Abstract
The function of the human proteome is defined by the proteostasis network (PN) (Science 2008;319:916; Science 2010;329:766), a biological system that generates, protects, and, where necessary, degrades a protein to optimize the cell, tissue, and organismal response to diet, stress, and aging. Numerous human diseases result from the failure of proteins to fold properly in response to mutation, disrupting the proteome. In the case of the exocytic pathway, this includes proteostasis components that direct folding, and export of proteins from the endoplasmic reticulum (ER). Included here are serpin deficiencies, a class of related diseases that result in a significant reduction of secretion of serine proteinase inhibitors from the liver into serum. In response to misfolding, variants of the serine protease α1-antitrypsin (α1AT) fail to exit the ER and are targeted for either ER-associated degradation or autophagic pathways. The challenge for developing α1AT deficiency therapeutics is to understand the PN pathways involved in folding and export. Herein, we review the role of the PN in managing the protein fold and function during synthesis in the ER and trafficking to the cell surface or extracellular space. We highlight the role of the proteostasis boundary to define the operation of the proteome (Annu Rev Biochem 2009;78:959). We discuss how manipulation of folding energetics or the PN by pharmacological intervention could provide multiple routes for restoration of variant α1AT function to the benefit of human health.
Keywords: alpha 1-antitrypsin deficiency, proteostasis, COPD, protein folding, endoplasmic reticulum
An understanding of how cells manage to fold a vast of array of polypeptides required for human health (the proteome) remains to be understood. We now appreciate that, to generate a natively folded protein, the cell uses a large number of assistants. These assistants comprise nearly a thousand components that make up the protein homeostasis, or “proteostasis network” (PN) (1, 2). They involve numerous chaperones and signaling pathways, such as the unfolded protein response (often referred to as “UPR”) (3), the heat shock response (generally referred to as “HSR”) (4), as well as Ca2+-sensing (5) and inflammatory pathways (6). Collectively, they function as a cohort of integrated activities to generate and protect the protein fold. These protective folding pathways are complemented by the components of the ubiquitin–proteasome system (referred to as “UPS”), and lysosomal and autophagic degradation pathways to control the composition and function of the highly variable proteome found in each cell type (2). Intriguingly, it is now recognized that folding and degradation are highly responsive to not only physiological stress (e.g., heat), but also to changes in the metabolite composition (the metabolome). The latter is strongly influenced by diet (7). These results suggest that the PN generates and protects a dynamic folding environment that biology uses to direct the function of each cell type, tissue, and, ultimately, organismal survival.
Work across a broad spectrum of organisms suggests that the PN is ancient, and coevolved with the remarkable diversity of polypeptide sequences and folds that direct human biology (4). Thus, the PN plays a key role to implement the capacity of polypeptides to fold and function in complex cell and tissue environments. Alterations in the polypeptide chain sequence as a consequence of mutation, co- and post-translational modifications, or in response to the concentration or the composition of the components of the PN or metabolites, challenges the fold and the PN, leading to a breakdown in the network, resulting in human pathologies (8).
Herein, we review evidence that a highly adaptive PN in each cell type is responsive to normal physiology and is adaptable to deal with overt challenges to the protein fold for mobilization of protein cargo, such as α1-antitrypsin (α1AT), through the exocytic trafficking pathway. We suggest that the combined effects of the kinetics and thermodynamics of folding, and the kinetics of misfolding at a defined PN capacity of a particular cell type, lies at the heart of the misfolding disease problem (2, 9). The biophysical properties of the fold and the composition of the PN allow the enabling concept of the proteostasis boundary (PB) (2). Using this generalized framework for folding and misfolding, we explore what may go awry in misfolding diseases, such as α1AT deficiency, in response to mutation. We discuss pharmacological strategies that may be used to enhance protein and proteome function to protect human biology from α1AT deficiency.
α1AT FUNCTION AND DISEASE
A large number of diseases are a consequence of the failure of a protein to exit the endoplasmic reticulum (ER) (10). In the case of soluble lumenal proteins, this includes α1AT deficiency, leading to lung disease and, in some cases, liver disease.
α1AT, a soluble 52-kD glycoprotein, is a member of the serine protease inhibitor (serpin) family, and is the most abundant antiprotease in the serum. α1AT is produced primarily by hepatocytes in the liver, although other tissues and cell types have been reported to express α1AT, including lung alveolar cells lining the interstitium, and lung macrophages. The structure of mature α1AT has been solved by X-ray crystallography, providing an important framework for molecular analyses (11). α1AT post-translationally acquires three N-linked oligosaccharides in the ER before being transported to the cell surface and secreted into the extracellular space. The adult liver secretes copious amounts of α1AT, producing more than a gram of α1AT per day. In the bloodstream, α1AT functions as a general inhibitor of serine proteases, including those secreted by neutrophils, such as neutrophil elastase, cathepsin G, and proteinase-3. In addition, α1AT is believed to move from the bloodstream into the alveolar interstitium, an extracellular matrix, which supports type I and type II epithelial cells lining the alveolus. In the lung, α1AT functions to inhibit neutrophil elastase and prevent degradation of the extracellular matrix in the alveolar interstitium (12, 13). Therefore, normal liver production of α1AT is required for maintenance of the protease–antiprotease balance in the serum and alveolar interstitium. α1AT is also believed to be synthesized and secreted directly into the interstitium by lung cells or macrophages (11, 13).
α1AT deficiency has been extensively reviewed (for representative reviews see References 11 and 13). Serum α1AT–deficient patients have an increased risk of developing severe lung disease and, depending on the particular α1AT variant, liver disease. The general hallmark of all forms of α1AT disease is panacinar emphysema as early as the third to fifth decade of life, reflecting reduction of serum pools of α1AT. In a subset of more severely affected patients with α1AT deficiency harboring the Z and other variants, mutant α1AT proteins are observed to form hepatic inclusion bodies (as well as inclusions in the lung) (11). The hepatic cell partially protects itself from these variants using autophagic pathways that clear aggregate polymers from the ER. When clearance is inadequate, accumulated aggregate triggers stress signaling pathways that contribute to further liver dysfunction, including neonatal hepatitis, juvenile cirrhosis, and hepatocellular carcinoma (13).
