Abstract
The prostaglandin (PG) receptors EP4 and FP have the potential to exert negative effects on adipogenesis, but the exact contribution of endogenous PG-driven receptor signaling to this process is not fully understood. In this study, we employed an adipocyte differentiation system from mouse embryonic fibroblasts (MEF) and compared the effects of each PG receptor-deficiency on adipocyte differentiation. In wild-type (WT) MEF cells, inhibition of endogenous PG synthesis by indomethacin augmented the differentiation, whereas exogenous PGE2, as well as an FP agonist, reversed the effect of indomethacin. In EP4-deficient cells, basal differentiation was upregulated to the levels in indomethacin-treated WT cells, and indomethacin did not further enhance differentiation. Differentiation in FP-deficient cells was equivalent to WT and was still sensitive to indomethacin. PGE2 or indomethacin treatment of WT MEF cells for the first two days was enough to suppress or enhance transcription of the Pparg2 gene as well as the subsequent differentiation, respectively. Differentiation stimuli induced COX-2 gene and protein expression, as well as PGE2 production, in WT MEF cells. These results suggest that PGE2-EP4 signaling suppresses adipocyte differentiation by affecting Pparg2 expression in an autocrine manner and that FP-mediated inhibition is not directly involved in adipocyte differentiation in the MEF system.
Keywords: prostanoid, receptor subtypes, adipogenesis, fat cell, aspirin-like drugs
Adipogenesis is a crucial aspect in controlling body fat mass (1, 2). Acquisition of the mature adipocyte phenotype is a highly regulated process in which mesenchymal stem cells (MSC) undergo differentiation, resulting in both an increase in size and number of mature adipocytes in adipose tissue. Adipose tissue is important not only for energy storage but also as an endocrine organ that regulates energy homeostasis by secreting various adipokines, such as cytokines, chemokines, growth factors, and lipid mediators (3). The presence of receptors for adipokines in preadipocytes and adipocytes has been shown, suggesting that secreted adipokines have autocrine effects and regulate their own differentiation and functions (4). Although it has been shown that a number of factors, including adipokines, regulate adipogenesis in various settings, most of the evidence comes from supraphysiological or pharmacological doses of these molecules to elicit a response. Hence, their physiological significance in local milieu has not been established.
Prostaglandins (PG) are arachidonate metabolites synthesized by the action of cyclooxygenase (COX) as the rate-limiting enzyme. COX has been shown to exist as two isomers, COX-1 and COX-2. PGs exert a wide range of actions through their binding to plasma membrane receptors (5, 6). For instance, PGF2α exerts its actions via specific interactions with the prostanoid FP receptor, which activates phopholipase C, resulting in phophatidylinositol breakdown (7). In contrast, PGE2 exerts action through its interaction with four PGE2 receptor subtypes (EP1, EP2, EP3, and EP4). The EP subtypes differ in their signal transduction pathways: EP1 is coupled to the mobilization of intracellular [Ca2+]; EP2 and EP4 are coupled to the stimulation of adenylyl cyclase and phosphoinositide 3-kinase (PI-3 kinase) (8); and EP3 is mainly coupled to the inhibition of adenylyl cyclase. The diverse actions of PGE2 can be explained by the existence of these multiple EP subtypes with different signal transduction pathways (6, 9). It has been shown that COX products, such as PGE2 and PGF2α, inhibit adipocyte development (10–12). A recent study suggested that COX-2 may be involved in body fat regulation (13). Mice heterozygous for the COX-2 gene showed approximately 30% increased body weight, with 2- to 3-fold larger fat pads compared with those of wild-type animals. PGE2 production in adipose tissue from COX-2 null mice was 20% of that of wild-type mice. These results suggest that COX-2 as well as PGE2 participates in the negative regulation of adipocyte differentiation. Indeed, we previously identified that PGE2-EP4 signaling suppresses adipocyte differentiation from 3T3-L1 preadipocytes (14, 15). In contrast, PGF2α has also been shown to suppress adipocyte differentiation from 3T3-L1 preadipocytes via the FP receptor (11). Thus, both PGF2α and PGE2 have the potential to suppress adipogenesis through FP and EP4, respectively. However, it has not been fully examined whether PGF2α and/or PGE2 are produced in preadipocytes as a kind of adipokine and control adipocyte differentiation in an autocrine manner.
As a first step to elucidate the physiological roles of EP4- and FP-mediated regulation of adipocyte differentiation and maturation, we employed an adipocyte differentiation system from mouse embryonic fibroblasts (MEF) and compared the effects of each receptor-deficiency on adipocyte differentiation.
MATERIALS AND METHODS
Mice
Specific-pathogen-free C57BL/6 mice were obtained from Japan SLC (Hamamatsu, Japan). Mice were maintained on a 12-h light, 12-h dark cycle under specific-pathogen-free conditions. Ptger4−/− and their control wild-type (WT) mice with a mixed background of 129SV and C57BL/6 were littermates of offspring from heterozygote crosses (16). Ptgfr−/− mice with a genetic background of C57BL/6 were generated as described (17, 18), and C57BL/6 mice were used as WT controls. All experimental procedures were approved by the Committee of Animal Research of Kyoto University Faculty of Pharmaceutical Sciences and Kumamoto University.
