Abstract
Acute lung injury can be induced indirectly (e.g., sepsis) or directly (e.g., chlorine inhalation). Because treatment is still limited to supportive measures, mortality remains high (∼74,500 deaths/yr). In the past, accidental (railroad derailments) and intentional (Iraq terrorism) chlorine exposures have led to deaths and hospitalizations from acute lung injury. To better understand the molecular events controlling chlorine-induced acute lung injury, we have developed a functional genomics approach using inbred mice strains. Various mouse strains were exposed to chlorine (45 ppm × 24 h) and survival was monitored. The most divergent strains varied by more than threefold in mean survival time, supporting the likelihood of an underlying genetic basis of susceptibility. These divergent strains are excellent models for additional genetic analysis to identify critical candidate genes controlling chlorine-induced acute lung injury. Gene-targeted mice then could be used to test the functional significance of susceptibility candidate genes, which could be valuable in revealing novel insights into the biology of acute lung injury.
Keywords: pulmonary edema, vascular permeability, terrorism countermeasures, acute respiratory distress syndrome
Even without signs of external injury, chemical exposure can produce severe trauma to internal target organs including the lungs, heart, gastrointestinal tract, eyes, and the central nervous system (1). Of these injuries, the extent of lung injury often is the most critical to survival (1–3). Chemical-induced acute lung injury (CIALI) can be viewed as a molecular cascade mounting over hours and days subsequent to even a transient incident. Unfortunately, CIALI is a likely consequence of terrorist attacks of multiple possible scenarios, including intentional detonation of chemical plants, railroad car derailment, or chemical truck hijacking (4). Chemicals of high concern include chlorine (2, 5), phosgene (6), sulfuric acid (7, 8), ammonia (9), and acrolein (10–13).
Realizing that past clinical trials based on predicted targets have had limited success, this study is designed to initiate a novel experimental strategy to gain new insights into this complex disease. Because CIALI can be induced by multiple chemicals and has diverse etiologies, common events must be identified that could be of value to an overall therapeutic approach. Our strategy requires the monitoring of multiple biochemical indicators forming complex molecular signatures (14–22). Thus, the overall goal is to understand the genetic, global transcriptomal, and molecular events that will provide insights into the mechanisms of CIALI that could redirect or strengthen emerging clinical approaches to diagnosis and treatment. Our central hypothesis is that the interplay between molecular signaling determines survival and controls the susceptibility to sequelae from CIALI. To explore this hypothesis, the long-term objectives of this study are: (1) to identify the genetic determinants and molecular mechanisms controlling CIALI common to exposure to five leading hazardous chemicals: chlorine, phosgene, sulfuric acid, ammonia, and acrolein; (2) to evaluate the therapeutic efficacy based on common events in cell signaling during CIALI; and (3) to identify the molecular mechanisms that are unique to each of the five leading hazardous chemicals during the early development of CIALI. This interim report presents our initial progress toward meeting these goals and supports the feasibility of the overall experimental approach.
METHODS
Selection of Mouse Strains
Female mice (6–8 wk old) were obtained from Jackson Laboratory (Bar Harbor, ME) and housed in barrier-protected microisolator cages, and all procedures were approved by the University of Pittsburgh Institutional Animal Use and Care Committee. Mouse strains selected for this study are the Tier 1 priority strains of the Mouse Phenome Database (MPD). The priority strains were classified based on strain availability, research trends, community input, and resources, including the NIEHS-Perlegen single nucleotide polymorphisms (SNPs) identification of 8.27 million genome-wide locations for a subset of these inbred strains. The Tier 1 strains included: 129X1/SvJ, A/J, AKR/J, BALB/cByJ, BTBR T+tf/J, C3H/HeJ, C57BL/6J, CAST/EiJ, DBA/2J, FVB/NJ, KK/HIJ, MOLF/EiJ, NOD/ShiLtJ, NZW/LacJ PWD/PhJ, and WSB/EiJ. These 16 strains are well distributed in the Mouse Family Tree, and strains that are difficult to breed (and become unavailable at times) or are redundant to other priority strains have been excluded.
Chlorine Exposure
Chlorine (45 ppm × 24 h) exposures were conducted in single pass, laminar, dynamic 0.32-m3 stainless steel inhalation chambers in HEPA-filtered air. Air samples were monitored continuously with a direct reading instrument (Polytron 3000; Dräger Safety Inc., Pittsburgh, PA) during exposure, and airflows adjusted to maintain exposure within 5% of the target concentration. Chlorine (Matheson Tri-Gas, Montgomery, PA) was introduced into the chamber using flow meters from cylinders placed in a vented safety cabinet. During exposure, room air also was continuously monitored and no readings above 0.5 ppm (current occupational threshold limit value) were observed.
