Abstract
Rationale: Cigarette smoking has been demonstrated in laboratory studies to have effects on lung epithelial and endothelial function similar to those observed in acute lung injury (ALI). However, the association between active and passive cigarette smoke exposure and susceptibility to ALI has not been prospectively studied.
Objectives: We hypothesized that both active and passive cigarette smoke exposure would be associated with increased susceptibility to ALI after severe blunt trauma.
Methods: We measured levels of cotinine, a metabolite of nicotine and validated biomarker of tobacco use, in plasma samples obtained immediately on arrival at the emergency department from 144 adult subjects after severe blunt trauma. Patients were then followed for the development of ALI.
Measurements and Main Results: Increasing quartiles of plasma cotinine were associated with the development of ALI (odds ratio [OR] for developing ALI in highest cotinine quartile, 3.25; 95% confidence interval [CI], 1.22–8.68; P = 0.017 for trend across quartiles). Moderate to heavy passive smoke exposure was associated with nearly the same odds of developing ALI as active smoking (OR for moderate to heavy passive smoking compared with no exposure or low level exposure, 3.03; 95% CI, 1.15–8.04; OR for active smoking, 2.77; 95% CI, 1.28–5.99). This association persisted after adjusting for other predictors of ALI, including Injury Severity Score and alcohol abuse.
Conclusions: Both moderate to heavy passive smoking and active smoking are independently associated with the development of ALI after severe blunt trauma. This finding has important implications both for public health and for understanding the pathogenesis of ALI.
Keywords: cigarette smoking, acute lung injury, acute respiratory distress syndrome, cotinine, secondhand smoke exposure
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Cigarette smoking has been demonstrated in laboratory studies to have deleterious effects on lung epithelial and endothelial function, similar to those observed in acute lung injury. However, the association between active and passive cigarette smoke exposure and susceptibility to acute lung injury has not been prospectively studied, in part because of a lack of reliable and quantitative approaches to measuring exposure in critically ill patients. We hypothesized that quantitative measures of both active and passive cigarette smoke exposure would be associated with increased susceptibility to acute lung injury after severe blunt trauma.
What This Study Adds to the Field
Both moderate to heavy passive smoking and active smoking, as measured by a well-validated biomarker of exposure (plasma cotinine), are strongly associated with the development of acute lung injury after severe blunt trauma, independent of other predictors of lung injury including alcohol abuse. This finding has important implications both for public health and for understanding the pathogenesis of acute lung injury.
Acute lung injury (ALI) is a common and frequently fatal cause of acute respiratory failure in critically ill patients, with an estimated U.S. incidence of 190,600 cases/year and a mortality of 30–40% (1). Acute lung injury frequently follows major trauma, which is itself a leading cause of morbidity and mortality worldwide (2); the development of ALI after severe trauma increases mortality by nearly threefold (3). Although some progress has been made over the past several decades in understanding the pathophysiology of ALI, it remains unclear why only a fraction of those patients with a clinical risk factor for ALI progress to develop the syndrome.
Chronic environmental exposures have not been well studied as potential risk factors for ALI, with the notable exception of alcohol abuse (4). Retrospective analyses have suggested that cigarette smoking may be associated with ALI, although these studies were limited by their use of nonspecific administrative data and insensitive measures of both smoking and ALI (5, 6). In addition, experimental studies have demonstrated that active smoking causes pathophysiological changes similar to those seen in ALI, including increased lung epithelial permeability, endothelial injury, and disordered platelet function (7–12). Likewise, cigarette smoke induces accumulation of neutrophils in the pulmonary circulation and potentiates leukocyte activation (13, 14), which may prime patients to develop ALI in the appropriate clinical setting (15). However, no prospective clinical studies have focused on the relationship between smoking and ALI. Furthermore, no studies have tested the impact of secondhand smoke exposure on the development of ALI, despite evidence from the cardiovascular literature that the effects of secondhand (or “passive”) smoke exposure on endothelial function and inflammation are nearly equivalent to those of active smoking (16). Meanwhile, global consumption of cigarettes continues to increase (17). One of every three people worldwide is an active smoker, and at least one-third of all adults and 40% of all children worldwide are regularly exposed to significant secondhand smoke (17, 18). Thus, exposure to cigarette smoke remains a major public health problem.