The α1AT locus is pleomorphic, with approximately 75 alleles identified to date that can be classified as normal or at risk, depending on the steady-state level of α1AT in the serum. α1AT deficiency is a disease that principally arises from mutations in the protein that result in less than 35% of the normal α1AT level in serum. Mutants, such as the α1AT-Saar or null Hong Kong variants, fail to fold properly, but remain soluble in the ER, and are efficiently targeted for degradation (14). Because such mutants do not accumulate, they do not trigger an unfolded protein response. This collection of folding and metabolic stress–responsive pathways promote translational attenuation, up-regulate ER folding and trafficking machineries, and, in the event that the cell is unable to eliminate the folding problem, initiate cell death pathways (15). These pathways and their contribution to α1AT disease are reviewed elsewhere Walter (38). Because the α1AT-Saar variant is expressed, but not efficiently secreted, the resulting serum has reduced levels of α1AT in homozygous patients. In general, reduced serum α1AT correlates with decreased levels of the protein in lung tissue. Reduced levels of serum α1AT generally lead to an increased risk for these patients of developing panacinar emphysema due to an imbalance in the extracellular proteostasis program (1, 2, 16). Interestingly, when secreted, variant α1AT often retains the ability to function as a protease inhibitor, although the level of residual activity is unique to each variant. Whereas wild-type α1AT has been shown to interact only transiently with the ER lumenal chaperones, binding immunoglobulin protein (BiP) and calnexin, monomer misfolded soluble intermediates can be detected to form robust complexes containing calnexin-endoplasmic reticulum protein 57 (ERp57)/BiP/glucose regulated protein 94 (GRP94) (13). The soluble misfolded pool is favored for degradation (13, 17). Current evidence suggests that degradation of the soluble misfolded α1AT variants involves carbohydrate-based folding sensing pathways targeting α1AT to the cytosolic proteasome through ER-associated degradation pathways involving the Sec61 translocon and cytosolic p97/valosin containing protein (VCP) complexes, Skp1–Cul1–F-box-protein (SCF)–ubiquitin lipase complexes, and the proteasome (17). Glycoproteins, in addition, use the calnexin-linked cycle and endoplasmic reticulum degradation-enhancing alpha-mannosidase-like (EDEM) family proteins to target misfolded cargo to the Sec61 translocon. These pathways have been the focus of work by Sifer and colleagues with α1AT (reviewed by Sifers elsewhere in this issue of the Journal, p. 376).
In contrast to the α1AT-Saar/null Hong Kong mutants, which are rare, the most common mutation causing α1AT deficiency is the Z variant, which affects approximately 1 in 1,600 live births of Northern European ancestry (13). Patients homozygous for a Glu342 to Lys substitution found in the Z variant have a folding defect in α1AT that leads not only to inefficient export from the ER of the soluble monomer pool, but accumulation of protein aggregates in the ER that form through loop-sheet polymerization of soluble monomers (13, 19). These aggregates form large hepatic inclusion bodies. In certain genetic backgrounds (15% of homozygous Z variant–expressing individuals), accumulation of polymer aggregates leads to early childhood liver disease. Because only a fraction of Z variant homozygous carriers have severe liver disease despite the appearance of aggregated polymer, it is likely that a number of (unknown) genetic modifiers may significantly influence the capacity of the cell to neutralize or dispose of aggregates (13). Interestingly, and in contrast to the clearance of the soluble α1AT monomer pool by the ER-associated degradation pathways, current efforts suggest that the Z variant aggregate is likely cleared from the ER by autophagic pathways (13, 20). Moreover, Z variant aggregates do not contribute to the unfolded protein response, but, rather, activate the ER-overload response that triggers apoptotic pathways through nuclear factor–κB signaling pathways (11, 13). Thus, the Z variant soluble monomer pool and Z variant polymer aggregates appear to engage different cellular degradation/stress pathways, and these contribute differentially to the disease etiology. Of particular note is the recent observation that the Z variant polymeric aggregate is largely devoid of cellular chaperones that bind the soluble misfolded form as indicated above. However, aggregates have been suggested to strongly bind protein disulfide isomerase and to elicit cellular responses that trigger the induction of cytosolic stress chaperone proteins, including heat shock protein (Hsp) 70 and Hsp90 (21). Moreover, the ER of Z variant–expressing hepatocytes has an altered redox potential (21), suggesting a potential defect in the normal oxidative environment of the ER that could influence mitochondrial function. In the lung, a newly recognized feature of aggregate prone variant disease is that the aggregate generated by either lung epithelial cells, macrophages, or neutrophils may in itself be proinflammatory and contribute to disease (22), an issue that will need to be assessed in approaches that modulate folding and trafficking of Z variant to the cell surface to provide benefit for α1AT deficiency.
In summary, the inability to export α1AT variants reflects the loss of folding stability, resulting in failure to exit the ER and targeting for degradation, contributing to liver and lung disease. In the case of the Z variant and related phenotypic variants, the accumulation of polymer aggregates can trigger induction of autophagic and signaling pathways. This likely contributes significantly to liver disease and possibly inflammatory response in the case of lung disease in a manner that is sensitive to the genetic background of the patient and the response of the cell, tissue, and organismal proteome to reduced levels of functional α1AT.
EXIT OF SOLUBLE CARGO FROM THE ER
Numerous components are involved in export of lumenal proteins from the ER for transport to and through the Golgi and for delivery to the cell surface or the extracellular space (23). Cumulative evidence suggests that there may be a high degree of specificity in the machinery directing both ER and Golgi exit, hence the importance of understanding the mechanism(s) by which α1AT traffics through these early pathways for secretion into serum. One of the more important and still unresolved challenges in the field is to understand how soluble proteins, such as α1AT, are selected for export from the ER. Cumulative evidence to date makes it unlikely that trafficking of even highly abundant cargo, such as α1AT, is left to nonspecific/bulk mechanisms (24). Several potential receptors for soluble cargo have been identified. For example, the ER-to-Golgi recycling lectin, endoplasmic reticulum-Golgi intermediate compartment 53 (ERGIC53), is required for export of the coagulation factors V and VII (25). Recent evidence suggests that ERGIC-53 may also be involved in the exit of α1AT through recognition of its N-linked carbohydrates (26). In yeast, Erv29p is essential for export of the soluble pheromone, pro–α-factor (27). Erv26p has been recently reported to be required for sorting of soluble yeast alkaline phosphatase in vesicles exiting the ER (28). These and other studies strongly suggest that export of soluble cargo is a receptor-mediated event. Consistent with this possibility, overexpression of human α1AT in transgenic mice resulted in the accumulation of murine α1AT in the ER, and hindered murine α1AT secretion by hepatocytes, although secretion of albumin and transferrin was unaffected (13). These data suggest the existence of a saturable, membrane-bound receptor(s) or receptor complex(es) that is required for the export of α1AT from the ER and trafficking through the Golgi to the cell surface through interaction with unknown carriers.
