Abstract
Inhalation experiments using laboratory animals are performed under controlled conditions to assess the toxicity of and to investigate interventional strategies to ameliorate injury resulting from oxidant gas exposures. A variety of dynamic inhalation exposure systems that use whole-body or nose-only exposure chambers have been developed for rodents. In a whole-body exposure chamber, the animals are immersed in the test atmosphere, whereas in nose-only or head-only exposure systems, exposures are localized primarily to the head and/or nasal regions. There are advantages and disadvantages with both types of exposure approaches. Considerations such as animal number, exposure duration, end points of study, and availability of test material should influence the selection of a particular exposure system.
Keywords: chlorine, inhalation exposure, exposure chamber
Inhalation experiments using laboratory animals have been conducted for many types of studies including toxicological, deposition and pharmacokinetic studies, and investigating mechanisms and efficacies of inhaled therapeutic agents. Investigations of inhaled oxidant gases have been broadly focused on mechanisms of toxicity, pharmacokinetics, and more recently on indentifying and testing potential countermeasures to treat injuries resulting from acute, high-concentration exposure to specific oxidants. Depending on the end points of such studies, the protocol of the inhalation exposure component including animal species and numbers, gas concentrations, and duration of exposure must be tailored to the study objectives. These considerations ultimately lead to the selection of an appropriate inhalation exposure system.
An inhalation exposure system encompasses toxicant gas phase generation and delivery, the exposure chamber, exposure concentration monitoring, and treatment of exhaust gases (1). Although static systems (no air flow) and recirculated flow systems have been previously employed, most gas phase inhalation exposure systems employ dynamic flow-through system, where the test gas is delivered to the exposure chamber, treated or removed, and exhausted under relatively steady-state (continuous) conditions. In dynamic inhalation exposure systems, two types of exposure approaches are generally used: whole body (WB) and nose-only. In WB exposure chambers, animals are immersed in the test atmosphere. The animals are unrestrained and the entire outer surface of the body exposed to the test atmosphere. Other routes of exposure including oral and dermal exposures may also occur. On the other hand, in nose- or head-only (NO-HO) exposure systems, animals are restrained in individual holding tubes with either their entire heads or just the snout exposed to the toxicant gas stream, which serves to reduce dermal exposure. Both types of exposure approaches have been used for a variety of investigations involving oxidant gas exposures. WB exposure chambers are usually operated at higher flow rates than NO-HO chambers with similar numbers of animals and thus require more test material. On the other hand, the animals in the holding tubes are stressed.
This article reviews these two approaches for conducting inhalation exposures to oxidant gases, safety and environmental considerations, and the rationale for considering nose-only versus whole body exposure systems, with a focus toward small animals or rodents. Inhalation systems for large animals such as dogs and primates are similar in the design principles, but they are mostly custom-designed systems and are limited to selected laboratories.
GENERATION OF GASES
Methods for generation of gases and vapors for inhalation exposures have been reviewed (2). For oxidant gases such as chlorine, nitrogen oxides, and sulfur oxides, which are available in compressed gas cylinders in pure or diluted form, generation of test atmosphere involves metering the appropriate amount of high-concentration source toxicant and diluting with air to deliver a test atmosphere with defined concentrations. The generation system usually consists of gas cylinders (toxicant, additional diluent gas, air, etc.), pressure gauges, and flow controller devices (flow meters or preferably mass flow controllers) as described in several reports on exposure experiments of chlorine gas (3–5), SO2 (6), and NO2 (7). With small exposure systems, it is preferable to inject the toxicant countercurrent to the flow of air to help ensure a well-mixed atmosphere at the chamber inlet. Furthermore, due to the complexities involved in reactive gas exposures, exposure systems, including the gas delivery and sampling lines, should be conditioned prior to experimental studies to decrease the rise time in inter-chamber toxicant concentrations.
Some oxidant gases for inhalation experiments can be prepared by chemical reaction of precursors. For example, ozone is usually generated by directly irradiating oxygen with a low-pressure mercury lamp or electrodes (7, 8), and NO2 can be generated by controlled vaporization of liquid nitrogen tetroxide dimer (N2O4) (9, 10).