This study was designed to determine the impact of both active and passive smoking on susceptibility to ALI in a prospective cohort of patients with severe trauma. Smoking history in the medical chart is frequently inaccurate, particularly in critically ill patients who are intubated and sedated (19); moreover, secondhand smoke exposure is difficult to quantify via history, even under optimal conditions (20–22). For these reasons, we used plasma cotinine to measure cigarette smoke exposure. Plasma cotinine is a metabolite of nicotine that accurately discriminates between active and passive smoking and quantifies cigarette smoke exposure (22, 23). We measured plasma cotinine levels in 144 patients with severe blunt trauma immediately on their arrival at the emergency department and analyzed the association between plasma cotinine levels and the development of ALI. Some of the results of these studies have been previously reported in the form of an abstract (24).
METHODS
Ethics Statement
The Institutional Review Board of the University of California at San Francisco (San Francisco, CA) approved the research protocol for this prospective cohort study and granted a waiver of consent for the blood sampling as a minimal risk intervention. Informed consent was subsequently obtained from patients or their surrogates for continued study participation.
Patients
Patients with severe blunt trauma admitted to San Francisco General Hospital (the only level 1 trauma center for the City and County of San Francisco) between February 2005 and February 2009 were studied. All adult patients with blunt trauma who met criteria for full trauma team activation and were subsequently admitted to the intensive care unit were eligible for enrollment. We focused on blunt trauma because the majority of trauma-related ALI follows blunt trauma (25). Patients who died within the first 24 hours before any arterial blood gases and/or chest radiographs could be obtained were excluded. Additional details on excluded subjects are available in the online supplement.
Sample Collection and Cotinine Measurements
The method of sample collection has been described previously in detail (26). Briefly, a 10-ml sample of blood was drawn within 10 minutes of arrival in the emergency department. The samples were immediately transferred to the central laboratory and centrifuged, and the plasma was extracted and stored at −80°C. Concentrations of free (unconjugated) cotinine in plasma were determined by liquid chromatography–tandem mass spectrometry (27). The limit of quantification for plasma cotinine was 0.02 ng/ml. Previous analyses have demonstrated that a plasma cotinine level of 3.08 ng/ml accurately discriminates active from passive smoking with a C-statistic of 0.991 (23); thus, we used this cutoff to determine whether patients with measurable plasma cotinine were active or passive smokers.
Data Collection and Outcome Measures
Data were collected prospectively on patient demographics, mechanism of injury and severity, and subsequent hospital course. The Injury Severity Score (ISS) was used as a measure of the degree of tissue injury (28). Smoking history was obtained from the medical chart and/or from patients or their surrogates whenever possible. Alcohol use history was obtained from chart review and, whenever possible, from patients or surrogates, using a previously validated survey instrument (the Alcohol Use Disorders Identification Test, AUDIT) (29, 30). Patients were monitored until hospital discharge or death. ALI was defined on the basis of the American–European Consensus Criteria (31) and was ascertained by two-physician review of all patient data for the first 7 days of hospitalization; the physicians were blinded to smoking status and cotinine measurements.
Statistical Analysis
Statistical significance was defined as P less than 0.05, using two-tailed tests of hypotheses. Categorical data were analyzed by chi-squared test or Fisher exact test. Normally distributed continuous variables were analyzed by t test or analysis of variance. Nonparametric continuous variables were analyzed by Wilcoxon rank-sum or Kruskal-Wallis test. Because cotinine is not normally distributed, cotinine levels were log-transformed or classified into ordinal variables for regression analysis. Tests for trend across categories were performed using linear contrast (32). Locally weighted scatterplot smoothing (Lowess) was used to analyze the smoothed, nonparametric relationship between cotinine and the development of ALI. Multivariable logistic regression was performed by a manual step-wise backward selection approach, initially including insurance status, race, sex, age, Injury Severity Score, and a composite variable reflecting several measures of alcohol abuse (any charted history of alcohol abuse, withdrawal, or alcoholic cirrhosis; high score on AUDIT questionnaire [≥8 points for men or ≥5 points for women (33)]; or blood alcohol level > 100 mg/dl), based on previous data showing the association of these variables with the development of ALI and/or associations of these variables with both cotinine and ALI in our sample. Covariates were then serially eliminated from the backward selection model on the basis of the highest P value, until all variables were statistically significant. Multivariable logistic regression model fit was checked with the Hosmer-Lemeshow test and the linktest (32). Statistical analysis was performed with STATA/MP 10.1 (Statacorp, College Station, TX).
