Abstract
Rationale: Beryllium sensitization (BeS) and chronic beryllium disease (CBD) are determined by at least one genetic factor, a glutamic acid at position 69 (E69) of the HLA-DPB1 gene, and by exposure to beryllium. The relationship between exposure and the E69 genotype has not been well characterized.
Objectives: The study goal was to define the relationship between beryllium exposure and E69 for CBD and BeS.
Methods: Workers (n = 386) from a U.S. nuclear weapons facility were enrolled into a case–control study (70 BeS, 61 CBD, and 255 control subjects). HLA-DPB1 genotypes were determined by sequence-specific primer-polymerase chain reaction. Beryllium exposures were reconstructed on the basis of worker interviews and historical exposure measurements.
Measurements and Main Results: Any E69 carriage increased odds for CBD (odds ratio [OR], 7.61; 95% confidence interval [CI], 3.66–15.84) and each unit increase in lifetime weighted average exposure increased the odds for CBD (OR, 2.27; 95% CI, 1.26–4.09). Compared with E69-negative genotypes, a single E69-positive *02 allele increased the odds for BeS (OR, 12.01; 95% CI, 4.28–33.71) and CBD (OR, 3.46; 95% CI, 1.42–8.43). A single non-*02 E69 allele further increased the odds for BeS (OR, 29.54; 95% CI, 10.33–84.53) and CBD (OR, 11.97; 95% CI, 5.12–28.00) and two E69 allele copies conferred the highest odds for BeS (OR, 55.68; 95% CI, 14.80–209.40) and CBD (OR, 22.54; 95% CI, 7.00–72.62).
Conclusions: E69 and beryllium exposure both contribute to the odds of CBD. The increased odds for CBD and BeS due to E69 appear to be differentially distributed by genotype, with non-*02 E69 carriers and E69 homozygotes at higher odds than those with *02 genotypes.
Keywords: berylliosis, genetics, case–control studies, occupational exposure, HLA-DP antigens
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Genetic studies repeatedly have linked beryllium sensitization and chronic beryllium disease to a glutamic acid at position 69 (E69) on HLA-DPB1. Few studies have examined beryllium exposure in combination with HLA-DPB1 E69.
What This Study Adds to the Field
The increased odds for chronic beryllium disease and beryllium sensitization due to HLA-DPB1 E69 appear to be differentially distributed by genotype, with non-*02 E69 carriers and E69 homozygotes at higher odds than those with *02 genotypes. Beryllium exposure and E69 genotype contribute individually to chronic beryllium disease odds.
In manufacturing nuclear weapons, workers at U.S. and international facilities have been exposed to beryllium aerosols. A subset of these workers have developed beryllium sensitization (BeS) and chronic beryllium disease (CBD). BeS is the demonstration of T-cell proliferation via presentation of an unknown beryllium antigen by human leukocyte antigen (HLA) class II molecules on antigen-presenting cells and is demonstrated clinically by the beryllium lymphocyte proliferation test (BeLPT) (1–4). In some individuals, BeS progresses to CBD, a progressive lung disease characterized by noncaseating granulomas (5, 6). The prevalence of BeS and CBD appears to vary depending on the workforce and beryllium exposure characteristics; prevalences of up to 15% for BeS and 8% for CBD have been reported in previous cross-sectional studies (2, 7). In general, the exposure–response relationship does not appear to be linear, with reports of BeS and CBD documented in individuals with low-exposure jobs (8–10). Differences in exposure characteristics (11–18) and host genetic factors (19–29) have been proposed as explanatory of the lack of linear exposure response.
Richeldi and colleagues (19) demonstrated overrepresentation of HLA-DPB1 alleles with a glutamic acid at position 69 of the β chain (E69) in CBD case subjects (97%) compared with control subjects (30%). More recent studies have verified these results, showing increased frequencies of E69 in CBD (61–97%) and BeS (39–90%) case subjects, compared with control subjects (30–47%) (20–27). The non-*02 E69 alleles have been associated with increased odds for CBD and BeS as compared with the *02 alleles and E69 homozygotes have been associated with greater odds of BeS and CBD as compared with heterozygotes (21, 24, 25, 27). Only one previous study has evaluated the combination of genetic and exposure factors on the odds of BeS and CBD (20). In this study, high beryllium exposure, and carriage of HLA-DPB1 E69, were each individually associated with increased odds of CBD. However, the study did not provide extensive characterization of exposure using industrial hygiene measurements to define exposure, nor did it evaluate risk of BeS and the effects of specific E69 genotype.
In the current study, we hypothesized that HLA-DPB1 E69 homozygosity, carriage of non-*02 E69 alleles, and higher beryllium exposure would confer increased odds of BeS and CBD. This hypothesis was addressed in a case–control study of a large population of beryllium-exposed nuclear weapons production workers. The aims of the study were as follows: (1) to investigate the interaction between E69 and beryllium exposure in the risk for BeS and CBD, (2) to determine whether E69 homozygosity and carriage of specific E69 alleles impact the odds of BeS and CBD, and (3) to define beryllium exposure levels important in the risk for BeS and CBD. Some of the results of these studies have been previously reported in the form of abstracts (30, 31).
METHODS
Study Recruitment and Design
The current investigation was conducted among workers from the Rocky Flats Environmental Technology Site (RFETS). Approximately 7,820 current and former workers at the plant had participated in medical surveillance based on the BeLPT between 1992 and 2001, with at least 117 having been diagnosed with CBD and 184 with BeS (32). All workers ever participating in RFETS beryllium medical surveillance were eligible to participate in the study. BeS case subjects had two or more abnormal blood BeLPT results and/or an abnormal bronchoalveolar lavage (BAL) BeLPT results. BeS case subjects were considered confirmed if they had undergone a medical evaluation with no evidence of CBD and unconfirmed if no medical evaluation had been performed. CBD case subjects had evidence of BeS along with either (1) granulomas on biopsy or (2) both an abnormal BAL BeLPT and greater than 15% lymphocytes in BAL fluid. Control subjects also worked at the RFETS facility and had at least two normal and no abnormal BeLPTs with one performed in the last 5 years. To the extent possible, control subjects were frequency matched approximately two to one to case subjects (combined BeS/CBD group), based on sex, race, and decade of hire at RFETS. The study protocol was approved by the National Jewish Health (Denver, CO) Institutional Review Board and included written informed consent from each study participant.
Genetic Assessment and Coding
Genomic DNA was extracted from peripheral blood, using a Wizard genomic purification kit (Promega, Madison, WI), with genotyping performed by sequence specific primer-polymerase chain reaction (SSP-PCR) for HLA-DPB1 as described by Gilchrist and colleagues (33). The technician performing genotyping was blinded to case and exposure status. Genes were typed to the allele level for the numerous HLA-DPB1 alleles. Participants were coded or grouped according to several strategies: (1) E69 positive or E69 negative, indicating carriage or noncarriage of at least one HLA-DPB1 allele containing the E69 marker (i.e., *0201, *0202, *0601, *0801, *0901, *1001, *1301, *1601, *1701, *1901); (2) HLA-DPB1 genotype specifying carriage of at least one of the indicated alleles (e.g., *0201 genotype, *0401 genotype, etc.); (3) *02 genotype specifying carriage of at least one *0201 or *0202 allele; (4) non-*02 E69-positive genotype specifying carriage of at least one non-*02 E69-positive allele (i.e., *0601, *0801, *0901, *1001, *1301, *1601, *1701, or *1901); or (5) additional coding indicating carriage of one or two copies of E69-positive alleles and pair-wise combinations of *02, non-*02, and E69-negative alleles.
Exposure Assessment
Each participant was interviewed by a trained interviewer, using a standardized exposure questionnaire. The interviewer was blinded to genotype and case status. From the exposure questionnaires, each of the unique beryllium exposure tasks was identified. Using published and unpublished beryllium exposure data from RFETS (34–36) and similar facilities (15, 37, 38), an arithmetic mean of the available task and time period–specific exposure measurements was calculated for each of the unique beryllium exposure tasks. Using participants' time and percent-beryllium estimates along with task exposure estimates from industrial hygiene data, cumulative and lifetime weighted mean beryllium exposure were calculated in units of micrograms per cubic meter times duration in years (μg/m3-years) or micrograms per cubic meter (μg/m3), respectively. The maximum task-based exposure (μg/m3) was used as a surrogate for short-term high exposure. Other exposure metrics included the highest reported exposure category and the percentage of work time at RFETS spent directly or indirectly exposed to beryllium. A more detailed description of the exposure assessment methods is included in the online supplement.