Transport of cargo, such as α1AT, through the exocytic pathway of eukaryotic cells involves the selective budding, targeting, and fusion of carrier vesicles (24). Vesicle formation involves the recruitment of specific cytosolic proteins that form, in part, the vesicle coat and drive membrane deformation. Evidence now demonstrates that cytosolic coat components play a critical role in cargo selection. In the exocytic pathway, these include the Coat protein (COPII) (involved in ER export), and the COPI and clathrin coats involved in Golgi transport (23). Insight into the basic features of COPII coat assembly have come from approaches that we and others have developed to follow transport in vivo using both genetic and molecular methodologies. We have also developed rigorous biochemical approaches to study export in vitro in permeabilized cells or ER microsomal membranes (prepared from cell homogenates) (23). Both systems faithfully reconstitute ER and Golgi export reactions in the test tube. Cytosolic components comprise the factors found in the COPII coat complex. This coat is assembled through the activity of the small regulatory GTPase, Sar1, a heterodimeric “adaptor” complex containing the proteins Sec23 and Sec24 (Sec23-24), and a second heterodimeric complex consisting of Sec13 and Sec31 (Sec13-31) that forms a scaffold for concentrating cargo–adaptor complexes in budding vesicles (23, 24). We have shown biochemically that cargo molecules are recruited by the COPII machinery through the formation of “prebudding complexes” involving only the activated Sar1 GTPase and the Sec23-24 adaptor. Both soluble and membrane-bound cargo are selected for export from the ER using similar principles to that observed for clathrin, where adaptor interaction “codes” found on cargo promote interactions with the Sec23-24. Evolutionarily conserved codes found in the cytoplasmic tail of a number of transmembrane proteins directly bind the Sec23-24 coat subunit, and are necessary for efficient ER exit (23, 24). The interplay between the machinery directing folding/degradation and cargo selection, which, in the case of the lumenal cargo, α1AT, will require a transmembrane receptor, and the recruitment and concentration by the COPII coat components defines a critical juncture in the secretory pathway for proteins, such as α1AT, to be able to be efficiently transported to the cell surface (23, 24).
THE ROLE OF ENERGETICS AND THE PN IN FOLDING
Proteins in the crowded proteome environment of a cell cannot fold without assistance, thus requiring the PN (1, 2, 16). The PN is an integrated biological system, consisting of general and specialized chaperones, folding enzymes, degradation components, and regulatory pathways that control composition and concentration of the network components in response to the environment. The proteostasis program is particularly challenged by mutation. These not only alter the inherited capacity of the proteostasis program, but are also sensed by signaling pathways, and either resolve the problem or promote cell death in the case of severe pathology. The PN can be controlled by biology both cell autonomously and cell nonautonomously, the latter involving neuronal and nonneuronal signaling pathways (29).
PN players include both highly conserved and highly specialized components that now number close to 1,000 components (1, 2, 16). Strikingly, the composition and capacity of the PN is unique to each cell type (16). This is because each cell presents a unique folding challenge to the PN based on its protein content that is responsible for unique cell and tissue physiology. An understanding of PN function in the exocytic pathway involves understanding the relationship between the energetics of protein folding and the role of PN components in the folding reaction. We have previously employed modeling to address this conceptual challenge, which we refer to as the FoldEx model (9). In that work, we described how the inherent energetics of the polypeptide chain fold are interpreted and influenced by the PN of the ER to achieve folding and export using Michaelis-Menten formalism (9). We have suggested that folding energetics and the individual steps involving translation, folding, degradation, and export together define a folding metabolic pathway to determine if a protein will fold and be exported from the ER, or be degraded. More recently, we generalized the FoldEx model to emphasize that all of biology uses folding energetics and the PN to achieve and maintain physiological function of cell, tissue, and organism. This model is referred to as FoldFx, and simply expands the concept of export to protein function. Thus, the composition and levels of proteostasis components defining the PN in a particular cell type dictate the capacity of the protein to function and drive cell, tissue, and organismal physiology (2).
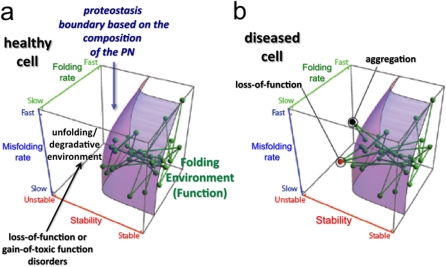
A critical feature of both the FoldEx and FoldFx models is the concept of the PB (2) (Figure 1). The boundary defines the minimal energetics that a protein must have to achieve folding and function in the context of a given PN capacity of a particular cell type. The PB forms a surface in three-dimensional space. The position of a protein relative to the boundary is defined by protein folding thermodynamics (from unstable to stable [x axis]), folding kinetics (from slow to fast [y axis]), and misfolding kinetics (from slow to fast [z axis]). The location and shape of the boundary is directly linked to the concentration/activity of the PN components. Thus, the position of the proteins in the biological network that drives human physiology (as indicated by the nodes (the proteins) and the edges (their links to other proteins within the network) are positioned based on the particular protein's folding energetics (their stabilities, folding rates, and misfolding rates). In a healthy cell, such as in a normal liver secreting α1AT, the position of the α1AT node and its edges lies beneath the boundary, indicating its stability in a given cell type for normal function (Figure 1a). Current evidence suggests that the position and shape of the boundary for each cell type is finely tuned, reflecting the composition of the PN. That is, it may be configured to provide just enough capacity for the folding load in a given cell type (2, 4, 16, 30). Thus, by setting the boundary as a threshold for folding and maintenance of the proteome by proteostasis in a particular cell type (1), the functional network dictating normal physiology becomes sensitive to the local environment. It can be adjusted in response to pathologic challenges to minimize damage and restore function via diet-, stress-, and aging-sensitive signaling programs.
Figure 1.