RESULTS
Strain Distribution Pattern
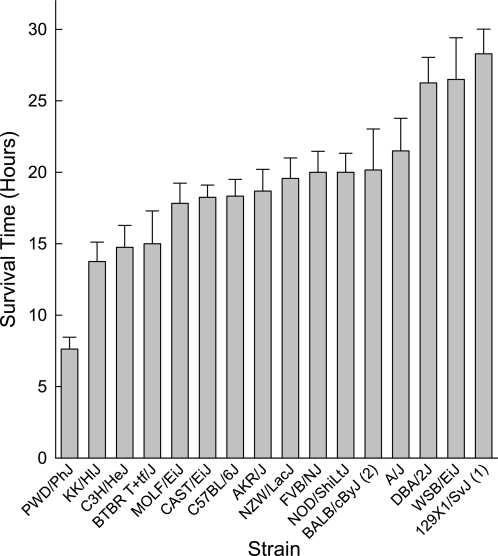
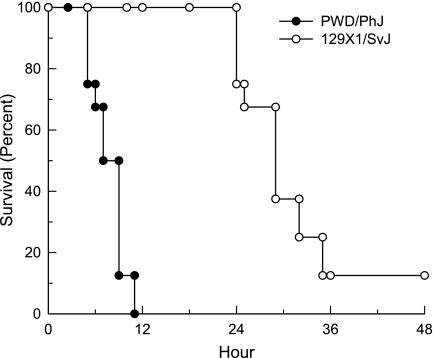
The first step in a genetic analysis of susceptibility to CIALI is to assess the survival time of various inbred mouse strains. In this intitial study, 16 inbred mouse strains were exposed to chlorine (45 ppm × 24 h) and survival time was recorded hourly. The most sensitive strain was PWD/PhJ (mean survival time = 7.6 ± 0.8 h), and the most resistant was 129X1/SvJ (mean survival time = 28.3 ± 1.3 h) (Figure 1). Strains included in this study have been used in the past for the generation of transgenic (e.g., FVB/NJ) and gene-targeted (C57BL/6J and 129X1/SvJ) mouse strains. Thus, the relative sensitivity should be of value to investigators in strain selection in future studies designed to evaluate the chlorine susceptibility. No overlap in the phenotype was noted between the most polar strains (Figure 2). Thus, crosses of PWD/PhJ and 129X1/SvJ strains could be very useful in future genetic analyses. Note that one of the eight 129X1/SvJ mice tested survived well past the exposure and longer than the observation period of 2 weeks. These results support the likelihood that there is an underlying genetic difference in murine susceptibility to CIALI.
Figure 1.
Strain distribution pattern of mean survival time of inbred mouse strains exposed to chorine. Inbred mouse strains were selected based on the Mouse Phenome Database priority Tier 1. Sixteen strains were exposed to chlorine (45 ppm × 24 h), and survival time was recorded hourly. The most sensitive strain was PWD/PhJ (mean survival time = 7.6 ± 0.8 h), and the most resistant was 129X1/SvJ (mean survival time = 28.3 ± 1.3 h). Values are mean ± SE (n = 8 mice/strain) of mice that died during the observation period (2 wk after exposure) and excluded the mice tested that survived longer than the observation period. The numbers in parentheses indicate the number of mice that survived past the observation period.
Figure 2.
Kaplan-Meier survival curves of the most sensitive strain (PWD/PhJ) and the most resistant strain (129X1/SvJ) exposed to chlorine (45 ppm x 24 h). Note that no overlap in the phenotype was observed between strains. These results support the likelihood that there is an underlying genetic difference in susceptibility of mice to chlorine-induce acute lung injury.
Future Directions
Two approaches can be used to further identify candidate genes that could contribute to the difference in susceptibility between mouse strains. The first approach is to generate the F1 cross of the two polar strains (PWD/PhJ x 129X1/SvJ) and determine the mean survival time of offspring (13). From this result, a set of backcross and F2 mice can be generated and phenotyped. Each member of these groups is genotyped with polymorphic makers (e.g., microsatellite or SNPs) distributed throughout the genome and a quantitative trait loci (QTL) analysis performed. The resulting linkage maps are likely to reveal chromosomal regions with significant logarithm (base 10) of odds (LOD) scores. The LOD score is the likelihood that a marker at a specific locus is linked (in disequilibrium) to the observed phenotype compared with the likelihood of observing the same data by chance. Typically, this analysis produces 1–5 chromosomal regions with significant linkage that span approximate 10–30 mega–base pairs (Mbp) each (14, 16). These regions can be narrowed by marker-assisted (speed congenics) or other breeding strategies (23) and candidate genes can then be selected based on microarray results (i.e., a genetical-genomics approach) (17, 24–26), biological plausibility (i.e., a bibliomics approach), or other methods.