Reagents
PGE2, fluprostenol, SC560, and NS398 were purchased from Cayman Chemical (Ann Arbor, MI). The EP-specific agonists ONO-DI-004 (EP1), ONO-AE1-259 (EP2), ONO-AE-248 (EP3), and ONO-AE1-329 (EP4) and the EP-specific antagonists ONO-8713 (EP1), ONO-AE3-240 (EP3), and ONO-AE3-208 (EP4) were generous gifts from Ono Pharmaceutical Co. (Osaka, Japan). Indomethacin was purchased from Sigma (St. Louis, MO). Mouse polyclonal anti-COX-1 antibody and mouse polyclonal anti-COX-2 antibody were purchased from Cayman Chemical. Mouse monoclonal anti-actin antibody was purchased from Chemicon (Temecula, CA). Intracellular cyclic AMP was measured using a radioimmunoassay kit (Yamasa, Choshi, Japan), and PGE2 was quantified using an enzyme immunoassay kit (Cayman Chemical).
MEF cell culture, adipocyte differentiation, and triglyceride content measurement
Mouse embryos at embryonic day 14.5 were harvested from WT, Ptger4−/−, and Ptgfr−/− mice. Embryos were minced, filtrated through a 95 µm nylon mesh, and washed. Then MEF cells were prepared. MEF cells were grown to confluency (2 × 106 cells per 60 mm dish) in Dulbecco's modified Eagle's medium (DMEM) high glucose supplemented with 10% calf serum. Differentiation was initiated by culturing the cells in differentiation-inducing cocktail (DIC) containing 10% fetal bovine serum (FBS), 0.5 mM isobutylmethylxanthine (IBMX), 0.25 µM dexamethasone, and 0.2 µM insulin. After two days, the culture medium was changed to adipocyte growth medium containing 10% FBS and 0.2 µM insulin and changed every two days for an additional six days. MEF cells grown in a 60 mm dish were harvested in 1 ml of 2-propanol and sonicated. Triglyceride levels in the cell lysate were measured using the Triglyceride E test kit according to the manufacturer's instructions (Wako, Tokyo, Japan). MEF cells were fixed with 4% paraformaldehyde and incubated in Oil Red O solution (0.05 g of Oil Red O, 6 ml of isopropanol, and 4 ml of water). The number of oil droplet-positive cells was counted.
RNA isolation and real time RT-PCR
Total RNA was isolated from MEF cells on the indicated days of the differentiation program with the RNeasy mini kit (QIAGEN, Venlo, Netherlands), subjected to the RT reaction with a Superscript II First-strand Synthesis Kit, and subjected to real time PCR with a LightCycler (Roche Applied Science, Penzberg, Germany) using Fast Start DNA Master SYBR Green I as reported previously (19). Crossing point values were acquired by using the second derivative maximum method. The expression level of each gene was quantified using external standardized dilutions. Relative expression levels of target genes between samples were normalized by those of β-actin (Actb). Primer sequences for each gene are shown in Table 1. The specificity of each primer set was confirmed by checking the product size by gel electrophoresis and it melting temperature. RT-PCR for the detection of mRNAs for mouse EP3 isoforms (α, β, and γ) was performed as reported previously (20, 21).
TABLE 1.
Primer sequences used for real-time RT-PCR
| Gene | Forward | Reverse |
| Ptger1 | 5′-cgtcgctctcgacgattccgaaagaccgca-3′ | 5′-cgatggccaacaccaccaacaccagcaggg -3′ |
| Ptger2 | 5′-ttcatattcaagaaaccagaccctggtggc-3′ | 5′-agggaagaggtttcatccatgtaggcaaag -3′ |
| Ptger3 | 5′-atcctcgtgtacctgtcacagcgacgctgg -3′ | 5′-tgctcaaccgacatctgattgaagatcatt-3′ |