RESULTS
Study Subjects
Table 1 describes the baseline characteristics of study subjects, stratified by the development of ALI. Comorbidities were generally uncommon in this sample, and there were no differences in the prevalence of HIV/AIDS, interstitial lung disease, coronary artery disease, congestive heart failure, chronic kidney disease, diabetes, hypoalbuminemia, or malignancy between those with and without ALI (data not shown). Of note, the prevalence of alcohol abuse, illicit drug use, and charted history of cigarette smoking did not differ between those with and without ALI. Table E1 in the online supplement presents similar demographic and clinical data stratified by plasma cotinine categories.
TABLE 1.
COMPARISON OF DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF PATIENTS WITH AND WITHOUT ACUTE LUNG INJURY
| Characteristic | Patients without ALI (n = 82) | Patients with ALI (n = 62) | P Value |
|---|---|---|---|
| Age, yr: mean ± SD | 52 ± 21 | 44 ± 18 | 0.02 |
| Female sex, n (%) | 26 (32%) | 12 (19%) | 0.10 |
| Private insurance, n (%) | 32 (41%) | 21 (35%) | 0.22* |
| Race, n (%) | |||
| White | 44 (54%) | 41 (66%) | |
| African-American | 10 (13%) | 8 (13%) | 0.26 |
| Asian-American | 23 (28%) | 12 (19%) | |
| Latino ethnicity | 15 (19%) | 13 (21%) | 0.80 |
| Traumatic brain injury, n (%) | 54 (66%) | 45 (73%) | 0.39 |
| History of alcohol abuse, n (%) | 20 (24%) | 14 (23%) | 0.80 |
| History of alcohol abuse, alcohol withdrawal, alcoholic cirrhosis, high score on AUDIT, or high blood alcohol level, n (%) | 24 (29%)† | 19 (31%)‡ | 0.86 |
| History of active illicit drug use, n (%) | 11 (13%) | 8 (13%) | 0.93 |
| History of active smoking, n (%) | 21 (26%) | 22 (36%) | 0.20 |
| History of asthma, n (%) | 3 (4%) | 6 (10%) | 0.19 |
| History of COPD, n (%) | 0 (0%) | 2 (3%) | 0.20 |
| Injury Severity Score, mean ± SD | 24 ± 15 | 33 ± 15 | 0.0003 |
| Chest component of injury score, mean ± SD | 0.9 ± 1.6 | 1.9 ± 1.9 | 0.0009 |
| Units pRBCs transfused in first 24 h, median (IQR) | 0 (0, 2) | 3 (0, 9) | <0.0001 |
| Units FFP transfused in first 24 h, median (IQR) | 0 (0, 2) | 2 (0, 8) | 0.0001 |
| Units platelets transfused in first 24 h, median (IQR) | 0 (0, 0) | 0 (0, 1) | <0.0001 |
| Day of ALI, median (IQR) | — | 1 (0, 3) | — |
Definitions of abbreviations: ALI = acute lung injury; AUDIT = Alcohol Use Disorders Identification Test; COPD = chronic obstructive pulmonary disease; FFP = fresh frozen plasma; IQR = interquartile range; pRBCs = packed red blood cells.
P value for comparison overall of types of insurance.
Of the four subjects with no chart history of alcohol abuse, two were classified as alcohol abusers based on AUDIT score and two based on high blood alcohol level.
Of the five subjects with no chart history of alcohol abuse, all five were classified as alcohol abusers based on high blood alcohol levels.
Prevalence and Correlates of Cigarette Smoke Exposure
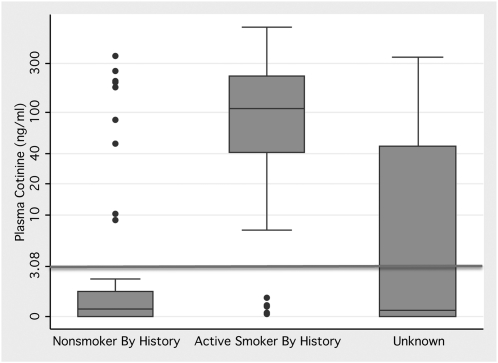
Plasma cotinine demonstrated evidence of active smoking in many patients who were either nonsmokers by history or had no documented or obtainable smoking history (Figure 1). Specifically, 41% of patients demonstrated to be active smokers by cotinine were not detected by smoking history.
Figure 1.
