Wei S, Song W, Ji HL, Janciauskiene S, Matalon S. α1-Antitrypsin inhibits epithelial Na+ transport in vitro and in vivo. Am J Respir Cell Mol Biol 2009;41:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Churg A, Wang X, Wang RD, Meixner SC, Pryzdial ELG, Wright JL. α1-Antitrypsin suppresses TNF-α and MMP-12 production by cigarette smoke-stimulated macrophages. Am J Respir Cell Mol Biol 2007;37:144–151. [DOI] [PubMed] [Google Scholar]
- 80.Mahadeva R, Atkinson C, Li Z, Stewart S, Janciauskiene S, Kelley DG, Parmar J, Pitman R, Shapiro SD, Lomas DA. Polymers of Z α1-antitrypsin co-localize with neutrophils in emphysematous alveoli and are chemotactic in vivo. Am J Pathol 2005;166:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greene CM, Miller SD, Carroll TP, Oglesby IK, Ahmed F, O'Mahony M, Taggart CC, McElvaney NG, O'Neill SJ. Anti-apoptotic effects of Z α1-antitrypsin in human bronchial epithelial cells. Eur Respir J 2010;35:1155–1163. [DOI] [PubMed] [Google Scholar]