| Ptger3 Isoform α/β | 5′-tctggtggtgacctttgcctgcaacctggc-3′ | 5′-ctgaggctggagatatttctgcactgagtc-3′ |
| Ptger3 Isoform γ | 5′-tctggtggtgacctttgcctgcaacctggc-3′ | 5′-tcagtccataagggttagggacattggctg-3′ |
| Ptger4 | 5′-ttccgctcgtggtgcgagtgttc -3′ | 5′-gaggtggtgtctgcttgggtacg -3′ |
| Ptgdr | 5′-aaaggaactgctgcctgcctcaggcaatca -3′ | 5′-gttctcaagtttaaaggctccatagtacgc -3′ |
| Ptgfr | 5′-gcatagctgtctttgtatatgcttgtgata-3′ | 5′-gtgtcgtttcacaggtcactggggaattat-3′ |
| Ptgs1 | 5′-tgcatgtggctgtggatgtcatcaa-3′ | 5′-cactaagacagacccgtcatctcca-3′ |
| Ptgs2 | 5′- agtgtgcgacatactca-3′ | 5′-gcgtttgcggtactca-3′ |
| Pparg | 5′- tctccagcatttctgctccacactatgaag-3′ | 5′- cggcagttaagatcacacctatcataaata-3′ |
| Fasn | 5′- ggcttctaaccgcaaaagt-3′ | 5′- gtctcgttgcgtttgtagt-3′ |
| Lipe | 5′- ctatggattacccaagcgg-3′ | 5′- agtgttcgttcctcgg-3′ |
| Actb | 5′- cctgtatgcctctggtcgta-3′ | 5′-ccatctcctgctcgaagtct-3′ |
Measurement of PGE2 production, cAMP formation, and Ca2+ mobilization
PGE2 levels were measured using the prostaglandin E2 EIA kit according to the manufacturer's instructions (Cayman Chemical). Cyclic AMP levels in MEF cells were determined as reported previously (14). Briefly, the MEF cells were washed with HEPES-buffered saline containing 140 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, 11 mM glucose, and 15 mM HEPES, pH7.4, and preincubated for 10 min. Reactions were started by the addition of test reagents along with 100 µM Ro-20-1724. After incubation for 10 min or 1 h at 37°C, reactions were terminated by the addition of 10% trichloroacetic acid. The content of cAMP in the cells was measured by radioimmunoassay with a cAMP assay system. Ca2+ mobilization was analyzed by FlexStation (Molecular Devices, Sunnyvale, CA) as follows: MEF cells were loaded with 4 µM Fura-2/AM, and then fluorescence was measured by illuminating the cells with alternating 340/380 nm light every 3 s, and fluorescence intensity was measured at 510 nm. Changes in intracellular Ca2+ concentration were presented as the change in the ratio of fluorescence intensity for excitation at 340 and 380 nm.
Immunoblot analysis
MEF cells grown in a 100 mm dish were harvested at the indicated h of the differentiation program in SDS sample buffer and sonicated. Aliquots (30 µg protein) were then subjected to polyacrylamide gel electrophoresis (10%), and the separated proteins were transferred to a PVDF membrane. The membrane was incubated with anti-COX-1 (1:1000), anti-COX-2 (1:1000), or anti-actin (1:3000) antibody, and then bands were visualized with the ECL reagent (GE Healthcare, Little Chalfont, UK).
Statistical analysis
Experiments were independently repeated three times, and their mean value ± SEM are shown. Comparison of two groups was analyzed by Student's t-test. For comparison of more than two groups with comparable variances, one-way ANOVA was performed first. Then, either the Dunnet's or Tukey's test was used to evaluate the pairwise group difference. P < 0.05 was considered to indicate a significant difference.
RESULTS
Indomethacin augments adipocyte differentiation of MEF cells
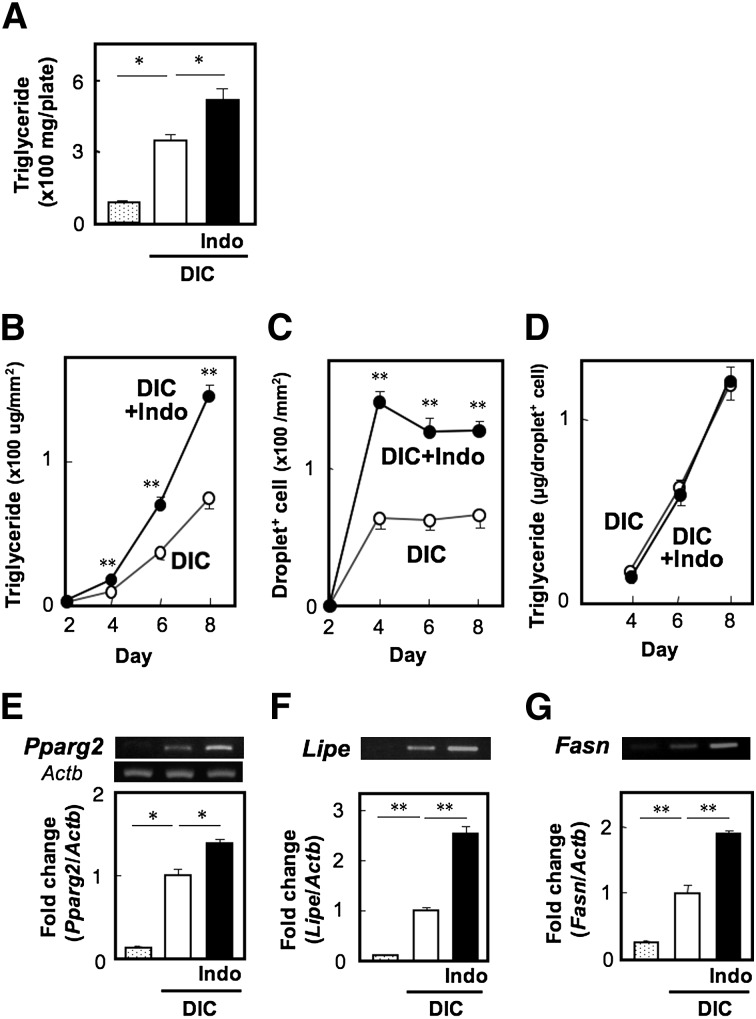
