25-Hydroxycholesterol (25OHC) is the most widely used oxysterol in connection with biochemical experiments designed to evaluate sensitivity of various biological systems against sterols. The levels of 25OHC are very low in the circulation and cells, however, and the physiological importance of this specific oxysterol is still uncertain. The possibility has been discussed that it may have a role in inflammation, and activation of the Toll-like receptors in macrophages induces formation of this oxysterol and its appearance in the circulation (1, 2).
The origin of the 25OHC in the circulation is likely to be mixed. A specific dioxygenase catalyzing conversion of cholesterol into 25OHC has been characterized and has a broad tissue distribution (3). In comparison with mice, the expression of this enzyme is very low in human tissues (3). Under in vitro conditions, both CYP27A1 and CYP46A1 catalyze conversion of cholesterol into 25OHC in parallel with the major products 27-hydroxycholesterol and 24S-hydroxycholesterol, respectively (4, 5). In addition, 25OHC may be formed from cholesterol by autoxidation (6).
According to the investigation by Honda et al. presented in this issue of the Journal of Lipid Research (7), 25OHC in the human circulation may have an additional origin. Cells overexpressing human recombinant CYP3A4 were shown to have a significant 25-hydroxylase activity toward cholesterol. Stimulation of Cyp3a by pregnenolone-16α-carbonitrile caused an accumulation of 25OHC in a cell line derived from mouse liver. In addition, treatment of the cells with troleandomycin, a specific inhibitor of CYP3A/Cyp3a, reduced the 25-hydroxylase activity. Finally, 25OHC levels in human sera correlated positively with levels of 4β-hydroxycholesterol (4OHC). The latter oxysterol is a well-established marker for CYP3A4 activity (8). The combined data is consistent with the possibility that a substantial part of 25OHC, normally present in the circulation, is a product of CYP3A4.
The present interesting finding that CYP3A4 is able to catalyze 25-hydroxylation of cholesterol must be evaluated in relation to the known very broad substrate specificity of this enzyme and the previous demonstrations that it is capable of carrying out hydroxylations in different positions in the same steroid structure. It has previously been shown that CYP3A4 is able to catalyze 25-hydroxylation of vitamin D, 1α-hydroxyvitamin D, and 5β-cholestane-3α,7α,12α-triol (9, 10). It is noteworthy, however, that a previous attempt to demonstrate 25-hydroxylase activity toward cholesterol by recombinant CYP3A4 failed (9). This study was not designed for studies on conversion of endogenous cholesterol into 25OHC, however, and a small conversion of added exogenous radioactive cholesterol that had been diluted with endogenous cholesterol may have been difficult to detect.
The broad substrate specificity of CYP3A4 and the ability of this enzyme to hydroxylate a substrate molecule in several positions is likely to be due to the presence of more than one mode of binding and a unique flexibility of the active site. It has been shown that there is a dramatic conformational change upon binding of some substrates to this enzyme with an increase in the active site volume by 80% (11). In one study on the rate of 24- and 25-hydroxylation of 1α-hydroxyvitamin D, there was a distinct difference in kinetics with 1α-hydroxyvitamin D2 as substrate but not with 1α-hydroxyvitamin D3 as substrate (10). Some residues at the active site of CYP3A4 important for modulation of the 24- and 25-hydroxylase activity were defined. At the present state of knowledge, it is not possible to evaluate if the same residues are of importance in connection with 25-hydroxylation of cholesterol.
A logical consequence of the fact that cholesterol is a substrate for CYP3A4 is that it may function as an inhibitor of the enzyme's activity. In accordance with this, Shinkyo and Guengerich (12) very recently reported that increasing levels of cholesterol in liver microsomes and in reconstituted systems inhibited CYP3A4 catalyzed hydroxylations of nifedipine and quinidine in a noncompetitive manner. A consequence of this is that treatment with statins, which may reduce cholesterol levels in the liver, may lead to less inhibition and a higher activity of CYP3A4. Whereas the in vitro data present in this article are convincing, clearly demonstrating that CYP3A4 has the potential to catalyze 25-hydroxylation of cholesterol, we believe that there is still an uncertainty with respect to the importance of this reaction under in vivo conditions.