A hypothetical network of interacting proteins as viewed relative to the proteostasis boundary (PB). Each node in the network facilitating biological function represents a corresponding protein's folding properties (folding kinetics [z axis], misfolding kinetics [y axis], and thermodynamic stability [x axis]), and each connection represents physical or functional interaction between two proteins in the system (1, 2, 16, 65). The highlighted surface represents the PB, which, for the sake of simplicity, is shown as being the same for all of the proteins in the network and is established by the composition of proteostasis compounds. It is likely that each compartment (endoplasmic reticulum [ER], Golgi, mitochondria, etc.) and each protein has a unique subset of proteostasis network (PN) components that facilitate function in the context of the general boundary defined by highly abundant, core PN components, such as heat shock protein (Hsp) 70 and Hsp90 in the case of folding, and the general proteasomal-based and lysosomal-based systems in the case of degradation. (a) All of the nodes are within the boundary in a healthy cell, and the cell, therefore, has normal function. (b) Mutations, such as those found in the Z variant of α1-antitrypsin, or aberrant post-translational modifications can alter the folding energetics of proteins, making their corresponding nodes fall outside (above in the diagram) the boundary surface. This space in the three-dimensional plot does not support function of the energetically destabilized variant, and can lead to either loss of function (red node) or protein aggregation (black node). In either case, the loss of connections to proteins within (below in the diagram) the PB can challenge the entire PN, leading to cell, tissue, and organismal disease. Figure modified with permission from Reference 2.
THE FOLDED VERSUS THE UNFOLDED PROTEIN—TRIGGERING LOSS-OF-FUNCTION DISEASE
When is a misfolded protein detected by the PN? This is a challenging question, as 30% of proteins are now thought to have some level of intrinsic disorder. Thus, quality control, if it exists, is unrelated to the status of a protein being “folded.” Indeed, the status of what is functional and, hence, useful to the cell is based largely on the contextual environment defined by the local PN of each cell type (2, 9, 16). How does a mutation alter a protein's ability to function in a biological program that is limited by the PN and the shape of the PB? We have suggested that a change in the amino acid sequence of a protein, as, for example, found in α1AT deficiency, can significantly alter protein-folding energetics, and therefore place the protein outside the protective environment of the PB for a particular cell type. Here, it would become susceptible to substantial misfolding, aggregation, and/or degradation (Figure 1b). Because all proteins interact with other proteins, the inability to fold the mutant protein is likely also to destabilize other proteins in the function network, resulting in ejection of many proteins from the embrace of the PB (Figure 1b). Indeed, a poor diet, oxidative stress that occurs in response to cigarette smoking, and aging have global effects on the folding of the proteome in that they all challenge the operation of the PN. A PN already challenged by a misfolded α1AT variant, which is additionally challenged by the environment, would necessarily be less capable of dealing with the loss of function/aggregation phenotypes observed in α1AT deficiencies (31, 32). Intriguingly, it is now established that signaling pathways involving the insulin growth factor receptor 1, through the activity Forkhead box, sub-group O (FOXO) and heat shock factor 1 transcription factors, can be used to protect the cell against misfolding disease and prolong organismal lifespan (4, 33, 34). Thus, the PN is a largely unanticipated but central player in human biology for managing health and the response to disease.
PROTEOSTASIS BOUNDARIES AND MISFOLDING DISEASE
Numerous diseases are a consequence of deficiencies in trafficking through the exocytic and endocytic pathways that disrupt the tissue and organismal proteome. Compartmentalization within the cell emphasizes that the cell operates in the context of multiple proteostasis boundaries—that is, the cell has evolved folding environments that are unique for each compartment, reflecting their role in folding and function (16). In the case of the secretory pathway for serum proteins, such as α1AT, after folding and export from the ER, the Golgi modifies the protein post-translationally by restructuring glycans thought to help stabilize the fold in the downstream intracellular or extracellular environments. We have speculated that the operation of the PN and the configuration of the boundary in a given compartment is likely closely linked to the operation of the trafficking components, such as vesicle budding and fusion factors, that dictate the overall dynamics of exocytic and endocytic compartment trafficking (35). This is because folding and trafficking are intricately linked. Unlike transmembrane proteins that must be folded and stabilized by PN components found within compartments and in the cytosol, secreted proteins, such as α1AT, that are in transit to the cell surface reside exclusively within the lumen of compartments, and are, therefore, currently thought to be uniquely subject to compartmentalized PNs (16). However, cytosolic signaling pathways are known to play a major role in controlling the PN composition of individual exocytic and endocytic compartments, as mentioned previously.
Mutations, such as those found in α1AT deficiency, that trigger misfolding and loss of function, are prototypical of many trafficking diseases. Missense mutations, such as those found in the Z variant or deletions found in the ΔF508 variant of the cystic fibrosis (CF) transmembrane regulator (CFTR) triggering CF (36), can slow folding, accelerate misfolding, and/or generate a thermodynamically unstable fold. As a result, the protein's folding energetics lie outside the capacity defined by the biochemical embrace of the PB, targeting the protein for degradation (Figure 1b). This is usually associated with a marked decrease in the mutant protein in its downstream environment, leading to organismal loss-of-function phenotype. As is observed in Z-variant α1AT disease, such mutations can also trigger aggregation in the ER. It is becoming appreciated that this can be managed by the autophagic branch of the PN—yet, when it fails, cell pathology general ensues (12, 31, 32, 37). Interestingly, such mutations may actually allow normal export from the ER and trafficking to the cell surface, but trigger rapid retrieval and targeting for degradation by the lysosome through the endocytic pathway. Here, the secreted protein, although stable in the secretory pathway, is unstable with respect to the PN defining post-ER compartments or the extracellular environment (16). Indeed, some mutations result in a loss of function, not because the protein is rapidly degraded or fails to be transported and stabilized in its downstream locale, but, rather, because the mutation is in a critical active site residue, rendering it simply dysfunctional. Finally, a mutant (or even a wild-type protein) may simply saturate the PN capacity of a cell type, triggering a more general disruption of cellular physiology that challenges the integrity and capacity of the PB and, hence, cell viability, tissue function, and organismal health.
Nearly all of the above phenotypes described have been observed in response to the 75 alleles recognized as contributing to α1AT deficiency. Thus, misfolding disease is, for the most part, a very heterogeneous problem that requires both specific and global therapeutic solutions that use endogenous protection pathways to restore health.