MEF cells were primed with differentiation-inducing cocktail containing insulin, dexamethasone, and IBMX for two days followed by treatment with insulin for an additional six days. Their differentiation into adipocytes was monitored by Oil Red O staining, and their triglyceride (TG) content was measured as an index of differentiation. Indeed, the differentiated cells contained 336.9 ± 15.7 mg TG/plate (2.0 × 106 cells per plate), but the MEF cells cultured in the absence of the differentiation cocktail exhibited only 87.4 ± 6.1 mg TG per plate. When the differentiation program was performed in the presence of 10 µM of indomethacin, an inhibitor of COX, the TG content in the cells was increased to approximately 1.5- to 2.0-fold of the control level (Fig. 1A). To examine whether indomethacin affects the number of differentiated cells and/or the TG content per differentiated cell, we assessed the time-dependent changes in TG content and the number of cells containing fat droplets (droplet+ cells) during the differentiation program (Fig. 1B–D). In both cell groups, TG was undetected on day 2, slight but significant levels of TG were detected on day 4, and then levels drastically increased on days 6 and 8. However, at every time point, the TG levels in indomethacin-treated cells were significantly higher by 2-fold than in control cells (Fig. 1B). In both groups, the oil droplets became visible on day 4, but the number of droplet+ cells were constant until day 8 (Fig. 1C). Interestingly, indomethacin increased the droplet+ cell number by approximately 2-fold. Indeed, the TG levels per droplet+ cell were indistinguishable between the two groups (Fig. 1D). These results suggest that indomethacin promotes adipocyte differentiation but not maturation. When we examined gene expression of PPARγ, a transcription factor playing a central role in adipocyte differentiation, its induction was observed upon DIC treatment, and such gene expression was augmented by indomethacin (Fig. 1E). Indomethacin accelerated the induction of lipogenic enzyme genes, such as fatty acid synthase Fasn (Fig. 1G), and lipolytic enzyme genes, such as hormone-sensitive lipase Lipe (Fig. 1F). These results suggest that PG endogenously synthesized by MEF cells suppresses adipocyte differentiation.
Fig. 1.
Indomethacin facilitates adipocyte differentiation of MEF cells. MEF cells grown to confluency (∼2 × 106 cells per plate) were treated with a standard DIC in the presence or absence of indomethacin (10 µM, Indo). On day 8 (A, E–G) or the indicated days of the differentiation program (B), the TG content of the cells (A, B) or RNA expression level of Pparg2 (E), Lipe (F), and Fasn (G) was measured as described in the Materials and Methods. On the indicated days of the differentiation program, cells were stained with Oil Red O and visualized by bright-field light microscopy. The number of droplet-positive (droplet+) cells was counted (C), and the average TG content per droplet+ cell was calculated (D). Values represent the means ± SEM of three independent experiments (n = 3). *P < 0.05, **P < 0.01.
Expression of PG receptors in MEF cells
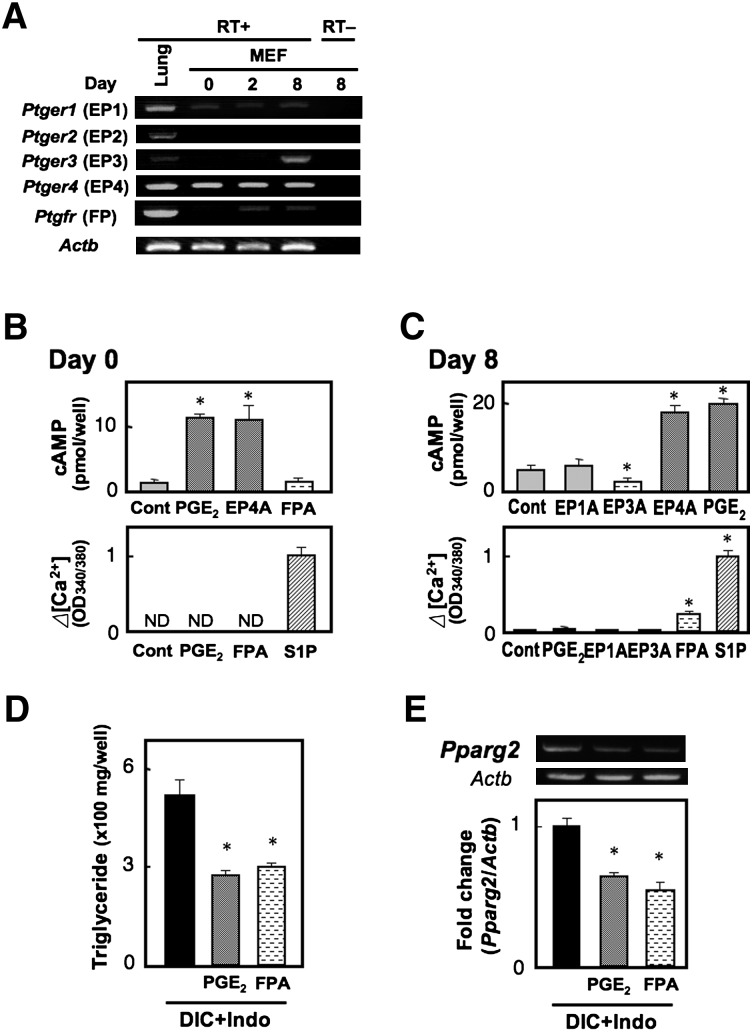
