Abstract
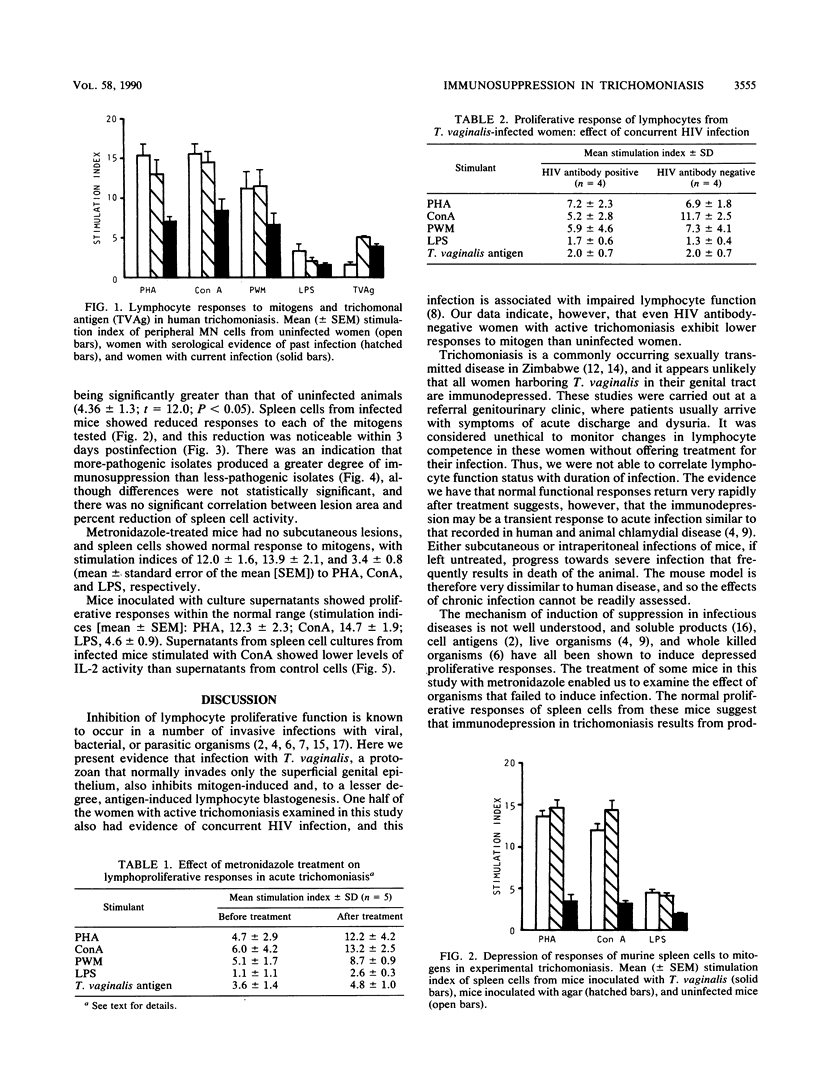
Proliferative responses to mitogens were determined by using peripheral blood mononuclear cells from women with active trichomoniasis, with serological evidence of past infection with Trichomonas vaginalis, and with no evidence of current or past infection. Even after the human immunodeficiency virus antibody status of the patients was taken into account, cells from women with active trichomoniasis showed reduced responses to phytohemagglutinin, concanavalin A, pokeweed mitogen, and bacterial lipopolysaccharide. Similar findings were obtained by using spleen cells from mice inoculated subcutaneously with live trichomonads. Reduction in proliferative responses by these cells could be detected 3 days after inoculation. There was some evidence to suggest that more-pathogenic strains of the parasite induced a greater degree of immunosuppression. The responses of spleen cells from mice inoculated with trichomonad-free culture supernatants were within normal limits, indicating that live trichomonads were needed to induce suppression. Support for this was gained from studies with cells from women who were treated successfully. Cells from these women rapidly regained normal lymphoproliferative function. Interleukin-2 (IL-2) production by spleen cells from infected mice was determined from measurements of mitochondrial activity in an IL-2-dependent T-cell line following incubation with stimulated spleen cell culture supernatants. These tests demonstrated lower IL-2 activity in supernatants from cell cultures from infected mice than in those from uninfected mice. The reduction in IL-2 activity did not, however, appear to correlate with the degree of reduction of mitogen-induced lymphoproliferation. Suppression of T-cell-mediated immunity may be one of the mechanisms by which T. vaginalis is able to evade host responses to infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Garza G. E. Soluble Trichomonas vaginalis antigens in cell-free culture supernatants. Mol Biochem Parasitol. 1984 Oct;13(2):147–158. doi: 10.1016/0166-6851(84)90109-9. [DOI] [PubMed] [Google Scholar]
- Brownback P. E., Barrow W. W. Modified lymphocyte response to mitogens after intraperitoneal injection of glycopeptidolipid antigens from Mycobacterium avium complex. Infect Immun. 1988 May;56(5):1044–1050. doi: 10.1128/iai.56.5.1044-1050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantstein T., Trissl D., Klos M., Gold D., Hahn H. Mitogenicity of Entamoeba histolytica extracts for murine lymphocytes. Immunology. 1980 Oct;41(2):347–352. [PMC free article] [PubMed] [Google Scholar]