WHOLE-BODY CHAMBER
Exposure chambers have been designed in various sizes and shapes (1, 11). In a WB exposure chamber, the animals are immersed in the test atmosphere. Because of the reactivity of oxidant gases it is paramount to consider the materials used throughout the exposure chamber, delivery lines, and other components of the composite exposure system that may contact test gas phase. For example, chlorine can react with aluminum and copper, and it is also reactive with steel and stainless steel, which is more facile in the presence of water vapor. Therefore only glass, polyester, Teflon, PVC, polyethylene, and polypropylene can be used (3–5). On the other hand, in ozone exposure systems, as an example of another reactive gas, high purity stainless steel (e.g., 316), Teflon, and glass are preferential for exposure system components.
Small WB exposure chambers are often custom-made or adapted from boxes or cabinets of appropriate sizes. Figure 1 shows a custom-made 3.5-L cylindrical glass chamber (catalog number X02AI99C15A57H5; Specialty Glass, Houston, TX) that was adapted from an in vitro exposure system (12), which can house two rats or six mice for chlorine inhalation experiments (5) and facilitates the rapid introduction and removal of the experimental animals. The diluted chlorine gas enters the chamber from the top and passes through a diffuser located inside the top lid to distribute the gas uniformly in the lower part of the chamber where animals are located. Gases exit the chamber via two large-bore diameter ports in its bottom portion and pass through a scrubber with rough filter impregnated with sodium hydroxide to remove chlorine. The total flow rate is 5 L/minute. Because this chamber is used for high-concentration, short-term exposures, it is important that the chamber turnover rate is very rapid to reduce the concentration rise and fall times.
Figure 1.

A 3.5-L glass whole body (WB) inhalation chamber for exposing small numbers of rodents to chlorine gas.
Another approach to enable exposure of greater animal numbers involves a 54-L clear polyester cabinet (catalog number F420741000J; Bel-Art Products, Pequannock, NJ) modified for exposing 24 mice in cages located on two levels within the chamber (Figure 2). Diluted chlorine gas flows from an eight-port Teflon manifold prior to entering the exposure chamber through eight inlets distributed across the face of one sidewall of the chamber (4). The test atmosphere passes the animals and exits from the opposite side of the chamber via a similar eight-port manifold. The total flow is 50 L/minute and the exhaust is passed through a Greenburg Smith impinger containing 400 ml of 10% aqueous sodium carbonate for removal and scavenging of chlorine prior to the exhaust pump. Here also, a large turnover rate is accomplished (∼1 vol change/min).
Figure 2.
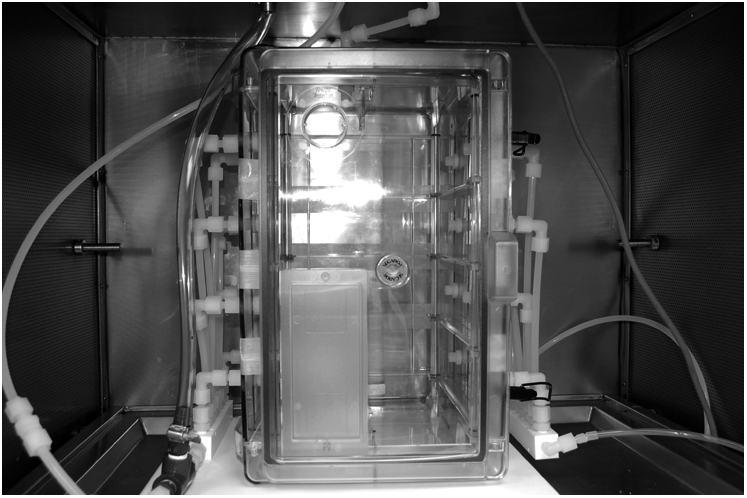
A 54-L polyester WB inhalation chamber for exposing 24 mice to chlorine gas.