We next examined the mRNA expression of PG receptors in MEF cells during the differentiation program (Fig. 2A). EP1 and EP4 mRNAs were expressed during the differentiation period. In addition, among the PGE receptor subtypes, significant expression of EP4 mRNA was detected throughout the differentiation process. We failed to detect a significant amount of EP2 receptor mRNA in these cells. Expression of FP and EP3 mRNA was undetectable in the untreated cells, but FP mRNA could be detected in the cells on days 2 and 8, and EP3 mRNA could be detected in cells on day 8. As we previously identified the existence of multiple EP3 isoforms in mouse, namely EP3α, EP3β, and EP3γ, which differ in their selectivity of G-protein coupling (20, 21), we investigated which EP3 isoforms are induced upon adipocyte differentiation by competitive RT-PCR (supplementary Fig. I). As a result, all three EP3 isoforms were equally detected on day 8 but not on day 0. To confirm the expression of functional PG receptors, we investigated whether each receptor agonist could induce signal transduction in undifferentiated (Fig. 2B) and differentiated cells (Fig. 2C). In undifferentiated MEF cells on day 0, PGE2 as well as an EP4 agonist (10−7 M each) induced cAMP accumulation to a similar extent, but EP1, EP2, and EP3 agonists failed to do so. These results indicate that the EP4 receptor is the dominant Gs-coupled PGE receptor in undifferentiated MEF cells. In contrast, PGE2 and all of the EP-specific agonists failed to induce intracellular Ca2+ mobilization. Since RT-PCR analysis showed a faint band for EP1 gene expression in MEF cells, EP1 may be expressed only in a very small population of undifferentiated MEF cells. An FP agonist also failed to stimulate intracellular Ca2+ mobilization, indicating that the FP receptor is not expressed in undifferentiated MEF cells. In differentiated cells on day 8, PGE2 and an EP4 agonist again induced cAMP accumulation, but EP1 and EP3 agonists failed to do so. Although we detected mRNAs for EP3γ, an isoform coupled to Gs, as well as EP3α and EP3β in the cells on day 8, an EP3 agonist decreased basal cAMP levels. The EP3 receptor appears to be mainly coupled to inhibition of adenylyl cyclase in differentiated adipocytes. On the other hand, an FP agonist induced intracellular Ca2+ mobilization, but PGE2 and all of the EP-specific agonists failed to do so. These results suggest that at least functional FP, EP3, and EP4 receptors are expressed in differentiated adipocytes.
Fig. 2.
Prostanoid EP4 and FP receptors have the potential to suppress adipocyte differentiation of MEF cells. A: Gene expression of PGE and PGF receptors in MEF cells. MEF cells grown to confluency were treated with DIC, and total RNA was extracted from untreated cells (day 0), cells on day 2, or cells on day 8. Total RNA was subjected to the reverse transcription reaction in the presence (RT+) or absence of reverse transcriptase (RT−) and subsequent PCR analysis. Mouse lung RNA was used as a positive control. B, C: Undifferentiated (B, day 0) or differentiated MEF cells (C, day 8) were subjected to the cAMP (top) and Ca2+ assay (bottom). PGE2 (0.1 µM), an EP1 agonist (0.1 µM, EP1A), an EP3 agonist (0.1 µM, EP3A), an EP4 agonist (0.1 µM, EP4A), and an FP agonist (0.1 µM, FPA) were used. In the Ca2+ assay, sphingosine 1-phosphate (10 µM, S1P) was used as a positive control. D, E: MEF cells were treated with DIC supplemented with vehicle, PGE2 (1 µM), or an FP agonist (1 µM, FPA) in the presence of indomethacin (+Indo). Triglyceride content of the cells was measured on day 8 (D), and total RNA was extracted on day 8 and subjected to real time RT-PCR analysis (E). The Pparg2 gene expression levels were normalized to the β-actin (Actb) mRNA levels. Values represent the means ± SEM of three independent experiments (n = 3). *P < 0.05.