Box-and-whisker plot demonstrating the range of plasma cotinine values in patients stratified by history of smoking. Horizontal line represents the cutoff value of 3.08 ng/ml, which accurately discriminates between active and passive smoking (23). The y axis is log-scale. Number in each group: Nonsmokers by history, n = 53; active smokers by history, n = 43; unknown smoking history, n = 48.
Using a plasma cotinine level of at least 3.08 ng/ml as a cutoff, 44% of patients were determined to be active smokers, markedly higher than the local population prevalence of 15% (34). In addition, 53 patients (66% of nonsmokers) had evidence of passive smoke exposure by plasma cotinine. In contrast, the prevalence of passive smoking in the United States was estimated at 40% of nonsmokers (35).
Subjects with a history of any evidence of alcohol abuse had markedly higher cotinine levels than those without a history of alcohol abuse (median, 26 ng/ml compared with 0.2 ng/ml; P < 0.001). Plasma cotinine levels were not associated with Injury Severity Score (Spearman r = 0.06; P = 0.48; Table E1). Of note, although severity of chest injury was associated with the development of ALI, it was not associated with plasma cotinine (Table E1).
Association between Cigarette Smoke Exposure and ALI
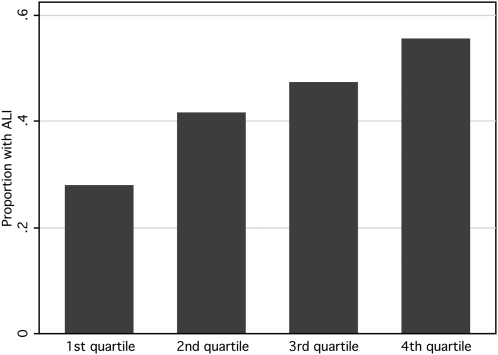
Increasing levels of plasma cotinine were associated with a significantly increased risk of developing ALI (odds ratio for ALI, 1.42 per 1-log increase in plasma cotinine; 95% confidence interval, 1.02–1.98; P = 0.036). Because plasma cotinine is not normally distributed, and because the dose–response effect of cigarette smoke exposure may not be linear (16), cotinine was divided into quartiles for further analysis of its association with the development of acute lung injury. Increasing quartiles of cotinine were associated with increasing odds of developing ALI (Table 2 and Figure 2). For subjects in the highest quartile of cotinine (cotinine, 108–691 ng/ml), the odds of developing ALI were 3.25 times higher than those for subjects in the lowest quartile of cotinine (cotinine, 0–0.088 ng/ml) (odds ratio, 3.25; 95% confidence interval, 1.22–8.68; P = 0.02 for quartile comparison and P = 0.017 for trend across quartiles). Analysis of the data by tertiles revealed a similar pattern (data not shown).
TABLE 2.
UNIVARIATE ANALYSES OF ASSOCIATION BETWEEN PLASMA COTININE AND ACUTE LUNG INJURY
| Measure of Exposure | Odds Ratio for Developing ALI (95% CI) | P Value |
|---|---|---|
| Quartiles of Cotinine (n = 36 for each quartile) | ||
| Lowest quartile (cotinine, 0–0.088 ng/ml) | (Referent) | NA |
| Second quartile (cotinine, 0.09–0.6 ng/ml) | 1.86 (0.69–4.97) | 0.22 |
| Third quartile (cotinine, 0.67–96 ng/ml) | 2.33 (0.87–6.20) | 0.09 |
| Fourth quartile (cotinine, 108–691 ng/ml) | 3.25 (1.22–8.68) | 0.02 |
| Combining Low-level Exposure with No Exposure | ||
| No exposure to low level exposure (cotinine < 0.19 ng/ml; n = 54) | (Referent) | NA |
| Moderate to heavy passive smoke exposure (cotinine, 0.19–3.08 ng/ml; n = 26) | 3.03 (1.15–8.04) | 0.026 |
| Active smoking (cotinine > 3.08 ng/ml; n = 64) | 2.77 (1.28–5.99) | 0.01 |
Definition of abbreviations: ALI = acute lung injury; CI = confidence interval; NA = not applicable.
Figure 2.
Bar chart demonstrating the proportion of patients who developed acute lung injury in increasing quartiles of plasma cotinine (n = 36 in each quartile; P value for trend = 0.017).