Statistical Analysis
We used SAS version 9.1 (SAS Institute, Inc., Cary, NC) for statistical analyses. Univariate tests of association between categorical variables were performed using chi-square tests with Fisher's exact tests used when any cell had an expected value less than five. The Bonferroni method was used to address multiple comparisons; as a result the reported unadjusted P values should be compared with α = 0.0006 (n = 87) for genetic comparisons based on all possible combinations and α = 0.017 (n = 3) for comparison of the many correlated exposure measures based on the three comparisons (BeS vs. control subjects, CBD vs. control subjects, and BeS vs. CBD). Because of skewed distributions, continuous variables were compared across the three groups (control subjects, BeS, and CBD), using the Kruskal-Wallis test followed by pair-wise comparisons using the Mann-Whitney test when significant (P ≤ 0.05). A purposeful model-building strategy (39) using unconditional logistic regression was used to model disease state as a function of multiple predictors, including gene and environment variables. All independent variables with univariate P values less than 0.25 were evaluated in multivariate models that included one genetic variable specifying E69 status or genotype and one continuous exposure variable. A significance level of 0.05 was required for a variable to remain in the model. First-order interactions with significance levels at or below 0.1 were included in the final model. All demographic variables were evaluated in the final model for confounding and included in the model when their presence resulted in at least a 10% change in any of the estimated regression coefficients. Two strategies for inclusion of genetic variables in logistic regression models were used: (1) carriage of any E69 allele, and (2) an allele-specific risk model. The allele-specific risk model used a set of classification variables coded as follows: (1) carriage of only E69-negative alleles, (2) carriage of a single copy of an *02 allele along with an E69-negative allele, (3) carriage of a single E69-positive non-*02 allele along with an E69-negative allele, or (4) carriage of two E69 allele copies, one *02 allele and one E69-positive non-*02 allele. For this model, the first variable (E69−) was modeled as the reference. To estimate the probability of CBD as a function of beryllium exposure meaningful for the whole of the Rocky Flats population, a weighted logistic regression was performed using the observed rate of CBD of 1.7% reported in a previous cross-sectional study (10). More detailed statistical methods are included in the online supplement.
RESULTS
Study Population
A total of 399 individuals were enrolled in this study. Thirteen subjects were excluded for the following reasons: 5 because they did not meet our criteria for diagnosis of CBD or BeS (2 with only one abnormal BeLPT, 1 diagnosed with sarcoidosis without abnormal BeLPTs, 2 with insufficient medical information to provide an accurate diagnosis), 4 with either a diagnosis of BeS or CBD before their hire date at RFETS or with long-term beryllium exposure at a facility other than RFETS, and 4 whose DNA was unavailable for genotyping. The final cohort consisted of 386 former RFETS workers including 255 control subjects with potential beryllium exposure, 61 subjects with CBD, 53 subjects with confirmed BeS, and 17 individuals who were classified as unconfirmed BeS on the basis of two abnormal BeLPTs, but who had not undergone a bronchoscopy to rule out CBD. Seven of the 61 CBD case subjects were diagnosed on the basis of an abnormal BeLPT BAL response and greater than 15% lymphocytes. As BeS and CBD are rare outcomes associated with specialized industrial operations, populations studied often overlap. Although this cohort was assembled specifically for this study, some of the case subjects and control subjects in this study also participated in previous genetic (24), cross-sectional industry-based (10, 32, 35, 36, 40), and longitudinal studies (5, 6) of BeS and CBD.
Demographic Characteristics
As shown in Table 1, there were no significant differences in age, year of hire at RFETS, and number of years worked at RFETS among the three groups. The cohort was predominantly male (88.3%), consistent with the traditionally male-dominated workforce at RFETS. However, there were proportionally more female case subjects with BeS (n = 15, 21.4%) compared with control subjects (n = 25, 9.8%; P = 0.009) and CBD case subjects (n = 5, 8.2%; P = 0.036). Although participants were predominantly white (97.7%) and non-Hispanic (93.8%), there was a significantly higher proportion of African-American CBD case subjects (n = 4, 6.6%) compared with control subjects (n = 3, 1.2%; P = 0.028). Hispanics were overrepresented among BeS case subjects (n = 8, 11.4%) compared with control subjects (n = 11, 4.3%; P = 0.039). Most of the differences in sex, race, and ethnicity are likely due to underenrollment of control subjects or frequency matching on the combined BeS/CBD group. A trend for increased smoking among BeS case subjects was apparent, with BeS case subjects more likely to be current smokers (n = 8, 11.4%) compared with control subjects (n = 13, 5.1%; P = 0.094) and CBD case subjects (n = 2, 3.3%; P = 0.104). BeS case subjects also worked fewer years at the facility (median, 12.6 yr) compared with control subjects (median, 15.0 yr; P = 0.020). Among those classified as having BeS, there were no differences in age, sex, race, ethnicity, smoking status, year of hire at RFETS, or years spent working at RFETS between those with confirmed BeS and those with unconfirmed BeS (data not shown). There were also no differences in these variables between those with biopsy-proven CBD and those diagnosed with CBD on the basis of abnormal BAL BeLPT and lymphocytosis (data not shown).
TABLE 1.
COMPARISON OF DEMOGRAPHIC CHARACTERISTICS AMONG SUBJECTS WITH CHRONIC BERYLLIUM DISEASE, SUBJECTS WITH BERYLLIUM SENSITIZATION, AND CONTROL SUBJECTS
| Total | Control Subjects | BeS | CBD | ||
|---|---|---|---|---|---|
| (n = 386) | (n = 255) | (n = 70) | (n = 61) | P Value | |
| Median age, yr (range)* | 67 (41–89) | 67 (41–89) | 65 (45–84) | 65 (49–86) | |
| Sex, n (%)† | |||||
| Male | 341 (88.3%) | 230 (90.2%) | 55 (78.6%) | 56 (91.8%) | |
| Female | 45 (11.7%) | 25 (9.8%)‡ | 15 (21.4%)‡,§ | 5 (8.2%)§ | 0.009,‡ 0.036§ |
| Race, n (%)† | |||||
| White | 377 (97.7%) | 252 (98.8%) | 68 (97.1%) | 57 (93.4%) | |
| African American | 9 (2.3%) | 3 (1.2%)‖ | 2 (2.9%) | 4 (6.6%)§ | 0.028§ |
| Ethnicity, n (%)† | |||||
| Hispanic | 24 (6.2%) | 11 (4.3%)‡ | 8 (11.4%)‡ | 5 (8.2%) | 0.039‡ |
| Non-Hispanic | 362 (93.8%) | 244 (95.7%) | 62 (88.6%) | 56 (91.8%) | |
| Smoking status, n (%)† | |||||
| Current | 23 (6.0%) | 13 (5.1%)‡ | 8 (11.4%)‡,§ | 2 (3.3%)§ | 0.094,‡ 0.104§ |
| Former | 203 (52.6%) | 135 (52.9%) | 34 (48.6%) | 34 (55.7%) | |
| Never | 160 (41.4%) | 107 (42.0%) | 28 (40.0%) | 25 (41.0%) | |
| Year of hire, median (range)* | 1969 (1952–1998) | 1968 (1952–1993) | 1972 (1952–1998) | 1969 (1952–1990) | |
| Years at facility, median (range)* | 15.0 (0.2–40.7) | 15.8 (0.2–40.7)‡ | 12.6 (0.5–33.7)‡ | 16.8 (1.0–40.0) | 0.020‡ |
Definition of abbreviations: BeS = beryllium sensitization; CBD = chronic beryllium disease.
Compared using Kruskal-Wallis test followed by pair-wise Mann-Whitney tests when significant.
Compared using chi-square or Fisher's exact method.
Comparison between BeS and control subjects.
Comparison between BeS and CBD.
Comparison between CBD and control subjects.
Exposure Characteristics
Qualitative self-reported exposure characteristics.
Qualitative self-reported exposure characteristics are shown in Table 2 comparing case subjects and control subjects. Overall, 86.3% of the cohort reported direct or indirect exposure to beryllium and 16.9% of the typical participants' time was spent working in jobs with direct or indirect beryllium exposure. Only 12.4% of the participants had ever worked as a beryllium machinist. CBD case subjects spent a greater percentage of their time directly exposed to beryllium (median, 4.3%) as compared with BeS case subjects (median, 0%; P = 0.009) and although the comparison was not significant compared with control subjects, a trend was apparent (median, 1.4%; P = 0.068). In addition, CBD case subjects were more likely to report direct exposure to beryllium (68.9%) as compared with BeS case subjects (45.7%; P = 0.008). Interestingly, BeS case subjects were less likely than control subjects to report direct exposure to beryllium (45.7% vs. 62.3%; P = 0.012), and there was a trend suggesting BeS case subjects were more likely to report “no known exposure to beryllium” compared with control subjects (22.9 vs. 12.5%; P = 0.031). Together, these data suggest that CBD case subjects had spent a greater percentage of their work time directly exposed to beryllium than did control subjects or BeS case subjects, and that BeS case subjects were less likely to have self-reported direct beryllium exposure than control subjects.