The second approach is to phenotype additional mouse strains and use the existing high-density SNP map generated for each strain (27). This method has advantages and disadvantages. The major advantage is that the resolution of the SNP linkage mapping is approximately 5,000 base pairs, which is often within a single gene. In many cases, 3–5 SNPs within a single gene can have significant associations. In addition, several SNPs can span a combination of alleles at multiple loci that are transmitted together, indicating a haplotype. In either instance, the region to be evaluated by subsequent analysis is narrow. The transcripts of candidate genes in this region can be assessed quickly by real-time–quantitative polymerase chain reaction and/or can be assessed by exon resequencing. The disadvantage of this method is that the false discovery rate is unknown and it is likely that several false positives will be detected. In addition, the traditional QTL method can provide insights into gene–gene interactions (i.e., epistasis) and can predict the percentage of the phenotypic difference that can be ascribed to the QTL region, an interval that may contain one or more relevant genes (23).
In summary, we have determined that inbred mouse strains vary in their sensitivity to chlorine-induced acute lung injury. The most polar strains vary by at least threefold in mean survival time, which is highly amenable to future genetic dissection and identification of novel candidate genes.
Supported by the National Institutes of Health (ES013781, ES015675, HL077763, HL085655, and HL091938).
The research is supported by the CounterACT Program, National Institutes Of Health Office of the Director, and the National Institute of Environmental Health Sciences, Grant Number ES015675.
Conflict of Interest Statement: G.D.L. received grant support from the National Institutes of Health (NIH) (more than $100,001). H.P.-V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.J.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.A.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.P.D. received grant support from the NIH (more than $100,001). A.-S.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Q.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.J.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.K. served as a consultant for Stomedix ($5,001–$10,000) and Genentech ($1,001–$5,000). He has received grant support from Centocor, Biogen Idec, and the NIH (more than $100,001). He owns patents on the Role of microRNAs in lung fibrosis, Peripheral blood biomarkers for idiopathic pulmonary fibrosis, and MMP activation peptides in urine. M.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.R.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Baker DJ. Critical care requirements after mass toxic agent release. Crit Care Med 2005;33(1, Suppl)S66–S74. [DOI] [PubMed] [Google Scholar]
- 2.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, et al. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 2009;27:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leikauf GD, McDowell SA, Wesselkamper SC, Hardie WD, Leikauf JE, Korfhagen TR, Prows DR. Acute lung injury: functional genomics and genetic susceptibility. Chest 2002;121:70S–75S. [DOI] [PubMed] [Google Scholar]
- 4.Howe D. The Homeland Security Council, planning scenarios: Executive Summaries. 2004.
- 5.Das R, Blanc PD. Chlorine gas exposure and the lung: a review. Toxicol Ind Health 1993;9:439–455. [DOI] [PubMed] [Google Scholar]
- 6.Pauluhn J, Carson A, Costa DL, Gordon T, Kodavanti U, Last JA, Matthay MA, Pinkerton KE, Sciuto AM. Workshop summary: phosgene-induced pulmonary toxicity revisited: appraisal of early and late markers of pulmonary injury from animal models with emphasis on human significance. Inhal Toxicol 2007;19:789–810. [DOI] [PubMed] [Google Scholar]
- 7.Leikauf GD, Spektor DM, Albert RE, Lippmann M. Dose-dependent effects of submicrometer sulfuric acid aerosol on particle clearance from ciliated human lung airways. Am Ind Hyg Assoc J 1984;45:285–292. [DOI] [PubMed] [Google Scholar]
- 8.Knapp MJ, Bunn WB, Stave GM. Adult respiratory distress syndrome from sulfuric acid fume inhalation. South Med J 1991;84:1031–1033. [DOI] [PubMed] [Google Scholar]
- 9.Benomran FA, Hassan AI, Masood SS. Accidental fatal inhalation of sulfuric acid fumes. J Forensic Leg Med 2008;15:56–58. [DOI] [PubMed] [Google Scholar]