For inhalation experiments with large numbers of rodents, large WB exposure chambers such as Hazelton-2000 or a smaller Hazelton-1000 multitier stainless steel chambers (Lab Products, Seaford, DE) have been used in inhalation exposure studies of ozone or nitrogen dioxide (8, 9). The Hazelton-2000 chamber has three levels of animal cages; each level is split into two tiers. The Hazelton-1000 chamber has single tier with a volume of 1 m3, and the Hazelton-2000 has a total volume of 2.3 m3. The Hazelton-2000 chamber provides enough space for simultaneous exposure of 144 rats, 360 mice, or 60 guinea pigs. A smaller Hazelton-1000 chamber has half the capacity. The total flow rates are 450 and 200 L/minute with a full load of animals in the Hazelton-2000 and Hazelton-1000 chamber, respectively. The chamber is designed to provide uniform distribution of test atmosphere through the chamber at these flow rates (13, 14). A major advantage of these chambers is that food and water are provided during the nonexposure period; therefore the animal can stay in the chamber throughout the study period. These chambers are useful for repeated sub-chronic or chronic exposure studies up to about 30 months.
NOSE-ONLY CHAMBERS
NO-HO inhalation exposure chambers generally incorporate the use of animal restraint tubes to minimize dermal exposure and have much smaller volumes relative to WB chambers for similar numbers of animals. A popular design is a cylindrical chamber with animal ports located around the central chamber. The test atmosphere enters from the top of the chamber and is delivered to the individual animal ports through a tube. A single-level, 12-port all glass exposure chamber was built for exposure of rats to oxidant gases (10). In this system, the gas flows past the rats' heads and exits through a tube at the bottom of the glass animal restraint tube. Exhaled air from the animals is entrained so that it also leaves with the exhaust air, thus minimizing the potential for inhalation by other animals. A similar single-level glass chamber system for exposure of five mice to chlorine has also been reported (15).
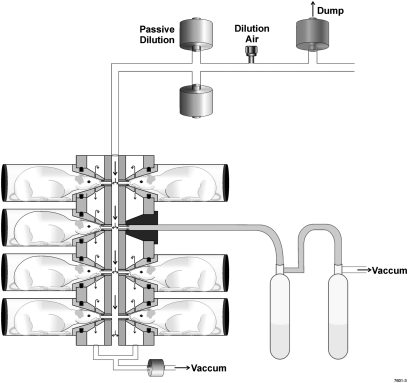
Stackable, multi-level NO-HO exposure chambers with concentric cylindrical chambers (In-Tox Products, Moriarty, NM) can accommodate larger numbers of animals (16, 17). As shown in Figure 3, the test atmosphere enters the inner cylinder and, via individual ports, each animal inhales gas from this common plenum, thereby assuring consistency in exposure concentration across experimental animals. The exhaled air from the animal enters an annular space between the inner and outer cylinder, which prevents rebreathing and cross-contamination with the common inspiratory plenum gas, and proceeds to the exhaust ports. This chamber design provides a uniform distribution of test materials across the individual animal ports irrespective of the different vertical levels, as has been documented in aerosol studies in the size range of 0.8 to 1.2 μm (16). One of the animal ports can serve as a convenient access to the inspiratory plenum to withdraw samples for test atmosphere analyses. A 52-port exposure chamber made of PVC has been used for inhalation exposure of chlorine to mice (3).
Figure 3.
A stackable multi-level nose-only exposure chamber for exposing rodents to oxidant gases. There are 12 animal ports in each level. A bubbler sampling system is connected to an animal port.
MONITORING CHAMBER CONCENTRATION
Maintaining a stable, well-mixed, well-characterized drug or toxicant concentration is essential for inhalation studies. The gas phase concentration is a major parameter for any exposure that attempts to calculate the inhaled dose, to establish dose–response relationships, to estimate such parameters as the LD50, and for conducting comparative studies across species or interventional strategies, for example. Gas phase concentrations should be measured by acquiring samples in the breathing zone of animals through a sampling port in the WB chamber or through an animal port in a NO-HO chamber. There are two techniques for concentration monitoring, a time-averaged sample and continuous real-time monitoring.
A time-averaged sample provides the average concentration during a given exposure period but it does not provide information on the stability of chamber concentrations. The oxidant gas is usually collected with impingers or bubblers, and the collected samples are then analyzed usually for a product formed (either directly or indirectly) from a relatively facile chemical reaction. For chlorine, a gas sample may be passed through two midget impingers in series, each containing 10 ml of 1mM sodium hydroxide. The collected fluid was then analyzed spectrophotometrically for total chlorine content (17). Similarly, chlorine gas in a WB exposure chamber (range, ∼200 to 300 ppm) was sampled by passing through a fritted glass bubbler containing 100 ml of 1% sulfamic acid solution at a flow rate of 1 L/minute. Aliquots of the collected samples were then analyzed using a modified ASTM method for airborne chlorine (4).