Because the threshold at which cigarette smoke exposure begins to have a biological effect on ALI is unclear, we used a locally weighted scatterplot smoothing (Lowess) technique to plot a smoothed, nonlinear curve depicting the relationship between plasma cotinine and risk for ALI (Figure E1). This analysis revealed that the risk for developing ALI increased sharply across the range of passive smoke exposure, with an inflection point at a relatively low level of exposure. Thus, we dichotomized plasma levels of cotinine consistent with passive smoking at the median (0.19 ng/ml), and classified all those with exposure below this level together with those patients with undetectable cotinine. In this analysis, subjects with cotinine levels consistent with moderate to high levels of passive smoke exposure (greater than the median level) had nearly the same odds of developing acute lung injury as those who were active smokers (Table 2). Sensitivity analyses using a cotinine cutpoint of 10 ng/ml to distinguish active from passive smokers, as had been used in prior studies when the intensity of secondhand smoke exposure was higher (21), revealed similar results (data not shown).
In a multivariable regression model, the factors that remained independently predictive of the development of ALI were moderate to heavy passive smoke exposure, active smoking, and Injury Severity Score (Table 3). The full initial model, before backward selection, is presented in Table E2.
TABLE 3.
MULTIVARIATE REGRESSION MODEL OF FACTORS ASSOCIATED WITH THE DEVELOPMENT OF ACUTE LUNG INJURY: BACKWARD SELECTION
| Predictor | Odds Ratio for Developing ALI (95% CI) | P Value |
|---|---|---|
| Moderate to heavy passive smoke exposure (cotinine, 0.19–3.08 ng/ml) | 2.88 (1.05–7.94) | 0.04 |
| Active smoking (cotinine > 3.08 ng/ml) | 2.83 (1.25–6.39) | 0.01 |
| Injury Severity Score, per one-point increase | 1.04 (1.02, 1.07) | 0.001 |
Definition of abbreviations: ALI = acute lung injury; CI = confidence interval.
Eliminated from the model (in order): Source of medical insurance; composite variable capturing any history of alcohol abuse, or alcohol withdrawal, or alcoholic cirrhosis, or high score on AUDIT, or high blood alcohol level; age; race; sex.
DISCUSSION
Cigarette smoking remains a major threat to public health (36), with increasing numbers of people smoking worldwide and a plateau in the U.S. rate of smoking at 20% (17, 37). In addition to the harmful effects of cigarette smoke on active smokers, there are increasing data demonstrating the significant biological effects of secondhand, or passive, exposure to cigarette smoke (21). In this study, we present the results of the first prospective study of the effect of active and passive cigarette smoking on the risk of developing acute lung injury after severe blunt trauma, a major cause of morbidity and mortality worldwide. These data demonstrate that both active smoking and moderate to heavy passive smoking are associated with increased susceptibility to ALI in this setting, even after controlling for other important predictors of ALI, such as injury severity and alcohol abuse. This finding has important implications for both public health and for understanding of the pathogenesis of ALI. Specifically, if replicated, this finding should strengthen support for tighter regulation of exposure to secondhand smoke in public venues; further, it suggests that chronic exposure to cigarette smoke may prime individuals to develop ALI in the setting of a second hit such as severe trauma.
The relationship between cigarette smoke exposure and ALI is both biologically plausible and strongly supported by previous experimental work. In experimental human and animal studies, active smoking has been linked to alterations in lung epithelial and endothelial function similar to those seen in ALI. Studies in human subjects and animal models have demonstrated that asymptomatic smokers have significantly greater lung epithelial permeability, a pathophysiological hallmark of ALI, as compared with nonsmokers (7–10). Active smoking also decreases expression of the primary ion channels responsible for resolving alveolar edema, which could impair alveolar fluid clearance and thus increase formation of lung edema (38, 39). Likewise, both endothelial injury and disordered platelet function are thought to be critical to the pathogenesis of ALI (40, 41), and the cardiovascular literature has demonstrated the potent effects of both active and secondhand smoke on both of these processes (16). Furthermore, cigarette smoking induces neutrophil accumulation in the pulmonary circulation, depresses humoral and cell-mediated immunity, impairs mucociliary clearance, and alters alveolar macrophage number and function, any of which could contribute to enhancing susceptibility to ALI (13, 42).