TABLE 2.
COMPARISON OF REPORTED EXPOSURE CHARACTERISTICS AMONG SUBJECTS WITH CHRONIC BERYLLIUM DISEASE, SUBJECTS WITH BERYLLIUM SENSITIZATION, AND CONTROL SUBJECTS
|
P Value |
|||||||
|---|---|---|---|---|---|---|---|
| Total(n = 386) | Control Subjects(n = 255) | BeS(n = 70) | CBD(n = 61) | BeS versus Control Subjects | CBD versus Control Subjects | BeS versus CBD | |
| Any reported exposure to Be, n (%)* | 333 (86.3%) | 223 (87.4%)‡ | 54 (77.1%) | 56 (91.8%) | 0.031 | 0.342 | 0.022 |
| Year of first Be exposure, median (range)† | 1970 (1952–1996) | 1969 (1952–1971) | 1976 (1952–1996) | 1969 (1953–1990) | n.s. | n.s. | n.s. |
| Highest reported Be exposure, n (%)* | |||||||
| Any direct Be exposure | 233 (60.4%) | 159 (62.3%) | 32 (45.7%) | 42 (68.9%) | 0.012‡ | 0.343 | 0.008‡ |
| a. Directly alter Be part | 112 (29.0%) | 72 (28.2%) | 15 (21.4%) | 25 (41.0%) | 0.255 | 0.052 | 0.015‡ |
| b. Contact with Be waste materials | 90 (23.3%) | 63 (24.7%) | 13 (18.6%) | 14 (23.0%) | 0.283 | 0.774 | 0.536 |
| c. Contact with finished Be part | 31 (8.0%) | 24 (9.4%) | 4 (5.7%) | 3 (4.9%) | 0.329 | 0.259 | 1.0 |
| Any indirect Be exposure | 100 (25.9%) | 64 (25.1%) | 22 (31.4%) | 14 (22.9%) | |||
| d. Work within 5 ft of Be operation | 25 (6.5%) | 18 (7.1%) | 2 (2.9%) | 5 (8.2%) | 0.266 | 0.784 | 0.250 |
| e. Work in same room as Be operation | 17 (4.4%) | 12 (4.7%) | 4 (5.7%) | 1 (1.6%) | 0.756 | 0.475 | 0.371 |
| f. Work in same building as Be operation | 58 (15.0%) | 34 (13.3%) | 16 (22.9%) | 8 (13.1%) | 0.050 | 0.964 | 0.150 |
| No known exposure to Be | 53 (13.7%) | 32 (12.5%) | 16 (22.9%) | 5 (8.2%) | 0.031 | 0.342 | 0.022 |
| Percentage of work time exposed to Be, median (range)† | |||||||
| Directly (categories a–c above) | 1.3% (0–100%) | 1.4% (0–95.0%) | 0% (0–100%) | 4.3% (0–100%) | 0.104 | 0.068 | 0.009 |
| Indirectly (categories d–f above) | 5.2% (0–100%) | 6.1% (0–100%) | 3.1% (0–100%) | 10.0% (0–100%) | n.s. | n.s. | n.s. |
| Directly or indirectly | 16.9% (0–100%) | 15.9% (0–100%) | 10.6% (0–100%) | 33.6% (0–100%) | n.s. | n.s. | n.s. |
| Ever exposed to Be oxide, n (%)* | 22 (5.7%) | 13 (5.1%) | 5 (7.1%) | 4 (6.6%) | 0.555 | 0.751 | 1.0 |
| Ever worked as a Be machinist, n (%)* | 48 (12.4%) | 33 (12.9%) | 6 (8.6%) | 9 (14.7%) | 0.319 | 0.708 | 0.268 |
Definition of abbreviations: BeS = beryllium sensitization; CBD = chronic beryllium disease; n.s. = not significant (Kruskal-Wallis P > 0.05).
* Compared by chi-square or Fisher's exact method; unadjusted P values reported.
Compared by Kruskal-Wallis test followed by pair-wise Mann-Whitney test when significant.
Significant after Bonferroni correction (n = 3), α = 0.017.
Reconstructed exposures.
Reconstructed beryllium exposures comparing case and control subjects are shown in Table 3. CBD case subjects had significantly higher cumulative exposures (median, 1.46 μg/m3-years) and lifetime weighted average exposures (median, 0.07 μg/m3) than either BeS case subjects (median, 0.11 μg/m3-years; P = 0.001 and 0.01 μg/m3; P = 0.001) or control subjects (median, 0.39 μg/m3-years; P = 0.011 and 0.03 μg/m3; P = 0.008). Conversely, BeS case subjects had lower cumulative exposures (median, 0.11 μg/m3-years) than control subjects (median, 0.39 μg/m3-years; P = 0.031) and a trend toward lower lifetime weighted average exposure (median, 0.01 vs. 0.03 μg/m3; P = 0.073). There were no significant differences in any exposure characteristics between confirmed BeS case subjects and those classified as unconfirmed BeS (data not shown). Separating the lifetime weighted exposures into quartiles (≤0.001 μg/m3, >0.001 to ≤0.03 μg/m3, >0.03 to ≤0.17 μg/m3, and >0.17 μg/m3) demonstrated that CBD case subjects had a higher percentage of subjects with exposures over 0.17 μg/m3 (41.0%) compared with control subjects (22.3%; P = 0.015). In addition, a greater percentage of CBD case subjects (32.8%) tended to work in the highest task-based exposures compared with control subjects (20.0%; P = 0.032). Interestingly, BeS case subjects were more likely to have maximum task-based exposures less than 0.02 μg/m3 (35.7% compared with 19.2% of control subjects, P = 0.003; and 13.1% of CBD case subjects, P = 0.003).
TABLE 3.
COMPARISON OF RECONSTRUCTED EXPOSURE CHARACTERISTICS AMONG SUBJECTS WITH CHRONIC BERYLLIUM DISEASE, SUBJECTS WITH BERYLLIUM SENSITIZATION, AND CONTROL SUBJECTS
|
P Value |
|||||||
|---|---|---|---|---|---|---|---|
| Total(n = 386) | Control Subjects(n = 255) | BeS(n = 70) | CBD(n = 61) | BeS versus Control Subjects | CBD versus Control Subjects | BeS versus CBD | |
| Cumulative Be exposure, μg/m3-years: median (mean)* | 0.35 (3.68) | 0.39 (2.43) | 0.11 (2.96) | 1.46 (9.71) | 0.031 | 0.011 | 0.001 |
| Lifetime weighted average Be exposure, μg/m3: median (mean)* | 0.03 (0.24) | 0.03 (0.15) | 0.01 (0.25) | 0.07 (0.64) | 0.073 | 0.008 | 0.001 |
| Lifetime weighted average exposure quartiles, n (%)† | |||||||
| ≤0.001 μg/m3 | 91 (23.6%) | 57 (22.3%) | 24 (34.3%) | 10 (16.4%) | 0.040 | 0.306 | 0.020 |
| >0.001 to ≤0.03 μg/m3 | 109 (28.2%) | 74 (29.0%) | 21 (30.0%) | 14 (22.9%) | 0.873 | 0.342 | 0.363 |
| >0.03 to ≤0.17 μg/m3 | 89 (23.1%) | 67 (26.3%) | 10 (14.3%) | 12 (19.7%) | 0.037 | 0.285 | 0.411 |
| >0.17 μg/m3 | 97 (25.1%) | 57 (22.3%) | 15 (24.4%) | 25 (41.0%) | 0.869 | 0.003‡ | 0.015‡ |
| Maximum task-based exposure, n (%)† | |||||||
| <0.02 μg/m3 | 82 (21.2%) | 49 (19.2%) | 25 (35.7%) | 8 (13.1%) | 0.003‡ | 0.266 | 0.003‡ |
| ≥0.02 and <0.05 μg/m3 | 30 (7.8%) | 17 (6.7%) | 7 (10.0%) | 6 (9.8%) | 0.345 | 0.411 | 0.975 |
| ≥0.05 and <0.10 μg/m3 | 7 (1.8%) | 5 (2.0%) | 2 (2.86%) | 0 (0%) | 0.646 | 0.587 | 0.498 |
| ≥0.10 and <0.20 μg/m3 | 27 (7.0%) | 18 (7.1%) | 6 (8.6%) | 3 (4.9%) | 0.668 | 0.776 | 0.502 |
| ≥0.20 and <0.50 μg/m3 | 24 (6.2%) | 16 (6.3%) | 4 (5.7%) | 4 (6.6%) | 1.0 | 1.0 | 1.0 |
| ≥0.50 and <1.0 μg/m3 | 29 (7.5%) | 24 (9.4%) | 2 (2.9%) | 3 (4.9%) | 0.073 | 0.259 | 0.663 |
| ≥1.0 and <2.0 μg/m3 | 100 (25.9%) | 75 (29.4%) | 8 (11.4%) | 17 (27.9%) | 0.002‡ | 0.812 | 0.017 |
| ≥2.0 μg/m3 | 87 (22.5%) | 51 (20.0%) | 16 (22.9%) | 20 (32.8%) | 0.601 | 0.032 | 0.204 |
Definition of abbreviations: BeS = beryllium sensitization; CBD = chronic beryllium disease.