- 10.Leduc D, Gris P, Lheureux P, Gevenois PA, De Vuyst P, Yernault JC. Acute and long term respiratory damage following inhalation of ammonia. Thorax 1992;47:755–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leikauf GD, Leming LM, O'Donnell JR, Doupnik CA. Bronchial responsiveness and inflammation in guinea pigs exposed to acrolein. J Appl Physiol 1989;66:171–178. [DOI] [PubMed] [Google Scholar]
- 12.Hales CA, Musto SW, Janssens S, Jung W, Quinn DA, Witten M. Smoke aldehyde component influences pulmonary edema. J Appl Physiol 1992;72:555–561. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh HS, Shaver C, Case LM, Dietsch M, Wesselkamper SC, Hardie WD, Korfhagen TR, Corradi M, Nadel JA, Borchers MT, et al. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol 2008;38:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prows DR, Shertzer HG, Daly MJ, Sidman CL, Leikauf GD. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat Genet 1997;17:471–474. [DOI] [PubMed] [Google Scholar]
- 15.Hardie WD, Prows DR, Leikauf GD, Korfhagen TR. Attenuation of acute lung injury in transgenic mice expressing human transforming growth factor-alpha. Am J Physiol 1999;277:L1045–L1050. [DOI] [PubMed] [Google Scholar]
- 16.Wesselkamper SC, Prows DR, Biswas P, Willeke K, Bingham E, Leikauf GD. Genetic susceptibility to irritant-induced acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol 2000;279:L575–L582. [DOI] [PubMed] [Google Scholar]
- 17.McDowell SA, Gammon K, Bachurski CJ, Wiest JS, Leikauf JE, Prows DR, Leikauf GD. Differential gene expression in the initiation and progression of nickel-induced acute lung injury. Am J Respir Cell Mol Biol 2000;23:466–474. [DOI] [PubMed] [Google Scholar]
- 18.McDowell SA, Gammon K, Zingarelli B, Bachurski CJ, Aronow BJ, Prows DR, Leikauf GD. Inhibition of nitric oxide restores surfactant gene expression following nickel-induced acute lung injury. Am J Respir Cell Mol Biol 2003;28:188–198. [DOI] [PubMed] [Google Scholar]
- 19.Wesselkamper SC, Case LM, Henning LN, Borchers MT, Tichelaar JW, Mason JM, Dragin N, Medvedovic M, Sartor MA, Tomlinson CR, et al. Gene expression changes during the development of acute lung injury: role of transforming growth factor beta. Am J Respir Crit Care Med 2005;172:1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesselkamper SC, McDowell SA, Medvedovic M, Dalton TP, Deshmukh HS, Sartor MA, Case LM, Henning LN, Borchers MT, Tomlinson CR, et al. The role of metallothionein in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol 2006;34:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tichelaar JW, Wesselkamper SC, Chowdhury S, Yin H, Berclaz PY, Sartor MA, Leikauf GD, Whitsett JA. Duration-dependent cytoprotective versus inflammatory effects of lung epithelial fibroblast growth factor-7 expression. Exp Lung Res 2007;33:385–417. [DOI] [PubMed] [Google Scholar]
- 22.Bein K, Wesselkamper SC, Liu X, Dietsch M, Majumder N, Concel VJ, Medvedovic M, Sartor MA, Henning LN, Venditto C, et al. Surfactant-associated protein B is critical to survival in nickel-induced injury in mice. Am J Respir Cell Mol Biol 2009;41:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prows DR, Hafertepen AP, Winterberg AV, Gibbons WJ Jr, Wesselkamper SC, Singer JB, Hill AE, Nadeau JH, Leikauf GD. Reciprocal congenic lines of mice capture the aliq1 effect on acute lung injury survival time. Am J Respir Cell Mol Biol 2008;38:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganguly K, Stoeger T, Wesselkamper SC, Reinhard C, Sartor MA, Medvedovic M, Tomlinson CR, Bolle I, Mason JM, Leikauf GD, et al. Candidate genes controlling pulmonary function in mice: transcript profiling and predicted protein structure. Physiol Genomics 2007;31:410–421. [DOI] [PubMed] [Google Scholar]
- 25.Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, Ikegami M, Wesselkamper SC, Davidson C, Dietsch M, et al. Genomic profile of matrix and vasculature remodeling in TGF-alpha induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2007;37:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganguly K, Depner M, Fattman C, Bein K, Oury TD, Wesselkamper SC, Borchers MT, Schreiber M, Gao F, von Mutius E, et al. Superoxide dismutase 3, extracellular (SOD3) variants and lung function. Physiol Genomics 2009;37:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P, Wang Y, Vikis H, Maciag A, Wang D, Lu Y, Liu Y, You M. Candidate lung tumor susceptibility genes identified through whole-genome association analyses in inbred mice. Nat Genet 2006;38:888–895. [DOI] [PubMed] [Google Scholar]