The continuous monitoring of a chamber atmosphere provides a real-time concentration profile that can be used to determine the stability of gas generation/delivery or provide a warning when there is problem in the generation and/or delivery system that affect the exposure conditions. Continuous monitoring is usually achieved by using real-time monitors, which operate using a variety of principles. For example, in a WB exposure system, an electrochemical reduction detector was used to monitor the chlorine concentration (5), while ozone has been monitored via ultraviolet photometers (7, 8) and NO2 concentration via chemiluminescent NO2/NO detectors (7, 9). The monitor output signal can also be used to regulate generation and delivery of the toxicant to achieve a desired exposure concentration within the chamber.
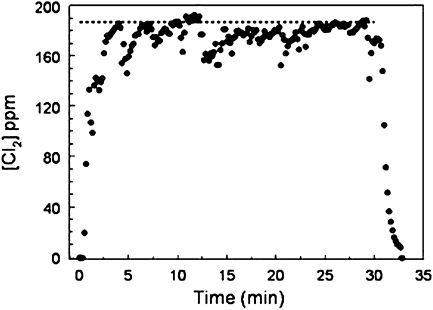
Figure 4 shows chlorine concentration monitored during a 30-minute inhalation exposure of chlorine gas in a small glass WB exposure chamber (5). When the exposure starts there is a rapid rise of Cl2 concentration, and in a few minutes it reaches a quasi–steady-state concentration. When the generation is stopped at 30 minutes, Cl2 concentration decreases within a few minutes to the background concentration. The rise and fall of chamber concentrations can be expressed by the theoretical Equation 1:
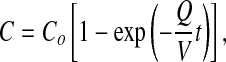 |
(1) |
where C is the chamber concentration at time, t, Co is the steady-state concentration, Q is the flow rate, V is the chamber volume, and t is time. When toxicant inflow stops, the concentration decreases exponentially from Co following the equation:
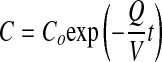 |
(2) |
Figure 4.
Cl2 concentration in the 3.5-L glass chambers (shown in Figure 1) with two rats.
The concentration–time characteristics of a chamber are most usefully expressed by stating the time, t99, required to attain 99% of the steady-state concentration by
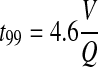 |
(3) |
These equations are derived on the basis of assumptions of constant gas concentration and flow rate entering the chamber (which can either be the high concentration during exposure initiation or zero when exposures are ended), and a single well-mixed compartment. Also, the volume occupied by the animals and gas losses due to the presence of animals are ignored. In practice, t99 is often obtained from the real-time monitoring such as that shown in Figure 4. The total exposure time for animals is then equal to T (duration of the test material delivered to the chamber) + t99.
SAFETY AND ENVIRONMENTAL CONSIDERATIONS
The most important consideration in designing an inhalation exposure system is to ensure a safe working environment for personnel within the laboratory environment and to prevent the discharge of pollutants to the outside atmosphere. Exposure of the operator and animal-care staff to oxidant gases can be minimized by having the exposure system operate under subatmospheric pressure so that any leakage is inward, or by placing the exposure chamber inside a fume hood or constantly vented containment chamber (4, 5). The chlorine exhaust from the exposure chambers should be treated by passing through a scrubber system as discussed in the previous section (4, 17).
Environmental factors including temperature, humidity, air flow, and CO2/ammonia levels inside an exposure chamber can influence the health, response, and exposure rate of animals. Recommended temperature and humidity ranges for rodents are 72–78°F and 40–70%, respectively (19). In NO-HO chambers, rodents are usually confined inside exposure tubes with little air circulation around the body, limiting heat transfer. Therefore, it is critical to maintain a low temperature around the tubes to prevent overheating of test animals, especially in the case of loaded multi-level chambers.