Compared using Kruskal-Wallis test followed by pair-wise Mann-Whitney test when significant.
Compared by chi-square or Fisher's exact method; unadjusted P values reported.
Significant after Bonferroni correction (n = 3), α = 0.017.
Genotype Characteristics
The E69 genotypic distribution was in Hardy-Weinberg equilibrium (HWE) for control subjects (χ2 = 0.03, P = 0.86), but not for BeS case subjects (χ2 = 10.69, P = 0.001) or CBD case subjects (χ2 = 10.69, P = 0.001). For BeS and CBD case subjects, there were more heterozygotes than expected and fewer E69 and non-E69 homozygotes than expected on the basis of HWE for the expected allele frequency for each of these groups. As shown in Table 4B, both BeS case subjects (92.9%; P < 0.0001) and CBD case subjects (83.6%; P < 0.0001) were more likely to carry an E69 allele compared with control subjects (38.0%). Case subjects were also more likely to carry two copies of E69 compared with control subjects (25.7% of BeS case subjects and 19.7% of CBD case subjects vs. 4.3% of control subjects, with P < 0.0001 for both). After correcting for multiple comparisons, the only significant allele-specific differences noted in Table 4A were that BeS case subjects were more likely to be carriers of the *0201 and *0601 alleles and CBD case subjects were more likely to be carriers of the *0601 allele compared with control subjects. When combined (Table 4B), the non-*02 E69 alleles were present at greater frequency in both BeS case subjects (55.7 vs. 14.1% of control subjects; P < 0.0001) and CBD case subjects (60.7% vs. control subjects; P < 0.0001). The differences were also significant when comparing the *02 genotype between BeS case subjects and control subjects (BeS, 52.9 vs. 26.7% of control subjects; P < 0.0001). Interestingly, the frequency of the *02 genotype in CBD case subjects (39.3%) did not differ significantly from control subjects after correcting for multiple comparisons. No significant differences were noted in any genotype frequency between BeS and CBD case subjects or between confirmed and unconfirmed cases of BeS.
TABLE 4.
COMPARISON OF HLA-DPB1 GENOTYPE FREQUENCY, AND OF GROUPED HLA-DPB1 E69 GENOTYPE FREQUENCY, AMONG SUBJECTS WITH CHRONIC BERYLLIUM DISEASE, SUBJECTS WITH BERYLLIUM SENSITIZATION, AND CONTROL SUBJECTS
|
P Value* |
||||||
|---|---|---|---|---|---|---|
| HLA-DPB1 Genotype | Control Subjects(n = 255) | BeS(n = 70) | CBD(n = 61) | BeS versus Control Subjects | CBD versus Control Subjects | BeS versus CBD |
| A. Comparison of HLA-DPB1 Genotype Frequency among Subjects with CBD, Subjects with BeS, and Control Subjects | ||||||
| E69-containing alleles | ||||||
| a. *0201 | 67 (26.3%) | 36 (51.4%) | 21 (34.4%) | <0.0001† | 0.202 | 0.050 |
| b. *0202 | 1 (0.4%) | 2 (2.9%) | 3 (4.9%) | 0.118 | 0.024 | 0.663 |
| c. *0601 | 3 (1.2%) | 9 (12.9%) | 11 (18.0%) | <0.0001† | <0.0001† | 0.411 |
| d. *0801 | 0 (0) | 1 (1.4%) | 0 (0) | 0.215 | n/a | 1.0 |
| e. *0901 | 2 (0.8%) | 4 (5.7%) | 4 (6.6%) | 0.021 | 0.014 | 1.0 |
| f. *1001 | 11 (4.3%) | 9 (14.8%) | 9 (12.9%) | 0.020 | 0.006 | 0.753 |
| g. *1301 | 14 (5.5%) | 7 (10.0%) | 5 (8.2%) | 0.177 | 0.382 | 0.721 |
| h. *1601 | 1 (0.4%) | 3 (4.3%) | 2 (2.9%) | 0.033 | 0.096 | 1.0 |
| i. *1701 | 4 (1.6%) | 7 (11.5%) | 7 (10.0%) | 0.003 | 0.001 | 0.785 |
| j. *1901 | 1 (0.4%) | 0 (0%) | 0 (0%) | 1.0 | 1.0 | n/a |
| Non–E69-containing alleles | ||||||
| k. *0101 | 20 (7.8%) | 7 (10.0%) | 5 (8.2%) | 0.562 | 1.0 | 0.721 |
| l. *0301 | 37 (14.5%) | 6 (8.6%) | 6 (9.8%) | 0.194 | 0.339 | 0.802 |
| m. *0401 | 187 (73.3%) | 35 (50.0%) | 26 (42.6%) | 0.0002† | <0.0001† | 0.398 |
| n. *0402 | 62 (24.3%) | 2 (2.9%) | 9 (14.8%) | <0.0001† | 0.108 | 0.014 |
| o. *0501 | 6 (2.3%) | 1 (1.4%) | 3 (4.9%) | 1.0 | 0.383 | 0.338 |
| p. *1101 | 8 (3.1%) | 1 (1.4%) | 2 (3.3%) | 0.690 | 1.0 | 0.598 |
| q. *1401 | 10 (3.9%) | 2 (2.9%) | 1 (1.6%) | 1.0 | 0.698 | 1.0 |
| r. *1501 | 1 (0.4%) | 1 (1.4%) | 0 (0%) | 0.385 | 1.0 | 1.0 |
| s. *2001 | 3 (1.2%) | 0 (0%) | 0 (0%) | 1.0 | 1.0 | n/a |
| t. *2301 | 2 (0.8%) | 0 (0%) | 0 (0%) | 1.0 | 1.0 | n/a |
| B. Comparison of Grouped HLA-DPB1 E69 Genotype Frequency among Subjects with CBD, Subjects with BeS, and Control Subjects | ||||||
| Any E69+ (one or two copies) | 97 (38.0%) | 65 (92.9%) | 51 (83.6%) | <0.0001† | <0.0001† | 0.097 |
| Any *02 (one or two copies) | 68 (26.7%) | 37 (52.9%) | 24 (39.3%) | <0.0001† | 0.050 | 0.122 |
| Single *02 with non-E69 | 57 (22.3%) | 21 (30.0%) | 13 (21.3%) | 0.184 | 0.850 | 0.258 |
| Any non-*02 E69+ (one or two copies) | 36 (14.1%) | 39 (55.7%) | 37 (60.7%) | <0.0001† | <0.0001† | 0.568 |
| Single E69+ non*02 with non-E69 | 29 (11.4%) | 26 (37.1%) | 26 (42.6%) | <0.0001† | <0.0001† | 0.522 |
| Any two E69+ copies | 11 (4.3%) | 18 (25.7%) | 12 (19.7%) | <0.0001† | <0.0001† | 0.412 |
| Two E69+ copies (*02 alleles) | 4 (1.6%) | 5 (7.1%) | 1 (1.6%) | 0.025 | 1.0 | 0.214 |
| Two E69+ copies (*02 plus non-*02) | 7 (2.75%) | 11 (15.7%) | 10 (16.4%) | 0.0002† | 0.0002† | 0.916 |
| Two E69+ copies (non-*02 alleles) | 0 (0%) | 2 (2.9%) | 1 (1.6%) | 0.046 | 0.193 | 1.0 |
Definition of abbreviations: BeS = beryllium sensitivity; CBD = chronic beryllium disease; n/a = not applicable, analysis not possible.