Over the past several decades, the deleterious effects of passive smoking have been increasingly appreciated. Secondhand smoke exposure has been conclusively linked to a variety of respiratory, cardiovascular, and neoplastic diseases, including coronary artery disease, lung cancer, sudden infant death syndrome, and exacerbations of obstructive lung disease (21). Moreover, the effects of passive smoking on endothelial function, platelet activation, and inflammation were estimated in a meta-analysis to be on average 80–90% of those of active smoking; in some cases, the effect of passive smoking was as or more potent than that of active smoking (16). Despite the demonstration of these adverse effects of secondhand smoke exposure, 40% of Americans are still exposed to secondhand smoke, including 54% of children between ages 3 and 11 years (35). In some of our analyses, we dichotomized passive smokers at the median into low-level (<0.19 ng/ml) and moderate- to high-level (≥0.19 ng/ml) exposure; a plasma cotinine level of 0.20 ng/ml is roughly equivalent to spending 1 hour in a moderately smoky room, such as a bar or restaurant (43, 44). Thus, the quantity of passive smoke exposure associated with increased risk of ALI in this study appears to be relatively modest, although this threshold for exposure will need to be further tested in other at-risk groups.
Why might the effects of active and passive smoking on susceptibility to ALI be so similar in our analysis? Several explanations are possible. First, the similar odds ratios observed for active smoking and moderate to heavy passive smoking may represent a threshold effect: once exposure to cigarette smoke passes a certain level, the increase in susceptibility reaches a plateau. Second, the chemical constituents of sidestream smoke, which is the primary type of cigarette smoke inhaled by nonsmokers, differ from those of mainstream smoke and are in many cases more toxic, because of the different burning temperature of the cigarette at its lit end (45). Thus, smaller doses of these more toxic by-products of tobacco smoke may have a similar effect to the larger doses of mainstream smoke inhaled by the active smoker. Third, as mentioned previously, passive smoking has nearly the same effect on indices of endothelial injury, platelet activation, and inflammation as active smoking (16); thus, these pathways of injury may be affected equally by active and passive exposure, leading to equivalent susceptibility to ALI. A final possibility is that some of the subjects with cotinine in the heavy passive range are actually intermittent or social smokers (46), and are thus exposed to similar risk as active smokers.
The use of plasma cotinine to quantify exposure to cigarette smoke in patients with severe trauma has several advantages. First, plasma cotinine has been extensively validated as a biomarker of both active and passive smoking, with excellent accuracy in discriminating active from passive exposure and high specificity (23). In the Surgeon General's report on secondhand smoking, the prevalence of secondhand smoke exposure was assessed largely via this biomarker and other related nicotine metabolites (21). Second, the trauma population provides an ideal setting for using plasma cotinine because most trauma victims are presumably healthy and engaged in their normal activities of daily living until shortly before arrival in the emergency department. Thus, plasma cotinine is likely to represent a random and accurate snapshot of their average tobacco exposure. Third, plasma cotinine has a relatively short half-life of 16 hours; however, given our sampling within 10 minutes of emergency department arrival, and the short transport times of patients by emergency medical services in San Francisco (<1 h), cotinine decay should not have a significant impact on our findings. The high prevalence of both active and passive smoking that we detected in this cohort, using plasma cotinine, echoes previous findings in both a randomly selected subset of all admitted patients at the same urban county hospital (47) as well as in a mixed medical–surgical intensive care unit population in Tennessee (19).
We considered alcohol abuse a priori to be a major potential confounder of the association between cigarette smoke exposure and ALI, given prior evidence that alcohol abuse increases susceptibility to ALI (4), and therefore we captured this comorbidity in several ways. First, we collected any historical information available on alcohol abuse, including any evidence of alcoholic cirrhosis or alcohol withdrawal, from the medical chart. This is the approach that was used in the original report linking alcohol abuse to the development of the acute respiratory distress syndrome (ARDS) (48). Second, we administered a previously validated survey instrument, the AUDIT questionnaire, to willing patients and their surrogates to further quantify alcohol use. Unfortunately, patient and surrogate refusal to answer this questionnaire was relatively common, as was patient death before questionnaire administration. Third, we captured blood alcohol levels in all patients in whom this test was performed for clinical reasons. In our multivariable model, we forced the inclusion of a composite variable capturing high blood alcohol levels along with the two previous covariates (chart history of alcohol abuse and high score on the AUDIT) into the initial multivariable regression; however, it was not significantly associated with the development of ALI in this model. Finally, the prevalence of alcohol abuse in our study sample (30%) is similar to the prevalence of alcohol abuse reported in previous studies of alcohol abuse and ALI (30–34%) (4, 48) and double the prevalence reported in the trauma subgroup in the original study linking alcohol and lung injury (15%) (48). Despite these best attempts, some residual confounding by alcohol abuse remains possible. However, it should also be appreciated that previous studies of the relationship between alcohol abuse and ALI/ARDS in critically ill patients either did not account for cigarette smoke exposure at all (48) or used historical data to measure cigarette smoke exposure (4), which we have demonstrated in this study to be insensitive.