CELLULAR APPROACHES TO DEALING WITH MISFOLDED α1AT
There are multiple ways in which misfolded proteins that are defective in trafficking and/or function are handled by the cell to reduce pathophysiological impact. The logic behind these pathways, although just becoming evident, remains a major challenge to understand. One possibility is that the variant, once made, is rapidly degraded, because its energetics are outside the local PB in a particular cell type. This would eliminate a potential toxic load of misfolded protein within the cell, yet still lead to a loss-of-function phenotype. Of course, if the protein is essential for viability of the producing cell, losing it would be detrimental to the cell, possibly triggering death. On the other hand, if the degraded protein is required at a site that is distal from the producing cell, such as α1AT, the disease will stem from a tissue or organismal response to the absence of the protein. Many secreted proteins traversing the secretory pathway of, for instance, the liver or pancreas are likely to fall in the latter category.
A second possibility is that the misfolded protein accumulates in the producing cell as observed for the Z variant. Curiously, in this case, it is apparent that the PB is adequate to support the initial fold, yet a secondary, abnormal folding intermediate (the aggregate) becomes the problem. Here, it seems that the Z variant is prematurely primed to generate its activated state (e.g., it is hair triggered for shutter activity) (11). The ensuing self-polymerization, leading to aggregated protein, sets off a cascade of cellular proteome responses to attempt to resolve the problem, which, in the case of Z-α1AT aggregate, involves autophagy-based clearance (11, 13).
A third possibility is that the accumulated/aggregated protein simply exceeds the capacity of the PN to mitigate the problem. Here, the ensuing chronic response sets off alarms in both the unfolded protein response and, potentially, the heat shock response branches of the PN that strongly influence cell, tissue, and/or host function. These often trigger cell death, tissue necrosis, and/or decrease organismal lifespan (38). The rationale behind a particular set of responses pursued by the cell with respect to specific challenge to the PN largely remains an enigma.
In summary, given the many mutations and polymorphisms that are found in all proteins, and their largely unknown impacts on the kinetics and/or thermodynamics of folding that must interface with the local PN, it is not surprising that each mutation, such as the many different α1AT variants, triggers distinct cellular, tissue, and organismal responses. This is exacerbated by the genetic differences within the human population and epigenetic differences that occur in response to the environment.
THE ROLE OF PHARMACOLOGIC CHAPERONES AND PROTEOSTASIS REGULATORS IN CORRECTION OF MISFOLDING DISEASE
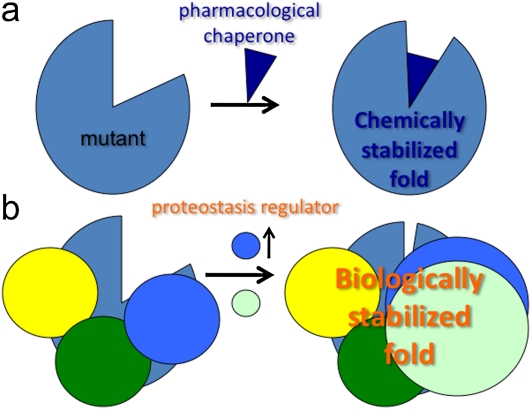
To treat misfolding diseases of the exocytic pathway, we need to understand why the fold is unacceptable to the PN and PB found in the ER (and other compartments) of the cell, and to determine if adjustments can be made pharmacologically that favor stability and function. This, in principle, could be accomplished by using small molecules referred to as a pharmacological chaperone (also called “correctors”), which directly bind to the misfolded protein and provide additional stability, as has been shown for G protein–coupled receptors (39), Gaucher's (40), and transthyretin (TTR) mutants (41) (Figure 2a). A pharmacological chaperone referred to as a “kinetic stabilizer” has completed successful phase III clinical trials. It is a first-in-class demonstration of the utility of such a class of molecules to correct disease (42) (http://www.foldrx.com/d05d85f8-f7dd-4942-9853-7e90e70563b6/news-press-detail.htm). An alterative class could involve adjustment of the loss of energetic stability by making further downstream adjustments to the stability of the fold. This is because the PN and, hence, the PB controlling stability and function in post-ER compartments and in the extracellular environment (16) is likely different from that at the ER. Here, a class of compounds referred to as a “potentiators,” such as Vertex 770 (http://www.cff.org/research/ClinicalResearch/FAQs/VX-770/), which increases the open channel probably of CFTR at the cell surface, favorably alters the channel defect in ΔF508 CFTR by promoting channel opening and restoration of chloride transport (36). A potentiator is similar, in principle, to agonists and antagonists for G protein–coupled receptors that bind and/or alter the activity of upstream and/or downstream signaling pathways. In the case of Vertex 770, its mode of action remains unknown (43).
Figure 2.
Comparison of role of pharmacological chaperones (PCs) and proteostasis regulators in restoration of misfolding disease. (a) Illustration that a PC can bind to a destabilized fold and stabilize the fold by providing additional energy, restricting the misfolded state either kinetically or thermodynamically to fewer conformational excursions from the normally folded state. (b) Illustration that a proteostasis regulator can alter the composition of the proteostasis network to increase the level of chaperones interacting with the wild-type and/or mutant fold to further stabilize the fold (blue circles) or elicit new chaperone components (yellow circles) to facilitate folding and stability, leading to increased function.
A second approach, which is based on the biological properties of the PN and the PB, would be to use a proteostasis regulator (1) (Figure 2b). Proteostasis regulators are molecules that adjust the composition and/or concentration of the PN through signaling pathways. In so doing, these compounds alter the composition of the PN and, hence, the shape of the boundary, which, in turn, may now be robust enough to prevent misfolding and subsequent targeting for degradation or aggregation, as in the case of the Z variant (31, 32). The utility of proteostasis regulators for correction of CF is supported by recent observations that correction can be achieved by adjusting the activity of the PN. Here, modulation of the Hsp90 chaperone/cochaperone–dependent folding steps associated with the cytosolic of the ER restores functionality to the ΔF508 CFTR mutant (44). Moreover, a proteostasis regulator that readjusts the boundary to support folding and trafficking of the protein from the ER could, in principle, also provide a more stabilizing environment for function in downstream compartments. For example, a proteostasis regulator that rescues ΔF508 from misfolding in the ER could also modify favorably the activity of PN/chaperone–dependent kinase activities that regulate channel gating (36). Thus, a compound could be both a pharmacological chaperone and a proteostasis regulator.