Compared by chi-square or Fisher's exact method; unadjusted P values reported.
Significant after Bonferroni correction (n = 87), α = 0.0006.
Multiple Logistic Regression
Increasing lifetime weighted exposure and E69 were associated with increased odds of CBD in our multiple logistic regression models (Table 6). In contrast, whereas E69 was highly predictive of BeS odds in logistic regression models, beryllium exposure metrics including those representing cumulative, average, and short-term high exposure were not associated with BeS odds (Table 5). Demographic variables including race, ethnicity, sex, age, and year of hire were not significant predictors of the odds of BeS or CBD, nor did their inclusion in the model significantly change the regression coefficients or odds ratios of the other predictors.
TABLE 6.
MULTIPLE LOGISTIC REGRESSION MODEL FOR CHRONIC BERYLLIUM DISEASE, CONSIDERING HLA-DPB1 E69 GENOTYPE AND EXPOSURE
| Independent Variable | Regression Coefficient | Standard Error | P Value | OR (95% CI) |
|---|---|---|---|---|
| Intercept | −2.92 | 0.34 | <0.001 | |
| HLA-DPB1 E69− genotype | ||||
| HLA-DPB1 E69− | Ref. | |||
| Single HLA-DPB1*02 allele (with E69− allele) | 1.24 | 0.45 | 0.006 | 3.46 (1.42–8.43) |
| Single HLA-DPB1 E69+ non-*02 allele (with E69− allele) | 2.48 | 0.43 | <0.001 | 11.97 (5.12–28.00) |
| E69 homozygote (*02 plus non-*02 E69+) | 3.11 | 0.60 | <0.001 | 22.54 (7.00–72.62) |
| Per unit increase in lifetime weighted average Be exposure, μg/m3 | 0.80 | 0.31 | 0.010 | 2.22 (1.21–4.07) |
Definition of abbreviations: CI = confidence interval; E69 = glutamic acid at position 69; OR = odds ratio; Ref. = reference group.
TABLE 5.
MULTIPLE LOGISTIC REGRESSION MODEL FOR BERYLLIUM SENSITIZATION, CONSIDERING HLA-DPB1 E69 GENOTYPE
| Independent Variable | Regression Coefficient | Standard Error | P Value | OR (95% CI) |
|---|---|---|---|---|
| Intercept | −3.72 | 0.48 | <0.001 | |
| HLA-DPB1 E69 genotype | ||||
| HLA-DPB1 E69− | Ref. | |||
| Single HLA-DPB1*02 allele (with E69− allele) | 2.48 | 0.53 | <0.001 | 12.01 (4.28–33.71) |
| Single HLA-DPB1 E69+ non-*02 allele (with E69− allele) | 3.39 | 0.54 | <0.001 | 29.54 (10.33–84.53) |
| E69 homozygote (*02 plus non-*02 E69+) | 4.01 | 0.68 | <0.001 | 55.68 (14.80–209.40) |
| Worked less than 5 yr at RFETS | 1.04 | 0.39 | 0.008 | 2.83 (1.31–6.13) |
Definition of abbreviations: CI = confidence interval; E69 = glutamic acid at position 69; OR = odds ratio; Ref. = reference group; RFETS = Rocky Flats Environmental Technology Site.
The significant predictors of BeS derived from multiple logistic regression are presented in Table 5. The model showed point estimates of increasing odds of BeS with carriage of a single *02 allele (odds ratio [OR], 12.01; 95% confidence interval [CI], 4.28–33.71), carriage of a single non-*02 E69 allele (OR, 29.54; 95% CI, 10.33–84.53), and E69 copy number with one *02 allele plus one non-*02 E69 allele (OR, 55.68; 95% CI, 14.8–209.40). In addition, increased odds of BeS was associated with having worked fewer than 5 years at the facility (OR, 2.83; 95% CI, 1.31–6.13). If a more simplistic model is used, adjusting for time spent working at the facility, the carriage of any E69 allele increases the odds of BeS 22-fold (OR, 21.89; 95% CI, 8.43–56.80) (data not shown). Beryllium exposure covariates including lifetime weighted average exposure, cumulative exposure, and short-term maximum task-based exposure were not significant predictors of BeS. Race, ethnicity, sex, smoking status, and first-order interactions were not significant predictors of the odds of BeS, nor did their inclusion in the model significantly change the regression coefficients or odds ratios of the other predictors.
Using a similar multiple logistic regression model, predictors of CBD are shown in Table 6. Of note, carriage of a single *02 allele (OR, 3.46; 95% CI, 1.42–8.43), carriage of a single non-*02 E69 allele (OR, 11.97; 95% CI, 5.12–28.00), and E69 copy number with one *02 allele plus one non-*02 E69 allele (OR, 22.54; 95% CI, 7.00–72.62) were associated with increased odds of CBD. In addition, each unit increase in lifetime weighted average beryllium exposure (OR, 2.22; 95% CI, 1.21–4.07) was associated with increased odds of CBD. In a simplified model, carriage of any E69 allele was associated with nearly eightfold increased CBD odds (OR, 7.61; 95% CI, 3.66–15.84) when adjusting for lifetime weighted average exposure (OR, 2.27; 95% CI, 1.26–4.09; data not shown in tables). In an alternative model, cumulative beryllium exposure was also a significant predictor of CBD, showing a small (<10%) increase in CBD odds per unit increase in cumulative exposure (OR, 1.04; 95% CI, 1.00–1.07; data not shown). As lifetime weighted average and cumulative exposure were highly correlated (r = 0.91), only a single exposure risk factor could be included in the model. In the lifetime weighted average exposure model, neither excluding an exposure outlier (10.7 μg/m3) nor excluding all those exposed above a lifetime weighted average of 2 μg/m3 impacted the genetic regression coefficients or odds ratios significantly, although the latter did impact the lifetime weighted average estimate, more than doubling the regression coefficient. As with BeS, race, ethnicity, sex, smoking status, and first-order interactions were not significant predictors of the odds of CBD, nor did their inclusion in the model significantly change the regression coefficients or odds ratios of the other predictors.
CBD odds ratio estimates were determined by genetic factor and exposure level to estimate how these varied with increasing exposure. The joint odds of CBD at increasing levels of beryllium exposure and varying E69 genotypes are illustrated in Table 7. Figure 1 demonstrates the output of a weighted logistic regression model using the predictors from Table 6 weighted by the previous site-wide prevalence estimate of 1.7% (10). Figure 1 shows that the baseline probability of CBD varies by specific E69 allele or E69 copy number and increases with increasing exposure. Considering the current Occupational Safety and Health Administration (OSHA) permissible exposure level (2.0 μg/m3), point estimates of CBD odds range from a 5-fold increase for E69-negative genotypes to a more than 100-fold increase for E69 homozygotes. Table 7 also illustrates that compliance with a reduced exposure level, such as 0.1 μg/m3, could potentially reduce the odds of CBD nearly fivefold across all levels of E69 status.
TABLE 7.
ODDS RATIO ESTIMATES BY BERYLLIUM EXPOSURE AND HLA-DPB1 E69 GENOTYPE FOR ODDS OF CHRONIC BERYLLIUM DISEASE
| Lifetime Weighted Average Beryllium Exposure (μg/m3) | HLA-DPB1 E69− Genotype | Single *02 (with E69− Allele) | Single E69+ non-*02 (with E69− Allele) | E69 Homozygote (*02 plus non-*02 E69+) |
|---|---|---|---|---|
| 0.02 | 1.02 (1.00–1.03) | 3.52 (1.45–8.57) | 12.16 (5.20–28.45) | 22.90 (7.11–73.83) |
| 0.05 | 1.04 (1.01–1.07) | 3.61 (1.48–8.77) | 12.45 (5.32–29.14) | 23.46 (7.27–75.69) |
| 0.10 | 1.08 (1.02–1.15) | 3.75 (1.54 −9.14) | 12.96 (5.53–30.37) | 24.41 (7.55–78.96) |
| 0.20 | 1.18 (1.04–1.32) | 4.06 (1.66–9.95) | 14.03 (5.95–33.07) | 26.43 (8.11–86.11) |
| 0.50 | 1.49 (1.10–2.02) | 5.16 (2.02–13.16) | 17.82 (7.25–43.81) | 33.56 (9.90–113.76) |
| 1.0 | 2.22 (1.21–4.07) | 7.68 (2.63–22.43) | 26.52 (9.38–75.02) | 49.95 (13.07–190.88) |
| 2.0 | 4.91 (1.46–16.56) | 17.01 (3.80–76.17) | 58.77 (13.43–257.2) | 110.7 (19.78–619.3) |
Definition of abbreviation: E69 = glutamic acid at position 69.