Limitations of our study include that it was conducted at a single center, had a relatively modest sample size, and lacked biomarkers reflective of longer term cigarette smoke exposure.
In conclusion, both active and moderate to heavy passive smoking are independently associated with the development of acute lung injury after severe blunt trauma. This finding has important implications for public health, because both major trauma and ALI are common causes of death, as well as for our understanding of the pathogenesis of ALI.
Supplementary Material
Supported by HL090833, the Flight Attendant Medical Research Institute, and KL2RR024130 from the NCRR, a component of the NIH (C.S.C.); by HL 51856 (M.A.M.); by GM085689 and a AAST Hemostasis and Resuscitation Scholarship (M.J.C.); and by NIH DA12393 (N.B.).
Author contributions: Study conception and design: C.C., M.M., M.E., N.B., M.C., J.F.P., M.J.C.; analysis and interpretation: C.C., M.M., M.E., N.B., M.J.C.; drafting the manuscript for important intellectual content: C.C., M.M., M.E., N.B., M.C., J.F.P., M.J.C.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201011-1802OC on March 18, 2011
Author Disclosure: C.C. received a sponsored grant from the Flight Attendants Medical Research Institute, and received consultancy fees from Ikaria and GlaxoSmithKline. N.B. received a sponsored grant from the Flight Attendants Medical Research Institute. M.E. is a full-time employee of Genentech Inc. M.M., M.C., M.C., and J.F.P. have no financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah CV, Localio AR, Lanken PN, Kahn JM, Bellamy S, Gallop R, Finkel B, Gracias VH, Fuchs BD, Christie JD. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med 2008;36:2309–2315. [DOI] [PubMed] [Google Scholar]
- 4.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 2003;31:869–877. [DOI] [PubMed] [Google Scholar]
- 5.Christenson JT, Aeberhard JM, Badel P, Pepcak F, Maurice J, Simonet F, Velebit V, Schmuziger M. Adult respiratory distress syndrome after cardiac surgery. Cardiovasc Surg 1996;4:15–21. [DOI] [PubMed] [Google Scholar]
- 6.Iribarren C, Jacobs DR Jr, Sidney S, Gross MD, Eisner MD. Cigarette smoking, alcohol consumption, and risk of ARDS: a 15-year cohort study in a managed care setting. Chest 2000;117:163–168. [DOI] [PubMed] [Google Scholar]
- 7.Jones JG, Minty BD, Lawler P, Hulands G, Crawley JC, Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet 1980;1:66–68. [DOI] [PubMed] [Google Scholar]
- 8.Jones JG, Minty BD, Royston D, Royston JP. Carboxyhaemoglobin and pulmonary epithelial permeability in man. Thorax 1983;38:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason GR, Uszler JM, Effros RM, Reid E. Rapidly reversible alterations of pulmonary epithelial permeability induced by smoking. Chest 1983;83:6–11. [DOI] [PubMed] [Google Scholar]
- 10.Li XY, Rahman I, Donaldson K, MacNee W. Mechanisms of cigarette smoke induced increased airspace permeability. Thorax 1996;51:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blann AD, McCollum CN. Adverse influence of cigarette smoking on the endothelium. Thromb Haemost 1993;70:707–711. [PubMed] [Google Scholar]
- 12.Fernandez JA, Gruber A, Heeb MJ, Griffin JH. Protein C pathway impairment in nonsymptomatic cigarette smokers. Blood Cells Mol Dis 2002;29:73–82. [DOI] [PubMed] [Google Scholar]
- 13.MacNee W, Wiggs B, Belzberg AS, Hogg JC. The effect of cigarette smoking on neutrophil kinetics in human lungs. N Engl J Med 1989;321:924–928. [DOI] [PubMed] [Google Scholar]
- 14.Lehr HA, Weyrich AS, Saetzler RK, Jurek A, Arfors KE, Zimmerman GA, Prescott SM, McIntyre TM. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J Clin Invest 1997;99:2358–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 2005;33:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 2005;111:2684–2698. [DOI] [PubMed] [Google Scholar]
- 17.Mackay J, Eriksen M. The tobacco atlas. Geneva, Switzerland: World Health Organization; 2002.