Although the above approaches may provide benefit for achieving production of a misfolded protein, they beg the resolution of the real problem in misfolding disease—that is, it is not just the cell, but often the tissue and organismal function that is disrupted. For example, mutations in diseases of myelinating cells (including Charcot-Marie-Tooth disease, Pelizaeus-Merzbacher disease, and multiple sclerosis [45]), diseases of connective tissues (ECM) (46), diseases of the eye, including retinitis pigmentosa (47), mutations in the low density lipoprotein (LDL) receptor, leading to athersclerosis (2), and defects in amyloid precursor protein processing, leading to Aβ1–42 production, resulting in Alzheimer's disease (48), challenge proteostasis boundaries, supporting distinct tissue environments (16). Thus, it may be important to target more global signaling pathways that control host responses. More global regulatory circuits that control the PN responsible for tissue and host function are now recognized to involve both endocrine and neuroendocrine signaling pathways, as shown recently in Caenorhabditis elegans models of heat stress (29). Thus, activation of PN signaling pathways by proteostasis regulators that integrate organismal physiology may significantly favor improved function.
PROGRESS IN TREATING SECRETORY DISEASE
A large class of misfolding diseases includes proteins that are synthesized as soluble proteins in the lumen of the ER. Misfolding is a particularly acute problem for tissues that are highly engaged in protein secretion, such as the liver (e.g., α1AT, albumin, etc.), pancreas (e.g., insulin and exocrine digestive enzymes), and the plasma cell (e.g., immunoglobuin), where the PN is fully activated to handle the protein folding load. Known secretory diseases include, in addition to α1AT deficiency, blood disorders of coagulation (49), congenital hyperthyroid goiter (50), procollagen disorders (51), and multiple systematic amyloidoses, including light chain amyloid (AL) disease (52), gelsolin (53) and TTR amyloid disease (41), among many others. It is also very reasonable to consider lysosomal storage diseases as “secretory” diseases. Here, the final destination is not serum or lymphatics—it is the lumen of the lysosome.
Gaucher's is an archetypal example of efficient removal of variant protein by the PN that invariably leads to loss of function. This class of disease can be now be partially corrected by pharmacological chaperones or proteostasis regulators. Interestingly, the most efficient rescue of Gaucher's required a combination of both pharmacologics. Here, the rapid degradation of mutant β-glucocerebrosidase could first be reduced by treatment of cells with a proteostasis regulator, stabilizing the protein fold, leading to accumulation in the ER, thus allowing it to engage a pharmacologic chaperone that promoted trafficking and function in the lysosome (40).
AL is a secretory disease involving the light chain of immunoglobulin (52). In AL, energetically destabilized variant κ or λ chains are generated by rare plasma cell populations. Although these variant light chains are sufficiently stable for export from the ER, once secreted, it is apparent that they become destabilized in response to unknown the extracellular stressors, resulting in deposition of polymeric AL in distal tissues. Thus, these variants fail to be recognized as “problematic” during nascent synthesis. One approach is to use a proteostasis regulator strategy to increase the stringency of the PB in the ER to reduce export of the variant and to promote its degradation. Alternatively, it may be possible to alter the PN environment of serum such that the variant fold is either stabilized by a proteostasis regulator (e.g., a kinetic stabilizer, as observed for TTR amyloid disease), or substantially destabilized so that it is targeted for degradation by the immune system, thereby eliminating amyloid deposition.
Intriguingly, it is not necessary to have a folding defect (e.g., a mutation) to challenge the PN in secretory disease. A prime example is type II diabetes. Here, excessive expression of wild-type insulin in response to diet can trigger disease (54). The major challenge to the PN in type II diabetes is to maintain the function of β cells in the islet of the pancreas to meet the excessive demand for insulin due to insulin resistance of peripheral tissues, such as muscle. When the rate of insulin production exceeds ER-associated PN capacity, insulin folding fails. More critically, the cellular PB collapses due to cellular overload of folding capacity (2, 16). This results in extensive deposition of aggregates of the peptide hormone, amylin, which is cosynthesized with insulin (55). Chronic abuse of the PN and unfolded protein response signaling as a consequence of excessive insulin production ultimately triggers β cell death and complete loss of capacity to generate insulin. To mitigate disease, such a system-wide organismal challenge clearly requires more global modulators of the PN to handle the pathophysiology. Such proteostasis regulators have recently been found, and belong to the histone deacetylase class of inhibitors that include both sodium 4-phenylbutyrate (4-PBA) (56, 57) and activators of sirtuins, such as resveratrol (58), glucagon-like peptide–1 receptor agonist, exenatide (59), and inhibitors of dipeptidyl peptidase–IV that block glucagon-like peptide–1 degradation (60). These regulators may function by rebalancing the PN in the β cell and in other tissue environments, reducing insulin resistance and protecting the β cell from apoptosis, and thereby attenuating the problem globally for organismal benefit.
As a final example, we need to consider approaches to mitigate α1AT disease. One possibility is that correction of the most common Z-variant disease could occur by application of folding stabilizers (e.g., pharmacologic chaperones). Indeed, progress has been made in the design of such compounds that inhibit Z-variant self-polymerization/aggregation (63), and provide an example of the potential feasibility of such an approach (11, 31, 32). Curiously, using this approach, little progress has been seen in generating a folded form of the Z variant that is secreted from the cell and is functionally active (31, 32).
A second approach that has considerable promise is to target the PN that regulates α1AT folding using proteostasis regulators (1, 2, 64). Here, the goal would be to alter the balance between folding and degradation to favor export—yet, we must also achieve production of a protein that has function in the extracellular environment. Thus, we need to focus on the PN that comprises the folding environment within the ER that contributes to misfolding and degradation of α1AT variants. Compared with the cytosolic PN, much less is generally known about the operation of folding by the ER PN. Considerable progress has been made in understanding the PN components that interact with wild-type and mutant α1AT that target α1AT for degradation, particularly by Sifers and colleagues (see article in this issue of the Journal, p. 376) (11, 13). One possibility to increase our understanding of restorative pathways is to use high-throughput screening methodologies to enable analysis of global small interfering RNA (siRNA) knockdown and overexpression of all ER (and potentially signaling cytosolic) PN components. Here, identification of specific PN components that improve the secretion of functional variant α1AT could be immensely informative of rescue pathways. A second possibility is to conduct a similar high-throughput screening approach using chemical libraries. Here, although mechanism of action will ultimately need to be elucidated, any compounds that elicit secretion of functional protein would be of great interest for further development of pharmacophores that could be used in the clinic to reduce the deficiency phenotype in patients. The Z variant of α1AT can also trigger aggregation in the ER (31, 32, 37), a condition that can exceed the capacity of autophagy pathways in some patients, triggering severe liver disease and cancer. Here promoting more efficient folding and export may relieve the aggregation phenotype and associated clinical pathologies. Alternatively, it may be possible simply to up-regulate the autophagic system to meet capacity needs for removing the aggregated pool in the ER (31, 32). Although this has the advantage of preventing the liver tissue–specific effects of Z-variant α1AT (61), simply removal of the aggregate does not solve the problem of α1AT deficiency in the lung (31, 32). Thus, a combination therapy may be required.