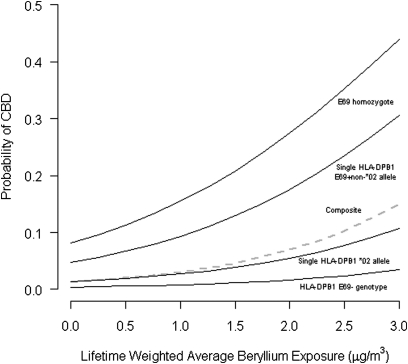
Figure 1.
Predicted probability of chronic beryllium disease (CBD) by HLA-DPB1 E69 (glutamic acid at position 69) genotype and lifetime-weighted average exposure based on weighted logistic regression using predictors described in Table 6. Regression weighted using previously documented Rocky Flats site-wide CBD prevalence of 1.7% (10). HLA-DPB1 E69− = carriage of only non–E69-containing alleles; single HLA-DPB1 *02 allele = carriage of one E69-containing *02 allele and one non–E69-containing allele; single HLA-DPB1 E69+ non-*02 allele = carriage of one E69-containing non–*02 allele and one non–E69-containing allele; E69 homozygote = carriage of one E69-containing non–*02 allele and one E69-containing *02 allele; Composite = average site-wide probability of CBD assuming the HLA-DPB1 characteristics of our case–control participants are representative of the site population.
DISCUSSION
In the largest case–control study of beryllium-exposed workers to date, we evaluated the relationship between quantitative beryllium exposure estimates in combination with HLA-DPB1 E69 genotype in determining the risk for BeS and CBD. We noted increased exposure associated with CBD as compared with control subjects, which was evident whether considering self-reported exposure assessments or quantitative exposure reconstructions. However, no exposure–response relationship was apparent for BeS, even with inclusion of genetic risk factors. For both CBD and BeS, E69 conferred increased odds as has been shown in other studies (19–25, 27). We found that the odds of BeS and CBD appear to be greater among carriers of the non-*02 HLA-DPB1 E69 alleles, and among HLA-DPB1 E69 homozygotes even after adjusting for beryllium exposure. Most importantly, we found evidence supporting individual contributions to CBD risk by increasing exposure and genetic susceptibility via E69 with no significant gene–environment interaction. Last, we provided evidence suggesting an exposure response for CBD and lack thereof for BeS after adjusting for E69 genetic risk factors.
The finding of an exposure–response relationship for CBD has implications for standard setting in the workplace, at a time when OSHA is reconsidering revising the currently out-of-date beryllium exposure standard. In this study, the odds of CBD were associated with higher lifetime weighted average and cumulative exposures, whereas increasing exposure was not a risk factor for BeS. This confirms the previous work by Viet and colleagues (36) at the RFETS facility showing significant relationships for both cumulative and mean exposures and CBD, but not BeS. Also, confirming previous reports (10, 40, 41), we identified CBD case subjects at low apparent exposures, with three CBD case subjects reporting no known beryllium exposure and an additional five reporting never having worked in areas or tasks where reconstructed exposures exceeded 0.02 μg/m3. Thus, this study, although clearly showing a higher prevalence of CBD at higher exposure levels, fails to demonstrate a threshold for the development of CBD. In contrast, for BeS, there was no evidence of either an exposure threshold or an exposure–response relationship as evidenced by the tendency for BeS case subjects to be overrepresented in the lowest lifetime weighted average exposure quartile and with more than one-third of BeS case subjects never having worked in areas or processes where reconstructed exposures exceeded 0.02 μg/m3. This frequent occurrence of BeS among workers with only minimal known exposure combined with evidence of an exposure–response relationship for CBD has important implications for worker protection both in terms of medical surveillance and removal from exposure. The findings could imply that workers who are sensitized and have not incurred sufficient beryllium exposure to develop CBD may remain sensitized indefinitely. In addition, the findings suggest that even minimally exposed workers should be screened, using the BeLPT, to detect BeS and facilitate early removal from exposure.
This study also confirms the individual contributions of exposure and genetics (E69 status) to the development of CBD. In our current study, carriage of any E69 allele conferred about eightfold increased odds of CBD and each unit increase in lifetime weighted average beryllium exposure increased the CBD odds approximately twofold. This 8-fold increased odds for carriage of any HLA-DPB1 E69 variant is within the confidence limits of the 12-fold increased odds from the initial gene–environment study (20). In comparing the risks from genetics and exposure, our current findings suggest that, in terms of CBD odds, carriage of any single E69 allele even in extremely low exposures incurs similar odds as exposure to an average beryllium concentration of 4 μg/m3 for those without an E69 allele.
The increased odds for carriage of any E69 allele appears to be differentially distributed when considering E69 genotype, with carriers of only a single copy of an *02 allele only at 3-fold increased odds, those with non-*02 genotypes at nearly 12-fold increased odds, and those with two E69 allele copies at more than 20-fold increased odds. Increased risks of BeS and CBD have been reported previously for carriers of non-*02 alleles and homozygotes (21, 24, 25, 27). It has been shown previously that carriage of E69 itself is critical to allow binding of beryllium or beryllium-bound peptides to the HLA-DP molecule (42, 43). The biological basis for the observed increased odds of BeS and CBD among carriers of non-*02 alleles is unclear, although it may be related to the previously identified more electronegative charge on the β chain of the HLA-DP molecule encoded by some of the non-*02 alleles (29), or it could be related to yet-to-be-determined differences in antigen or T-cell receptor–MHC binding affinity or kinetics. An important limitation of the allele grouping strategy used in this analysis is that the increased odds of BeS and CBD conferred by the non-*02 E69 alleles is likely driven by one or more of the alleles in the group and is not a true group effect. We are also limited in our ability to draw firm conclusions about the differential risks by genotype, due to the wide and overlapping confidence intervals for the genotype odds ratios resulting from the limited number of individuals in each of the strata. Larger studies evaluating the effects of HLA-DPB1 genotypes in combination with exposure and HLA-DRB1 are necessary to more precisely evaluate gene–environment effects.
In terms of policy development, exposure reduction has the potential to provide a greater public health benefit than preemployment genetic testing. As has been presented previously (44, 45), the low prevalence of BeS and CBD among those exposed and the high carrier frequency of the E69 allele combine to produce an unacceptable positive predictive value for using the E69 marker to determine eligibility for employment in the beryllium industry. Results from this study continue to support this assertion. From this study, considering the greatest genetic risk factors, non-*02 E69 genotype or E69 homozygosity, for the odds of CBD and assuming a generous CBD prevalence rate of 5%, a non-*02 genotype frequency of 15%, and a 4% frequency of E69 homozygotes, the positive predictive value of genetic testing is only 23% for the non-*02 genotype and only 59% for E69 homozygotes. This low positive predictive value implies that for every 100 individuals denied employment because of this genetic trait, the majority of them would not have developed CBD. Exposure reduction, on the other hand, reduces the odds for all exposed, regardless of E69 status, and might reduce the progression from BeS to CBD.
Using a weighted logistic regression, the models from our case–control study can be extrapolated to project the probability of CBD for workers at RFETS given the facility prevalence of CBD of 1.7% identified in a stratified sample by Kreiss and colleagues (10), and assuming the population characteristics of the participants in this study were representative of all workers at the site (Figure 1). The probability of CBD predicted by our model at a lifetime weighted average exposure of 0.2 μg/m3 is 0.4% for those with E69-negative genotypes, 1.5% for those with a single *02 allele, 5.4% for those with a single non-*02 E69 allele, and 9.1% for E69 homozygotes. Assuming the genotype frequencies of the workers at the entire site are similar to those of the participants in this study, the composite probability of CBD for all workers at the site would be 1.5% at a lifetime weighted average exposure of 0.2 μg/m3. From an occupational exposure limit point of view, this suggests strict compliance with an exposure limit of approximately 0.8 μg/m3 (assuming a log-normal distribution and a geometric standard deviation of three), using an upper tolerance limit approach as described by Mulhausen and colleagues (46) would result in a CBD prevalence of about 1.5% in an exposed population. This estimate is much higher than the 1-in-200 (0.5%) odds of CBD at an occupational exposure limit of 2 μg/m3 suggested by Viet and colleagues (36).