Both EP4 and FP agonists suppress adipocyte differentiation in MEF cells
We next examined the effect of exogenously added PGs on adipocyte differentiation. PGE2, as well as an FP agonist fluprostenol (1 µM each), significantly reduced the indomethacin-augmented TG content to the levels of the control group (Fig. 2D). In contrast, both EP1 and EP3 agonists failed to affect TG content (data not shown). Similar results were obtained regarding Pparg2 gene expression (Fig. 2E). Thus, both EP4 and FP receptors have the potential to suppress differentiation. As indomethacin treatment facilitates differentiation, either EP4 and/or FP signaling may endogenously suppress adipocyte differentiation in MEF cells.
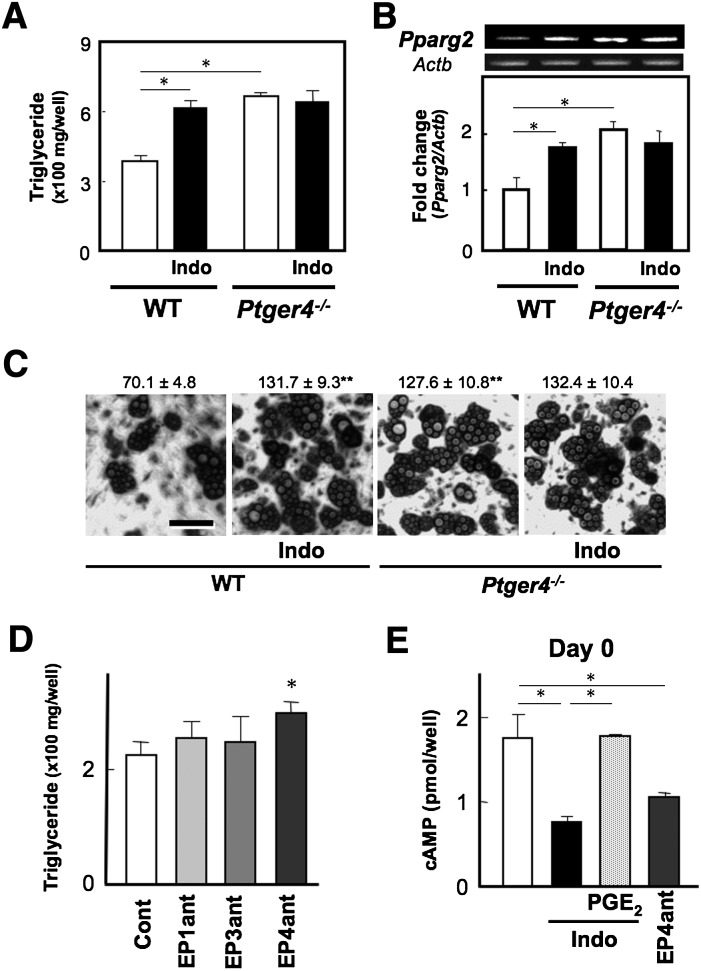
EP4-deficiency but not FP-deficiency mimics the enhancing effect of indomethacin on adipocyte differentiation
To examine which receptor signaling suppresses adipocyte differentiation in MEF cells, we prepared MEF cells isolated from Ptger4−/− and Ptgfr−/− mice, and then examined the outcome of DIC-induced adipocyte differentiation. Interestingly, EP4-deficient cells revealed higher levels of TG content on day 8 than WT cells, and such levels were equivalent to those of indomethacin-treated WT cells (Fig. 3A). Similar results were obtained regarding Pparg2 gene expression levels in the cells on day 8 (Fig. 3B). Moreover, the number of droplet+ cells in EP4-deficient cells was higher than in WT cells and similar to that of indomethacin-treated WT cells (Fig. 3C). Indeed, EP4 deficiency increased the total expression levels of lipolytic (Lipe) and lipogenic (Fasn) genes and did not affect TG content per cell as observed in indomethacin-treated WT cells (data not shown). Indomethacin did not further augment the total TG content, droplet+ number, or Pparg2 expression levels in EP4-deficient cells (Fig. 3A–C). Moreover, an EP4 antagonist (1 µM), but not EP1 or EP3 antagonists, mimicked the enhancing effect of indomethacin on differentiation (Fig. 3D). When we measured cAMP content in the cells on day 0, indomethacin as well as an EP4 antagonist attenuated cAMP levels, and PGE2 reversed the indomethacin-suppressed cAMP levels (Fig. 3E). These results suggest that endogenous PGE2-EP4 signaling suppresses adipocyte differentiation via the cAMP pathway in WT cells. On the other hand, Ptgfr−/− cells on day 8 showed TG levels similar to those of WT cells, and indomethacin still increased the TG levels as observed in WT cells (supplementary Fig. IIA). FP gene deficiency essentially did not affect the Pparg2 gene expression levels in MEF cells (supplementary Fig. IIB). These results indicate that PGF2α-FP signaling is not involved in the suppression of adipocyte differentiation, although FP signaling has the potential to be involved. Thus, endogenous PGE2-EP4 signaling appears to suppress adipocyte differentiation in MEF cells.
Fig. 3.