The circulating levels of 25OHC reported in the Honda et al. article are considerably higher than those reported in previous recent publications. We have reported plasma levels of 25OHC in a healthy population to range from 2 to 6 ng per mg cholesterol (13). In the investigation by Honda et al., the levels varied from 2 to 28 ng/mg cholesterol (see Fig. 6 in ref. 7). We have reported that the levels of 4OHC vary from 9 to 49 ng/ml plasma in a healthy population (14). When corrected for cholesterol, this corresponds to about 5–25 ng/mg cholesterol. In the investigation by Honda et al., the levels of 4OHC in the healthy population ranged from 10 to 60 ng/mg cholesterol. It is not possible to exclude the possibility that the difference between our results and those by Honda et al. may be due to genetic differences in the populations studied. In Fig. 6 in the publication by Honda et al., however, one gets the impression that there are two populations, one with 25OHC and 4OHC levels within the range we have defined for healthy subjects and one population with considerably higher levels of both 25OHC and 4OHC. There is little or no correlation between the two oxysterols in the population with the lower levels whereas there is a clear correlation in the population with the higher levels. Because both 25OHC and 4OHC are products of autoxidation, there is a possibility that the samples with the higher levels of the two oxysterols may have been products of autoxidation rather than by the action of enzymes. If so, autoxidation may explain the correlation between the two steroids. It should be emphasized that Honda et al. analyzed the oxysterols in serum. Autoxidation occurs more readily in this matrix than in EDTA-plasma, in which there is an effective protection against autoxidation of the endogenous cholesterol. In addition to 4OHC, human serum contains the isomer 4α-hydroxycholesterol. The 4α-isomer is not formed by CYP3A enzymes but is assumed to be formed mainly by cholesterol autoxidation. We have found that autoxidation of serum cholesterol yields a higher ratio between 4α-hydroxycholesterol and 4OHC than normal. The ratio between the two steroids can thus be used as a quality control. Unfortunately, the levels of 4α-hydroxycholesterol were not reported in the study by Honda et al.
If CYP3A4 is responsible for a substantial part of the 25OHC present in human circulation, as suggested by the study by Honda et al., the interesting possibility must be considered that circulating levels of this oxysterol may be used as a marker for the activity of CYP3A4.
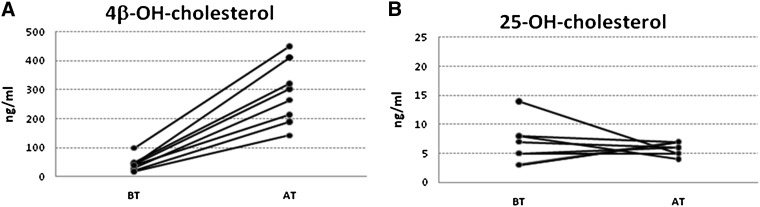
In order to study this possibility, we analyzed the levels of 25OHC in a population of infants before and after treatment with carbamazepine,a potent inducer of CYP3A4. The dramatic effect of this treatment on plasma levels of 4OHC has been published previously (15) and is shown in Fig. 1A. We analyzed levels of 25OHC in the same plasma samples, but failed to demonstrate a stimulatory effect by the treatment (Fig. 1B).
Fig. 1.
Plasma levels of 4β-hydroxycholesterol and 25-hydroxycholesterol in children before (BT) and after (AT) treatment with carbamazepine. The levels of 4β-hydroxycholesterol have been published previously (15).
To summarize, it is evident that CYP3A4 is able to catalyze 25-hydroxylation of cholesterol in parallel with 4β-hydroxylation of the same steroid. The role of this hydroxylation under in vivo conditions is uncertain, however, and according to our small pilot study, it is not possible to use circulating levels of 25OHC as a marker for the activity of CYP3A4.