- 18.World Health Organization. WHO report on the global tobacco epidemic. Geneva, Switzerland: World Health Organization; 2009.
- 19.Hsieh SJ, Ware LB, Eisner MD, Yu L, Jacob P, Havel C, Goniewicz ML, Matthay MA, Benowitz NL, Calfee CS. Biomarkers increase detection of active smoking and secondhand smoke exposure in critically ill patients. Crit Care Med 2011;39:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res 2009;11:12–24. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [PubMed]
- 22.Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4:149–159. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 2009;169:236–248. [DOI] [PubMed] [Google Scholar]
- 24.Calfee CS, Matthay MA, Eisner MD, Benowitz NL, Call M, Pittet JF, Cohen MJ. Active smoking and secondhand smoke exposure are associated with increased susceptibility to acute lung injury following severe blunt trauma [abstract]. Presented at the Flight Attendant Medical Research Institute 9th Scientific Symposium, Miami, FL, 2010.
- 25.Treggiari MM, Hudson LD, Martin DP, Weiss NS, Caldwell E, Rubenfeld G. Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med 2004;32:327–331. [DOI] [PubMed] [Google Scholar]
- 26.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg 2007;245:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dempsey D, Tutka P, Jacob P III, Allen F, Schoedel K, Tyndale RF, Benowitz NL. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther 2004;76:64–72. [DOI] [PubMed] [Google Scholar]
- 28.Baker SP, O'Neill B, Haddon W Jr, Long WB. The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 1974;14:187–196. [PubMed] [Google Scholar]
- 29.Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcohol Clin Exp Res 2002;26:272–279. [PubMed] [Google Scholar]
- 30.Donovan DM, Dunn CW, Rivara FP, Jurkovich GJ, Ries RR, Gentilello LM. Comparison of trauma center patient self-reports and proxy reports on the Alcohol Use Identification Test (AUDIT). J Trauma 2004;56:873–882. [DOI] [PubMed] [Google Scholar]
- 31.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American–European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed] [Google Scholar]
- 32.Vittinghoff E, Glidden D, Shiboski SC, McCulloch CE. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. Statistics for biology and health. New York, NY: Springer Science + Business Media; 2005.
- 33.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res 2007;31:185–199. [DOI] [PubMed] [Google Scholar]
- 34.Al-Delaimy WK, Lin L, Messer K, Sigman R, Pierce JP, Winglee M, White MM, Aiken M, Bobbitt H, Hubbell K, et al. California Tobacco Survey: 2005. San Diego, CA: California Department of Health Services (University of California San Diego); 2008.
- 35.Centers for Disease Control and Prevention. Nonsmokers' exposure to secondhand smoke–United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2010;59:1141–1146. [PubMed] [Google Scholar]
- 36.Gu D, Kelly TN, Wu X, Chen J, Samet JM, Huang JF, Zhu M, Chen JC, Chen CS, Duan X, et al. Mortality attributable to smoking in China. N Engl J Med 2009;360:150–159. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Current cigarette smoking among adults aged ≥ 18 years–United States, 2009. MMWR Morb Mortal Wkly Rep 2010;59:1135–1140. [PubMed] [Google Scholar]
- 38.Xu H, Ferro TJ, Chu S. Cigarette smoke condensate inhibits ENaC α-subunit expression in lung epithelial cells. Eur Respir J 2007;30:633–642. [DOI] [PubMed] [Google Scholar]
- 39.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 2006;173:1139–1144. [DOI] [PubMed] [Google Scholar]
- 40.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 41.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest 2009;119:3450–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med 2004;164:2206–2216. [DOI] [PubMed] [Google Scholar]
- 43.Repace J, Hughes E, Benowitz N. Exposure to second-hand smoke air pollution assessed from bar patrons' urinary cotinine. Nicotine Tob Res 2006;8:701–711. [DOI] [PubMed] [Google Scholar]
- 44.Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P III. Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res 2009;11:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.California Environmental Protection Agency. Proposed identification of environmental tobacco smoke as a toxic air contaminant. Sacramento, CA: California Environmental Protection Agency; 2005.
- 46.Schane RE, Glantz SA, Ling PM. Nondaily and social smoking: an increasingly prevalent pattern. Arch Intern Med 2009;169:1742–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benowitz NL, Schultz KE, Haller CA, Wu AH, Dains KM, Jacob P III. Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol 2009;170:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 1996;275:50–54. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.