Endogenous PGE2-EP4 signaling suppresses adipocyte differentiation in MEF cells. MEF cells from WT and Ptger4−/− mice (A–C) grown to confluency were treated with DIC in the presence or absence of indomethacin (10 µM, Indo). Triglyceride content of the cells was measured on day 8 (A), and total RNA was extracted on day 8 and subjected to real time RT-PCR analysis (B). The Pparg2 gene expression levels were normalized to the β-actin (Actb) mRNA levels. C: WT and Ptger4−/− cells on day 8 were stained with Oil Red O and visualized by bright-field light microscopy. The number of droplet-positive cells is shown at the top. D: MEF cells grown to confluency were treated with DIC in the presence of an EP1, EP3, or EP4 antagonist (1 µM each, EP1ant, EP3ant, or EP4ant). Triglyceride content of the cells was measured on day 8. E: MEF cells were treated with vehicle, an EP4 antagonist (1 µM, EP4ant), or indomethacin (10 µM, Indo) in the presence or absence of PGE2 (1 µM) for 1 h, and then subjected to the cAMP assay. Values represent the means ± SEM of three independent experiments (n = 3). *P < 0.05, **P < 0.01.
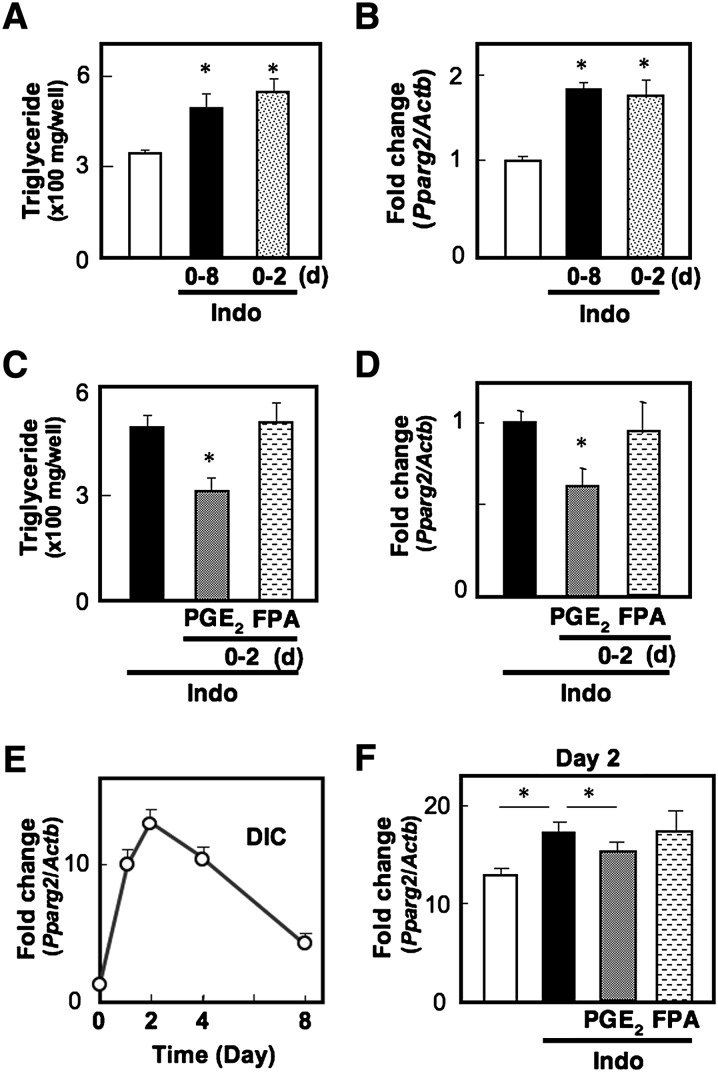
Endogenous PGE2-EP4 signaling suppresses transcription of the Pparg2 gene on day 2 of adipocyte differentiation
It has been considered that the destiny of each cell is determined during the first two days of the adipocyte differentiation program as a result of chronic exposure to DIC and that, thereafter, the committed cells gradually start to fulfill their function as adipocytes, which is adipocyte maturation. If endogenous EP4 signaling suppresses the differentiation stage, treatment of cells with indomethacin only for the first two days may be enough to facilitate the differentiation. As expected, cells treated with indomethacin for the first two days showed TG levels and Pparg2 expression levels as high as those in cells treated with indomethacin for eight days (Fig. 4A, B). We then examined whether exposure of cells to exogenous PGs during the differentiation stage could reverse the effect of indomethacin. PGE2 treatment for the first two days significantly suppressed the levels of TG content and Pparg2 expression, but an FP agonist failed to alter these levels (Fig. 4C, D). These results indicate that endogenous PGE2-EP4 signaling suppresses the differentiation stage of adipogenesis. We next investigated the time course of induction of the Pparg2 gene, which is a prerequisite for the commitment of individual cells to adipocyte differentiation in the MEF system (Fig. 4E). Pparg2 expression was drastically induced by DIC treatment, reaching a peak level on day 2 until the DIC was removed, and then expression gradually decreased until day 8. If suppressive PG signaling dominates the fate of differentiation during the first two days, indomethacin may alter the peak level of Pparg2 gene expression on day 2. As expected, indomethacin significantly augmented Pparg2 gene expression on day 2. Moreover, PGE2, but not an FP agonist, reversed the enhancing effect of indomethacin on Pparg2 transcription (Fig. 4F). These results indicate that endogenous PGE2-EP4 signaling suppresses adipocyte differentiation by attenuating transcription of the Pparg2 gene.