In general, an adjustment of the PN environment through proteostasis regulators to promote folding, reduce degradation, and enhance autophagy raises the possibility that multiple opportunities exist to tackle the deficiency phenotype found in α1AT disease.
THE NEXT STEP IN PROTEOSTASIS DISEASE MANAGEMENT—REPROGRAMMING THE PN?
Although we have specifically focused on the role of the PN and stabilization of the proteome for correction of α1AT deficiency through different classes of pharmacophores, we now know that the composition and operation of the PN is highly flexible and programmable (2). For example, changes in the PN are very evident during development and generation of the many specialized environments that generate tissue function—for example, plasma cells use the multiple stress signaling pathways to upgrade the PN for high levels of antibody production. It certainly responds to daily stress challenges as outlined previously here. Moreover, the success or failure of viral, bacterial, and fungal pathogens to sustain a productive infection is now recognized in many cases to involve the PN (62). Thus, we speculate that the (re)programmable nature of proteostasis may offer many unanticipated approaches to reset the folding environment. That is, rebalance the proteome proteostasis (65) in a more permanent fashion to mitigate the disease etiology found in serpin deficiencies and environmental challenges to the lung including chronic obstructive pulmonary disease (COPD) and emphysema, diseases that globally perturb folding and proteostasis through physical and oxidative stress mechanisms.
Supported by National Institutes of Health grants GM42336, GM33301, HL67201, and NS067643 (W.E.B.), and by an Alpha-1 Fellowship (J.J.C.).
Author Disclosure: M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.J.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.E.B. was a consultant for FoldRX and PTI, and he was on the Board or Advisory Board for PTI.
References
- 1.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 2008;319:916–919. [DOI] [PubMed] [Google Scholar]
- 2.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 2009;78:959–991. [DOI] [PubMed] [Google Scholar]
- 3.Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol 2008;3:399–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 2008;22:1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 2005;38:303–310. [DOI] [PubMed] [Google Scholar]
- 6.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008;454:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregor MG, Hotamisligil GS. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 2007;48:1905–1914. [DOI] [PubMed] [Google Scholar]
- 8.Ideker T, Sharan R. Protein networks in disease. Genome Res 2008;18:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiseman RL, Powers ET, Buxbaum JN, Kelly JW, Balch WE. An adaptable standard for protein export from the endoplasmic reticulum. Cell 2007;131:809–821. [DOI] [PubMed] [Google Scholar]
- 10.Wu JC, Liang ZQ, Qin ZH. Quality control system of the endoplasmic reticulum and related diseases. Acta Biochim Biophys Sin (Shanghai) 2006;38:219–226. [DOI] [PubMed] [Google Scholar]
- 11.Gooptu B, Ekeowa UI, Lomas DA. Mechanisms of emphysema in α1-antitrypsin deficiency: molecular and cellular insights. Eur Respir J 2009;34:475–488. [DOI] [PubMed] [Google Scholar]
- 12.Gooptu B, Lomas DA. Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu Rev Biochem 2009;78:147–176. [DOI] [PubMed] [Google Scholar]
- 13.Perlmutter DH, Brodsky JL, Balistreri WF, Trapnell BC. Molecular pathogenesis of α1-–antitrypsin deficiency–associated liver disease: a meeting review. Hepatology 2007;45:1313–1323. [DOI] [PubMed] [Google Scholar]
- 14.Kamimoto T, Shoji S, Hidvegi T, Mizushima N, Umebayashi K, Perlmutter DH, Yoshimori T. Intracellular inclusions containing mutant α1-antitrypsin Z are propagated in the absence of autophagic activity. J Biol Chem 2006;281:4467–4476. [DOI] [PubMed] [Google Scholar]
- 15.Schroder M, Kaufman RJ. Divergent roles of IRE1α and PERK in the unfolded protein response. Curr Mol Med 2006;6:5–36. [DOI] [PubMed] [Google Scholar]
- 16.Hutt DM, Powers ET, Balch WE. The proteostasis boundary in misfolding diseases of membrane traffic. FEBS Lett 2009;583:2639–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCracken AA, Brodsky JL. Recognition and delivery of erad substrates to the proteasome and alternative paths for cell survival. Curr Top Microbiol Immunol 2005;300:17–40. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Termine DJ, Swulius MT, Moremen KW, Sifers RN. Human endoplasmic reticulum mannosidase I is subject to regulated proteolysis. J Biol Chem 2007;282:4841–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomas DA. The selective advantage of α1-antitrypsin deficiency. Am J Respir Crit Care Med 2006;173:1072–1077. [DOI] [PubMed] [Google Scholar]
- 20.Kruse KB, Brodsky JL, McCracken AA. Autophagy: an ER protein quality control process. Autophagy 2006;2:135–137. [DOI] [PubMed] [Google Scholar]
- 21.Papp E, Szaraz P, Korcsmaros T, Csermely P. Changes of endoplasmic reticulum chaperone complexes, redox state, and impaired protein disulfide reductase activity in misfolding α1-antitrypsin transgenic mice. FASEB J 2006;20:1018–1020. [DOI] [PubMed] [Google Scholar]
- 22.Mulgrew AT, Taggart CC, McElvaney NG. α1-Antitrypsin deficiency: current concepts. Lung 2007;185:191–201. [DOI] [PubMed] [Google Scholar]
- 23.Stagg SM, LaPointe P, Razvi A, Gurkan C, Potter CS, Carragher B, Balch WE. Structural basis for cargo regulation of COPII coat assembly. Cell 2008;134:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurkan C, Stagg SM, Lapointe P, Balch WE. The COPII cage: unifying principles of vesicle coat assembly. Nat Rev Mol Cell Biol 2006;7:727–738. [DOI] [PubMed] [Google Scholar]
- 25.Nyfeler B, Zhang B, Ginsburg D, Kaufman RJ, Hauri HP. Cargo selectivity of the ERGIC-53/MCFD2 transport receptor complex. Traffic 2006;7:1473–1481. [DOI] [PubMed] [Google Scholar]
- 26.Nyfeler B, Reiterer V, Wendeler MW, Stefan E, Zhang B, Michnick SW, Hauri HP. Identification of ERGIC-53 as an intracellular transport receptor of α1-antitrypsin. J Cell Biol 2008;180:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otte S, Barlowe C. Sorting signals can direct receptor-mediated export of soluble proteins into COPII vesicles. Nat Cell Biol 2004;6:1189–1194. [DOI] [PubMed] [Google Scholar]