Sufficient air flow is required to supply oxygen to and remove CO2 from animals in exposure chambers, although with reactive gases chamber turnover rates should be sufficiently rapid to more than compensate for these. The flow rate in the WB chamber is related to the rise and fall time, t99, as shown in Equation 3. To minimize t99, we recommend a minimum flow rate such that t99 is equal or shorter than 15% of the intended exposure time, Texposure. Following Equation 3, the minimum flow rate in terms of chamber volume per unit time (or air exchange rate) can be expressed as:
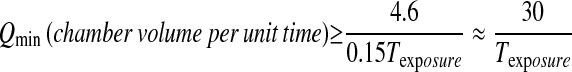 |
(4) |
For example, if the exposure time is 1 hour, then the minimum flow rate recommended is 30 air exchanges per hour. For a 6-hour exposure, the minimum flow rate is 5 air exchanges per hour. For large WB chambers such as Hazelton-1000 and Hazelton-2000 chambers the flow rates are usually set at 10 to 15 (chamber volume)/hour. Because of the large chamber volume to animal volume ratio, these flow rates supply enough air for animals. On the other hand, this air exchange rate may not provide sufficient air for animals in a NO-HO chamber. A flow of 2.5 times the minute volume of animals in the NO-HO chamber has been recommend as a minimum flow rate based on consideration of oxygen depletion and test material usage (11).
CONCLUSIONS
Both NO-HO and WB exposure chambers have been employed for chlorine gas exposure studies. In selection of an exposure system, one should consider the animal number, duration of exposure, whether it is a single or repeated exposure, cost and availability of test material, and finally, whether monitoring of animal breathing during the exposure is needed. Animals in a WB chamber are unrestrained, less stressed, and may be able to thermoregulate more easily. Animal handling is also minimized. Therefore, it is useful for both large and small numbers of animals, and good for long-term repeated exposure experiments. However, because dermal and oral exposures also occur, they may influence the extent of absorption of the test material. More test material is also required for WB chambers due to the relatively large dead space. It is also important to have a well-mixed condition in a WB chamber (1, 11). On the other hand, studies in the NO-HO chamber uses less test material and other routes of exposure are minimized. In addition, monitoring of animal breathing rate can be achieved by adapting the restraint tubes to function as plethysmographs (20). Primary disadvantages of NO-HO chambers are that animals are restrained and stressed, body core temperatures may raise due to thermoregulation constraints, size of the restraint tube must be matched to the size of animals being exposed, and it is also labor intensive to manipulate large numbers of animals, especially for repeated long-term exposure protocols (11). Training animals in the restraint tubes prior to the inhalation experiment may reduce the stress (21).
The research is supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Environmental Health Sciences, Grant Number U54 ES017218.
Supported by National Institutes of Health Grants U54 NS058185 (to Y.S.C.), U54 ES017218 (to L.B.), U01 ES015673 (to R.J.R.), and U01 ES015676 (to E.M.P.).
Conflict of Interest Statement: Y.S.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.J.R. received grant support from the National Institutes of Health (NIH) and the Centers for Disease Control (more than $100,001). E.M.P. received grant support from the NIH (more than $100,001) and Phillip Morris ($10,001–$50,000). E.M.P. attests that no research conducted in the laboratory and no authorships associated with the manuscript were derived from funding from Phillip Morris. G.L.S. received grant support from the NIH as a co-investigator on various grants (more than $100,001), NIOSH as a co-investigator ($1,001–$5,000), and Phillip Morris as a co-investigator (2000–2003; more than $100,001). G.L.S. attests that no funding for this paper was derived from Phillip Morris. S.M. served as a consultant for Sepracor, Inc. and received a $2,000 honorarium for participation in their annual scientific meeting; received grant support from Talecris to identify the interactions of alpha antitrypsin with ion channels ($10,001–$50,000; grant expires in 2009) and Inspire Pharmaceuticals to identify the role of P2Y inhibitors on RSV injury to the respiratory system ($50,001–$100,000; grant expired in 2008), and Sepracor Inc., to test the efficacy of BROVANA in chlorine-induced injury (more than $100,001; grant expired June 2009). He has a pending patent through the University of Alabama, Birmingham, for methods for using pyrimidine synthesis inhibitors to increase airway epithelial cell fluid uptake, and his spouse/life partner has a pending patent through Purdue University for DPPC formulations and methods for using. He received fees from Elsevier for editing a book on Free Radical Effects on Membranes ($5,001–$10,000).
References
- 1.Cheng YS, Moss O. Inhalation exposure systems. Toxicol Methods 1995;5:161–197. [Google Scholar]
- 2.Wong BA. Generation and characterization of gases. In: McClellan RO, Henderson RF, editors. Concepts in inhalation toxicology. Washington, DC: Taylor & Francis; 1995. pp. 129–149.