Our multiple logistic regression model for BeS suggested increased odds for those working fewer than 5 years at RFETS. Although there have been reports of BeS occurring within a short period of time after first exposure (15, 47, 48) and others have reported similar protective effects (49), this study would likely not detect early BeS as most of the case subjects were first screened many years after first exposure to beryllium. Only 20% of BeS case subjects in this study were diagnosed as current workers. These case subjects were diagnosed on average 17 years after starting work at the facility, with all diagnosed more than 6 years after starting work at the facility. It is more likely that these increased odds for short-term workers is an artifact of study design, as our study did not include frequency matching for the number of years worked. It is also possible that this effect was a result of the increased participation by long-term workers in the control group. However, this finding suggests that workers exposed for only a short time are at risk of BeS.
Misclassification of disease status and exposure could have impacted our results. The main source of disease misclassification was the inclusion of 17 unconfirmed BeS case subjects. All of the unconfirmed BeS case subjects met the definition of BeS with repeat abnormal BeLPTs; however, as they had not undergone complete medical evaluations, many of them likely had CBD rather than BeS. Analyses of significant exposure effects, using this mixed group of BeS case subjects, would likely be biased away from the null as CBD was found to be associated with exposure. Thus, the lack of significant association between exposure and BeS in this mixed group is noteworthy.
One of the strengths of this study is its detailed exposure reconstruction, in which the use of individual interviews accounts for the large variation of work composition within a single job classification. This attention to exposure at the individual level was likely one of the reasons that this study was able to identify an exposure–response relationship for CBD, whereas others using grouping strategies at the job classification level have failed. It is unclear whether the small differences in CBD odds identified at exposure levels less than 0.2 μg/m3 were accurate or the result of imprecision in exposure reconstruction at the lower levels. Furthermore, cumulative exposures may have been overestimated for case subjects, as exposures accrued until the date of BeS or CBD diagnosis, which was likely much later than the date of disease development. The use of reported time percentages to calculate average and cumulative exposures likely resulted in lower exposure estimates than would have been assigned using methods relying on grouping strategies at the job classification level. The use of industrial hygiene data from other time periods and facilities in the development of the task exposures likely resulted in misclassification on an absolute micrograms per cubic meter scale, but less misclassification on a relative scale for comparing study participants. In assigning exposure estimates to tasks rather than individuals, the misclassification on both the absolute and relative scales should have been nondifferential. In spite of these potential misclassifications, we did find exposure–response relationships for CBD in our multiple logistic regression models. Future studies will be needed to address interactions with other genes in the HLA region and the effects of exposure on CBD severity as higher exposures may be more important with increasing CBD-related impairment. The use of contemporary exposure data in future studies will be critical to determine the CBD risk at average exposures less than 0.2 μg/m3.
Supplementary Material
Acknowledgments
The authors thank the former Rocky Flats workers who participated in this research; Gina Mondello and Holly Christenson, who facilitated the study process; May Gillespie, Starza Duskin, Alexas Jonth, and Jill Elliot for their efforts in genotyping the study participants; Shawn Arbuckle and Katie Finnie for their assistance with exposure interviews; Dr. Bill Stange for assistance with Rocky Flats exposure data; and Dr. Stephen Reynolds, Del Sandfort, Dr. Tom Keefe, and Dr. Tracy Nelson for their review of this manuscript.
Supported by grant P01 ES011810 (Dr. Newman) from the NIEHS (NIH) and by grant 1 UL1 RR025780 (Dr. Sokol) from the NCRR (NIH).
From a dissertation submitted to the Academic Faculty of Colorado State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201002-0254OC on March 11, 2011
Author Disclosure: M.V.D. is employed by National Jewish Health; his institution has received fees for consultation on beryllium exposure control at industrial facilities, lecture fees from Tecniplast, expert witness fees related to a nonoccupational beryllium case, and sponsored grants not related to beryllium from the Electric Power Research Institute. J.W.M. is employed by National Jewish Health; his institution has received fees for consultation on beryllium exposure control at industrial facilities, and expert witness fees from Golomb & Honik, PC. M.M.M. is employed by National Jewish Health. L.S.N. and his institution have received travel support from the World Association for Sarcoidosis and Other Granulomatous Disorders; he is employed by the University of Colorado. L.A.M. is employed by National Jewish Health and has received royalties from UpToDate; her institution has received grants from Centocor and MondoBIOTECH. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kreiss K, Newman LS, Mroz M. Blood testing for chronic beryllium disease. J Occup Med 1991;33:1188–1189. [DOI] [PubMed] [Google Scholar]
- 2.Kreiss K, Newman LS, Mroz MM, Campbell PA. Screening blood test identifies subclinical beryllium disease. J Occup Med 1989;31:603–608. [DOI] [PubMed] [Google Scholar]
- 3.Mroz MM, Kreiss K, Lezotte DC, Campbell PA, Newman LS. Reexamination of the blood lymphocyte transformation test in the diagnosis of chronic beryllium disease. J Allergy Clin Immunol 1991;88:54–60. [DOI] [PubMed] [Google Scholar]
- 4.Saltini C, Winestock K, Kirby M, Pinkston P, Crystal RG. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T cells. N Engl J Med 1989;320:1103–1109. [DOI] [PubMed] [Google Scholar]
- 5.Mroz MM, Maier LA, Strand M, Silviera L, Newman LS. Beryllium lymphocyte proliferation test surveillance identifies clinically significant beryllium disease. Am J Ind Med 2009;52:762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman LS, Mroz MM, Balkissoon R, Maier LA. Beryllium sensitization progresses to chronic beryllium disease: a longitudinal study of disease risk. Am J Respir Crit Care Med 2005;171:54–60. [DOI] [PubMed] [Google Scholar]
- 7.Rosenman K, Hertzberg V, Rice C, Reilly MJ, Aronchick J, Parker JE, Regovich J, Rossman M. Chronic beryllium disease and sensitization at a beryllium processing facility. Environ Health Perspect 2005;113:1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanton ML, Henneberger PK, Kent MS, Deubner DC, Kreiss K, Schuler CR. Sensitization and chronic beryllium disease among workers in copper–beryllium distribution centers. J Occup Environ Med 2006;48:204–211. [DOI] [PubMed] [Google Scholar]
- 9.Kreiss K, Mroz MM, Newman LS, Martyny J, Zhen B. Machining risk of beryllium disease and sensitization with median exposures below 2 micrograms/m3. Am J Ind Med 1996;30:16–25. [DOI] [PubMed] [Google Scholar]
- 10.Kreiss K, Mroz MM, Zhen B, Martyny JW, Newman LS. Epidemiology of beryllium sensitization and disease in nuclear workers. Am Rev Respir Dis 1993;148:985–991. [DOI] [PubMed] [Google Scholar]
- 11.Stefaniak AB, Hoover MD, Day GA, Dickerson RM, Peterson EJ, Kent MS, Schuler CR, Breysse PN, Scripsick RC. Characterization of physicochemical properties of beryllium aerosols associated with prevalence of chronic beryllium disease. J Environ Monit 2004;6:523–532. [DOI] [PubMed] [Google Scholar]
- 12.McCawley MA, Kent MS, Berakis MT. Ultrafine beryllium number concentration as a possible metric for chronic beryllium disease risk. Appl Occup Environ Hyg 2001;16:631–638. [DOI] [PubMed] [Google Scholar]
- 13.Kent MS, Robins TG, Madl AK. Is total mass or mass of alveolar-deposited airborne particles of beryllium a better predictor of the prevalence of disease? A preliminary study of a beryllium processing facility. Appl Occup Environ Hyg 2001;16:539–558. [DOI] [PubMed] [Google Scholar]