- 28.Bue CA, Barlowe C. Molecular dissection of Erv26p identifies separable cargo binding and coat protein sorting activities. J Biol Chem 2009;284:24049–24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 2008;320:811–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prahlad V, Morimoto RI. Integrating the stress response: lessons for neurodegenerative diseases from C. elegans. Trends Cell Biol 2009;19:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, Maurice N, Mukherjee A, Goldbach C, Watkins S, et al. An autophagy-enhancing drug promotes degradation of mutant α1-antitrypsin Z and reduces hepatic fibrosis. Science 2010;329:229–232. [DOI] [PubMed] [Google Scholar]
- 32.Kaushal S, Annamali M, Blomenkamp K, Rudnick D, Halloran D, Brunt EM, Teckman JH. Rapamycin reduces intrahepatic α1-antitrypsin mutant Z protein polymers and liver injury in a mouse model. Exp Biol Med (Maywood) 2010;235:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science 2006;313:1604–1610. [DOI] [PubMed] [Google Scholar]
- 34.Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 2009;139:1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurkan C, Lapp H, Alory C, Su AI, Hogenesch JB, Balch WE. Large-scale profiling of Rab GTPase trafficking networks: the membrome. Mol Biol Cell 2005;16:3847–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem 2008;77:701–726. [DOI] [PubMed] [Google Scholar]
- 37.Perlmutter DH. Autophagic disposal of the aggregation-prone protein that causes liver inflammation and carcinogenesis in α1-antitrypsin deficiency. Cell Death Differ 2009;16:39–45. [DOI] [PubMed] [Google Scholar]
- 38.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007;8:519–529. [DOI] [PubMed] [Google Scholar]
- 39.Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein–coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev 2007;59:225–250. [DOI] [PubMed] [Google Scholar]
- 40.Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR III, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell 2008;134:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurshman Babbes AR, Powers ET, Kelly JW. Quantification of the thermodynamically linked quaternary and tertiary structural stabilities of transthyretin and its disease-associated variants: the relationship between stability and amyloidosis. Biochemistry 2008;47:6969–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi S, Reixach N, Connelly S, Johnson SM, Wilson IA, Kelly JW. A substructure combination strategy to create potent and selective transthyretin kinetic stabilizers that prevent amyloidogenesis and cytotoxicity. J Am Chem Soc 2009;132:1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulloa-Aguirre A, Conn PM. Targeting of G protein–coupled receptors to the plasma membrane in health and disease. Front Biosci 2009;14:973–994. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, et al. Hsp90 cochaperone AHA1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell 2006;127:803–815. [DOI] [PubMed] [Google Scholar]
- 45.Lin W, Popko B. Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci 2009;12:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bateman JF, Boot-Handford RP, Lamande SR. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat Rev Genet 2009;10:173–183. [DOI] [PubMed] [Google Scholar]
- 47.Kosmaoglou M, Schwarz N, Bett JS, Cheetham ME. Molecular chaperones and photoreceptor function. Prog Retin Eye Res 2008;27:434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matus S, Lisbona F, Torres M, Leon C, Thielen P, Hetz C. The stress rheostat: an interplay between the unfolded protein response (UPR) and autophagy in neurodegeneration. Curr Mol Med 2008;8:157–172. [DOI] [PubMed] [Google Scholar]
- 49.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA 2008;105:18525–18530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim PS, Lee J, Jongsamak P, Menon S, Li B, Hossain SA, Bae JH, Panijpan B, Arvan P. Defective protein folding and intracellular retention of thyroglobulin-R19K mutant as a cause of human congenital goiter. Mol Endocrinol 2008;22:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myllyharju J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann Med 2008;40:402–417. [DOI] [PubMed] [Google Scholar]
- 52.Baden EM, Sikkink LA, Ramirez-Alvarado M. Light chain amyloidosis—current findings and future prospects. Curr Protein Pept Sci 2009;10:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page LJ, Suk JY, Bazhenova L, Fleming SM, Wood M, Jiang Y, Guo LT, Mizisin AP, Kisilevsky R, Shelton GD, et al. Secretion of amyloidogenic gelsolin progressively compromises protein homeostasis leading to the intracellular aggregation of proteins. Proc Natl Acad Sci USA 2009;106:11125–11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev 2008;29:317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev 2008;29:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol 2007;17:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006;313:1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metoyer CF, Pruitt K. The role of sirtuin proteins in obesity. Pathophysiology 2008;15:103–108. [DOI] [PubMed] [Google Scholar]
- 59.Salehi M, Aulinger BA, D'Alessio DA. Targeting β-cell mass in type 2 diabetes: promise and limitations of new drugs based on incretins. Endocr Rev 2008;29:367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halimi S. DPP-4 inhibitors and GLP-1 analogues: for whom? Which place for incretins in the management of type 2 diabetic patients? Diabetes Metab 2008;34:S91–S95. [DOI] [PubMed] [Google Scholar]
- 61.Marcus NY, Brunt EM, Blomenkamp K, Ali F, Rudnick DA, Ahmad M, Teckman JH. Characteristics of hepatocellular carcinoma in a murine model of α1-antitrypsin deficiency. Hepatol Res 2010;40:641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cowen LE, Singh SD, Kohler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci USA 2009;106:2818–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miranda E, Pérez J, Ekeowa UI, Hadzic N, Kalsheker N, Gooptu B, Portmann B, Belorgey D, Hill M, Chambers S, et al. A novel monoclonal antibody to characterize pathogenic polymers in liver disease associated with α1-antitrypsin deficiency. Hepatology 2010;52:1078–1088. [DOI] [PubMed] [Google Scholar]
- 64.Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, Hoch B, Kellner W, Kelly JW, Schmidt A, et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol 2010;6:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hutt DM, Balch WE. The proteome in balance. Science 2010;329:766–767. [DOI] [PMC free article] [PubMed] [Google Scholar]