- Guagliardi L. E., Byrne G. I., Paulnock D. M. Differential modulation of lymphocyte proliferative responses and lymphokine secretion in mice during development of immunity to Chlamydia psittaci. Infect Immun. 1989 May;57(5):1561–1567. doi: 10.1128/iai.57.5.1561-1567.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Koster F. T., Williams J. C., Goodwin J. S. Cellular immunity in Q fever: modulation of responsiveness by a suppressor T cell-monocyte circuit. J Immunol. 1985 Aug;135(2):1067–1072. [PubMed] [Google Scholar]
- Lane H. C., Masur H., Gelmann E. P., Longo D. L., Steis R. G., Chused T., Whalen G., Edgar L. C., Fauci A. S. Correlation between immunologic function and clinical subpopulations of patients with the acquired immune deficiency syndrome. Am J Med. 1985 Mar;78(3):417–422. doi: 10.1016/0002-9343(85)90332-8. [DOI] [PubMed] [Google Scholar]
- Levitt D., Corlett R. Patterns of immunoenhancement and suppression induced by Chlamydia trachomatis in vivo and in vitro. J Immunol. 1988 Jan 1;140(1):273–276. [PubMed] [Google Scholar]
- Mason P. R., Gwanzura L., Latif A. S., Marowa E. Genital infections in women attending a genito-urinary clinic in Harare, Zimbabwe. Genitourin Med. 1990 Jun;66(3):178–181. doi: 10.1136/sti.66.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. R., Gwanzura L. Mouse spleen cell responses to trichomonal antigens in experimental Trichomonas vaginalis infection. J Parasitol. 1988 Feb;74(1):93–97. [PubMed] [Google Scholar]
- Mason P. R., Patterson B. A. Proliferative response of human lymphocytes to secretory and cellular antigens of Trichomonas vaginalis. J Parasitol. 1985 Jun;71(3):265–268. [PubMed] [Google Scholar]
- Mason P. R. Serodiagnosis of Trichomonas vaginalis infection by the indirect fluorescent antibody test. J Clin Pathol. 1979 Dec;32(12):1211–1215. doi: 10.1136/jcp.32.12.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChesney M. B., Altman A., Oldstone M. B. Suppression of T lymphocyte function by measles virus is due to cell cycle arrest in G1. J Immunol. 1988 Feb 15;140(4):1269–1273. [PubMed] [Google Scholar]
- Nutman J., Berger M., Chase P. A., Dearborn D. G., Miller K. M., Waller R. L., Sorensen R. U. Studies on the mechanism of T cell inhibition by the Pseudomonas aeruginosa phenazine pigment pyocyanine. J Immunol. 1987 May 15;138(10):3481–3487. [PubMed] [Google Scholar]
- Street D. A., Taylor-Robinson D., Ackers J. P., Hanna N. F., McMillan A. Evaluation of an enzyme-linked immunosorbent assay for the detection of antibody to Trichomonas vaginalis in sera and vaginal secretions. Br J Vener Dis. 1982 Oct;58(5):330–333. doi: 10.1136/sti.58.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Yano A., Yui K., Aosai F., Kojima S., Kawana T., Ovary Z. Immune response to Trichomonas vaginalis. IV. Immunochemical and immunobiological analyses of T. vaginalis antigen. Int Arch Allergy Appl Immunol. 1983;72(2):150–157. [PubMed] [Google Scholar]