- 3.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo J, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 2003;168:568–574. [DOI] [PubMed] [Google Scholar]
- 4.Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol 2008;20:783–793. [DOI] [PubMed] [Google Scholar]
- 5.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait.E., Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 2008;295:L733–L743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao H, Xu X, Na J, Hao L, Huang L, Li G, Xu Q. Protective effects of salicylic acid and vitamin C on sulfur dioxide-induced lipid peroxidation in mice. Inhal Toxicol 2009;20:865–871. [DOI] [PubMed] [Google Scholar]
- 7.Keeberger SR, Zhang LY, Jakab GJ. Differential susceptibility to oxidant exposure in inbred strains of mice: nitrogen dioxide versus ozone. Inhal Toxicol 1997;9:621. [Google Scholar]
- 8.Johnson NF, Hotchkiss JA, Harkema JR, Henderson RF. Proliferative responses of rat nasal epithelia to ozone. Toxicol Appl Pharmacol 1990;103:143–155. [DOI] [PubMed] [Google Scholar]
- 9.Mauderly JL, Cheng YS, Gillett NA, Griffith WC, Henderson RF, Pickrell JA, Wolff RK. Influence of pre-existing pulmonary emphysema on susceptibility of rats to chronic inhalation exposure to nitrogen dioxide. Inhal Toxicol 1990;2:129–150. [Google Scholar]
- 10.Stavert DM, Lehnert BE. Nitric oxide and nitrogen oxide as inducers of acute pulmonary injury when inhaled at relatively high concentrations for brief periods. Inhal Toxicol 1990;2:53–67. [Google Scholar]
- 11.Wong BA. Inhalation exposure system: design, methods and operation. Toxicol Pathol 2007;35:3–14. [DOI] [PubMed] [Google Scholar]
- 12.Ballinger CA, Cueto R, Squadrito G, Connor LM, Coffin JJ, Velsor LW, Pryor WA, Postlethwait EM. Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic Biol Med 2005;38:515–526. [DOI] [PubMed] [Google Scholar]
- 13.Moss OR, Decker JR, Cannon WC. Aerosol mixing in an animal exposure chamber having three levels of caging with excreta pan. Am Ind Hyg Assoc J 1982;43:244–249. [Google Scholar]
- 14.Cheng YS, Barr EB, Carpenter RL, Benson JM, Hobbs CH. Improvement of aerosol distribution in whole-body inhalation exposure chambers. Inhal Toxicol 1989;1:153–166. [Google Scholar]
- 15.Gunn MD. Prevention of inflammatory lung injury after chlorine exposure. Presented at the 3rd CounterACT Network Research Symposium, Washington, DC, April 14–16, 2009.
- 16.Cannon WC, Blanton EF, McDonald KE. The flow-past chamber: an improved nose-only exposure system for rodents. Am Ind Hyg Assoc J 1983;44:923–928. [DOI] [PubMed] [Google Scholar]
- 17.Morris JB, Wilkie WS, Shusterman DJ. Acute respiratory responses of the mouse to chlorine. Toxicol Sci 2005;83:380–387. [DOI] [PubMed] [Google Scholar]
- 18.Benson BM, Hahn FF, March TH, McDonald JD, Gomez AP, Sopori MJ, Bourdelais AJ, Naar J, Zaias J, Bossart GD, et al. Inhalation toxicity of brevetoxin 3 in rats exposed for twenty-two days. Environ Health Perspect 2005;113:626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NIH. Guide for care and use of laboratory animals. Publication No. 85–23. Bethesda, MD: National Institutes of Health; 1985.
- 20.Medinsky MA, Dutcher JS, Bond JA, Henderson RF, Mauderly JL, Snipes MB, Mewhinney JA, Cheng YS, Birnbaum LS. Uptake and excretion of 14C-methyl bromide as influenced by exposure concentration. Toxicol Appl Pharmacol 1985;78:215–225. [DOI] [PubMed] [Google Scholar]
- 21.Barley J, Mikkelsen LF, Francis R, Johnston H, Einhardt V. Enforced restraint of rodents. Animal Technol Welfare 2007;6:11–13. [Google Scholar]