What then is the origin of the 25OHC present in human circulation? We found that a Swedish patient with the genetic disease cerebrotendinous xanthomatosis and a lack of CYP27 had undetectable plasma levels of 25OHC (Björkhem et al., unpublished observation). Because plasma levels of 25OHC may be absent also in healthy subjects, it is difficult to draw firm conclusions concerning the role of CYP27 in the generation of 25OHC. According to the results presented in Fig. 1B (15), CYP3A4 is not likely to be important, at least not under conditions with an upregulation of CYP3A4. As upregulation of the cholesterol 25-hydroxylase by lipopolysaccharide is paralleled by increased levels of 25OHC in the circulation (1), cholesterol 25-hydroxylase may be responsible for a significant part of the levels in the circulation. At the present state of knowledge it is not possible to exclude autoxidation as an additional significant contributor.
Footnotes
This article is available online at http://www.jlr.org
REFERENCES
- 1.Diczfalusy U., Olofsson KE., Carlsson A-M., Gong M., Golenbock DT., Rooyackers O., Fläring U., Björkbacka H. 2009. Marked up-regulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 50: 2258–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauman DR., Bitmansour AD., McDonald JG., Thompson BM., Liang G., Russell DW. 2009. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. USA. 106: 16764–16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund EG., Kerr TA., Sakai J., Li W-P., Russell DW. 1998. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J. Biol. Chem. 273: 34316–34327. [DOI] [PubMed] [Google Scholar]
- 4.Lund E., Björkhem I., Furster C., Wikvall K. 1993. 24-, 25- and 27-hydroxylation of cholesterol by a purified preparation of 27-hydroxylase from pig liver. Biochim. Biophys. Acta. 1166: 177–182. [DOI] [PubMed] [Google Scholar]
- 5.Lund EG., Guileyardo JM., Russell DW. 1999. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA. 96: 7238–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith LL. 1981. Cholesterol autoxidation. Plenum Press, New York: 1–12. [Google Scholar]
- 7.Honda A., Miyazaki T., Ikegami T., Iwamoto J., Maeda T., Hirayama T., Saito Y., Teramoto T., Matsuzaki Y. 2011. Cholesterol 25-hydroxylation activity of CYP3A4. J. Lipid Res. 52: 1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diczfalusy U., Nylén H., Elander P., Bertilsson L. 2011. 4β-Hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br. J. Clin. Pharmacol. 71: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furster C., Wikvall K. 1999. Identification of CYP3A4 as the major enzyme responsible for 25-hydroxylation of 5β-cholestane-3α,7α,12α-triol in human liver microsomes. Biochim. Biophys. Acta. 1437: 46–52. [DOI] [PubMed] [Google Scholar]
- 10.Gupta RP., He YA., Patrick KS., Halpert JR., Bell NH. 2005. CYP3A4 is a vitamin D-24- and 25-hydroxylase: analysis of structure function by site-directed mutagenesis. J. Clin. Endocrinol. Metab. 90: 1210–1219. [DOI] [PubMed] [Google Scholar]
- 11.Ekroos M., Sjögren T. 2006. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc. Natl. Acad. Sci. USA. 103: 13682–13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinkyo R., Guengerich FP. 2011. Inhibition of human cytochrome P450 3A4 by cholesterol. J. Biol. Chem. 286: 18426–18433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babiker A., Diczfalusy U. 1998. Transport of side-chain oxidized oxysterols in the human circulation. Biochim. Biophys. Acta. 1392: 333–339. [DOI] [PubMed] [Google Scholar]
- 14.Bodin K., Bretillon L., Aden Y., Bertilsson L., Broomé U., Einarsson C., Diczfalusy U. 2001. Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterol in humans. Evidence for involvement of cytochrome P450 3A4. J. Biol. Chem. 276: 38685–38689. [DOI] [PubMed] [Google Scholar]
- 15.Wide K., Larsson H., Bertilsson L., Diczfalusy U. 2008. Time course of the increase in 4β-hydroxycholesterol concentration during carbamazepine treatment of pediatric patients with epilepsy. Br. J. Clin. Pharmacol. 65: 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]