- 14.Martyny JW, Hoover MD, Mroz MM, Ellis K, Maier LA, Sheff KL, Newman LS. Aerosols generated during beryllium machining. J Occup Environ Med 2000;42:8–18. [DOI] [PubMed] [Google Scholar]
- 15.Kelleher PC, Martyny JW, Mroz MM, Maier LA, Ruttenber AJ, Young DA, Newman LS. Beryllium particulate exposure and disease relations in a beryllium machining plant. J Occup Environ Med 2001;43:238–249. [DOI] [PubMed] [Google Scholar]
- 16.Tinkle SS, Antonini JM, Rich BA, Roberts JR, Salmen R, DePree K, Adkins EJ. Skin as a route of exposure and sensitization in chronic beryllium disease. Environ Health Perspect 2003;111:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day GA, Dufresne A, Stefaniak AB, Schuler CR, Stanton ML, Miller WE, Kent MS, Deubner DC, Kreiss K, Hoover MD. Exposure pathway assessment at a copper–beryllium alloy facility. Ann Occup Hyg 2007;51:67–80. [DOI] [PubMed] [Google Scholar]
- 18.Day GA, Stefaniak AB, Weston A, Tinkle SS. Beryllium exposure: dermal and immunological considerations. Int Arch Occup Environ Health 2006;79:161–164. [DOI] [PubMed] [Google Scholar]
- 19.Richeldi L, Sorrentino R, Saltini C. HLA-DPB1 glutamate 69: a genetic marker of beryllium disease. Science 1993;262:242–244. [DOI] [PubMed] [Google Scholar]
- 20.Richeldi L, Kreiss K, Mroz MM, Zhen B, Tartoni P, Saltini C. Interaction of genetic and exposure factors in the prevalence of berylliosis. Am J Ind Med 1997;32:337–340. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, White PS, Petrovic M, Tatum OL, Newman LS, Maier LA, Marrone BL. Differential susceptibilities to chronic beryllium disease contributed by different Glu69 HLA-DPB1 and -DPA1 alleles. J Immunol 1999;163:1647–1653. [PubMed] [Google Scholar]
- 22.Saltini C, Richeldi L, Losi M, Amicosante M, Voorter C, van den Berg-Loonen E, Dweik RA, Wiedemann HP, Deubner DC, Tinelli C. Major histocompatibility locus genetic markers of beryllium sensitization and disease. Eur Respir J 2001;18:677–684. [DOI] [PubMed] [Google Scholar]
- 23.Rossman MD, Stubbs J, Lee CW, Argyris E, Magira E, Monos D. Human leukocyte antigen class II amino acid epitopes: susceptibility and progression markers for beryllium hypersensitivity. Am J Respir Crit Care Med 2002;165:788–794. [DOI] [PubMed] [Google Scholar]
- 24.Maier LA, McGrath DS, Sato H, Lympany P, Welsh K, Du Bois R, Silveira L, Fontenot AP, Sawyer RT, Wilcox E, et al. Influence of MHC class II in susceptibility to beryllium sensitization and chronic beryllium disease. J Immunol 2003;171:6910–6918. [DOI] [PubMed] [Google Scholar]
- 25.McCanlies EC, Ensey JS, Schuler CR, Kreiss K, Weston A. The association between HLA-DPB1Glu69 and chronic beryllium disease and beryllium sensitization. Am J Ind Med 2004;46:95–103. [DOI] [PubMed] [Google Scholar]
- 26.Gaede KI, Amicosante M, Schurmann M, Fireman E, Saltini C, Muller-Quernheim J. Function associated transforming growth factor-β gene polymorphism in chronic beryllium disease. J Mol Med 2005;83:397–405. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Farris GM, Newman LS, Shou Y, Maier LA, Smith HN, Marrone BL. Beryllium sensitivity is linked to HLA-DP genotype. Toxicology 2001;165:27–38. [DOI] [PubMed] [Google Scholar]
- 28.Snyder JA, Demchuk E, McCanlies EC, Schuler CR, Kreiss K, Andrew ME, Frye BL, Ensey JS, Stanton ML, Weston A. Impact of negatively charged patches on the surface of MHC class II antigen–presenting proteins on risk of chronic beryllium disease. J R Soc Interface 2008;5:749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder JA, Weston A, Tinkle SS, Demchuk E. Electrostatic potential on human leukocyte antigen: implications for putative mechanism of chronic beryllium disease. Environ Health Perspect 2003;111:1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Dyke M, Martyny J, Mroz PM, Silveira LJ, Strand M, Fingerlin TE, Sato H, Maier L. Exposure and genetics in beryllium sensitization and chronic beryllium disease: a case control study at Rocky Flats in Arvada, CO [abstract]. Am J Respir Crit Care Med 2010;181:A4007. [Google Scholar]
- 31.Van Dyke M, Martyny J, Mroz M, Silveria L, Strand M, Fingerlin T, Sato H, Maier L. Exposure and genetics increase risk of chronic beryllium disease in the nuclear weapons industry. Abstract presented at the American Industrial Hygiene Association Conference/Exhibition (AIHce), May 22–27, 2010, Denver, CO. Available from: http://www.aiha.org/aihce10/docs/10abstractbook.pdf
- 32.Stange AW, Furman FJ, Hilmas DE. The beryllium lymphocyte proliferation test: relevant issues in beryllium health surveillance. Am J Ind Med 2004;46:453–462. [DOI] [PubMed] [Google Scholar]
- 33.Gilchrist FC, Bunce M, Lympany PA, Welsh KI, du Bois RM. Comprehensive HLA-DP typing using polymerase chain reaction with sequence-specific primers and 95 sequence-specific primer mixes. Tissue Antigens 1998;51:51–61. [DOI] [PubMed] [Google Scholar]
- 34.Ruttenber AJ, Schonbeck M, McCrea J, McClure D, Martyny J. Improving estimates of exposures for epidemiologic studies of plutonium workers. Occup Med 2001;16:239–258. [PubMed] [Google Scholar]
- 35.Barnard AE, Torma-Krajewski J, Viet SM. Retrospective beryllium exposure assessment at the Rocky Flats Environmental Technology Site. Am Ind Hyg Assoc J 1996;57:804–808. [DOI] [PubMed] [Google Scholar]
- 36.Viet SM, Torma-Krajewski J, Rogers J. Chronic beryllium disease and beryllium sensitization at Rocky Flats: a case–control study. Am Ind Hyg Assoc J 2000;61:244–254. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JS, Foote K, McClean M, Cogbill G. Beryllium exposure control program at the Cardiff Atomic Weapons Establishment in the UK. Appl Occup Environ Hyg 2001;16:619–630. [DOI] [PubMed] [Google Scholar]
- 38.Madl AK, Unice K, Brown JL, Kolanz ME, Kent MS. Exposure–response analysis for beryllium sensitization and chronic beryllium disease among workers in a beryllium metal machining plant. J Occup Environ Hyg 2007;4:448–466. [DOI] [PubMed] [Google Scholar]
- 39.Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons; 2000.
- 40.Stange AW, Furman FJ, Hilmas DE. Rocky Flats beryllium health surveillance. Environ Health Perspect 1996;104S:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welch L, Ringen K, Bingham E, Dement J, Takaro T, McGowan W, Chen A, Quinn P. Screening for beryllium disease among construction trade workers at Department of Energy nuclear sites. Am J Ind Med 2004;46:207–218. [DOI] [PubMed] [Google Scholar]
- 42.Fontenot AP, Keizer TS, McCleskey M, Mack DG, Meza-Romero R, Huan J, Edwards DM, Chou YK, Vandenbark AA, Scott B, et al.. Recombinant HLA-DP2 binds beryllium and tolerizes beryllium-specific pathogenic CD4+ T cells, J Immunol 2006;177:3874–3883. [DOI] [PubMed] [Google Scholar]
- 43.Amicosante M, Sanarico N, Berretta F, Arroyo J, Lombardi G, Lechler R, Colizzi V, Saltini C. Beryllium binding to HLA-DP molecule carrying the marker of susceptibility to berylliosis glutamate β69. Hum Immunol 2001;62:686–693. [DOI] [PubMed] [Google Scholar]
- 44.Silver K, Sharp RR. Ethical considerations in testing workers for the -Glu69 marker of genetic susceptibility to chronic beryllium disease. J Occup Environ Med 2006;48:434–443. [DOI] [PubMed] [Google Scholar]
- 45.Weston A, Ensey J, Kreiss K, Keshava C, McCanlies E. Racial differences in prevalence of a supratypic HLA-genetic marker immaterial to pre-employment testing for susceptibility to chronic beryllium disease. Am J Ind Med 2002;41:457–465. [DOI] [PubMed] [Google Scholar]
- 46.Mulhausen JR, Damiano J. Quantitative exposure data: interpretation, decision making, and statistical tools: a strategy for assessing and managing occupational exposures, 2nd ed. Fairfax, VA: AIHA Press; 1998. pp. 117–150.
- 47.Cummings KJ, Deubner DC, Day GA, Henneberger PK, Kitt MM, Kent MS, Kreiss K, Schuler CR. Enhanced preventive programme at a beryllium oxide ceramics facility reduces beryllium sensitisation among new workers. Occup Environ Med 2007;64:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henneberger PK, Cumro D, Deubner DD, Kent MS, McCawley M, Kreiss K. Beryllium sensitization and disease among long-term and short-term workers in a beryllium ceramics plant. Int Arch Occup Environ Health 2001;74:167–176. [DOI] [PubMed] [Google Scholar]
- 49.Kreiss K, Mroz MM, Zhen B, Wiedemann H, Barna B. Risks of beryllium disease related to work processes at a metal, alloy, and oxide production plant. Occup Environ Med 1997;54:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.