Fig. 4.
PGE2-EP4 signaling suppresses transcription of the Pparg2 gene. A, B: MEF cells grown to confluency were treated with DIC in the presence (Indo) or absence of indomethacin. On day 2, DIC was replaced with media containing insulin in the presence (0-8) or absence of indomethacin (0-2). On day 8, triglyceride content of the cells was measured (A), and Pparg2 gene expression in the cells was measured by real time RT-PCR (B). C, D: MEF cells were treated with DIC containing indomethacin (Indo) supplemented with vehicle, PGE2, or an FP agonist (FPA). On day 2, DIC was replaced with media containing insulin and indomethacin in the absence of PG receptor agonists. On day 8, triglyceride content (C) and Pparg2 gene expression (D) were measured. E: Time course of induction of Pparg2 gene transcripts in MEF cells. Cells were treated with DIC, harvested at the various time points (0 h, 9 h, day 2, day 4, and day 8) of the differentiation program, and then subjected to Pparg2 gene expression analysis. F: MEF cells were treated with DIC containing indomethacin (Indo) supplemented with vehicle, PGE2, or an FP agonist (FPA). On day 2, the cells were harvested and subjected to Pparg2 gene expression analysis. The Pparg2 gene expression levels were normalized to the β-actin (Actb) mRNA levels. Values represent the means ± SEM of three independent experiments (n = 3). *P < 0.05.
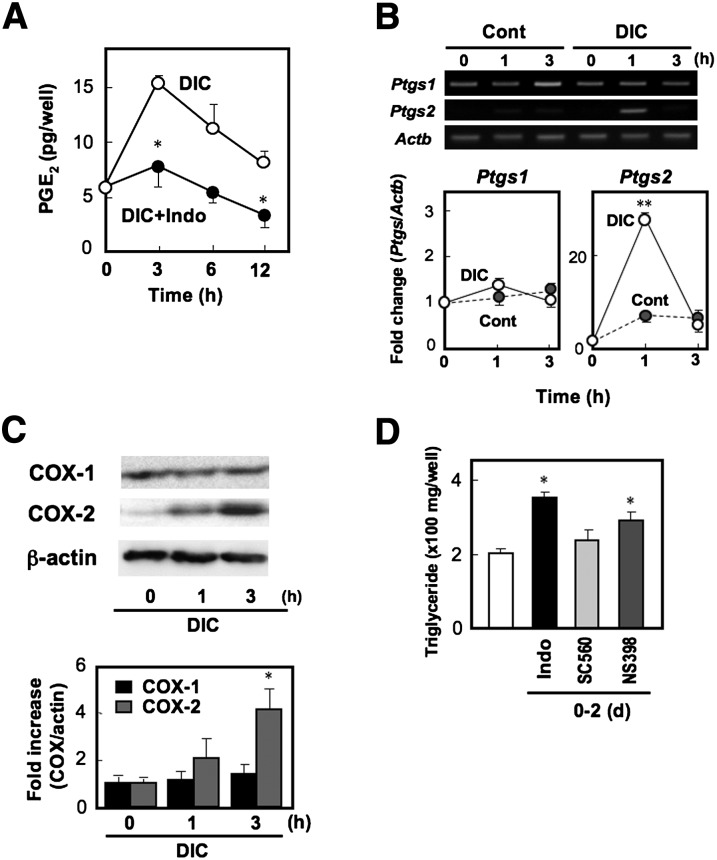
DIC treatment induces COX-2 expression and PGE2 production
The preceding experiments demonstrated that endogenous PGE2-EP4 signaling reduces the peak level of Pparg2 gene expression induced by DIC. Then, is PGE2 really produced by MEF cells in an indomethacin-sensitive manner? To assess this, we examined the time course of PGE2 production and gene expression of COX isozymes in MEF cells just after the addition of DIC. DIC stimulated PGE2 production, which reached a peak at 3 h and then gradually decreased. Such DIC-induced PGE2 production was inhibited by indomethacin (Fig. 5A). In accordance with this, DIC transiently induced Ptgs2 gene expression in MEF cells, reaching a peak at 1 h. In contrast, Ptgs1 gene expression was observed in MEF cells irrespective of DIC treatment (Fig. 5B). Indeed, COX-1 protein expression was detected in MEF cells before DIC treatment, and DIC did not alter its levels. COX-2 protein could barely be detected in MEF cells before DIC treatment, but a faint and significant amount was detected at 1 and 3 h after the addition of DIC (Fig. 5C). Thus, DIC rapidly induces COX-2 and PGE2 production in MEF cells in an indomethacin-sensitive manner. If COX-2-derived PGE2 is involved in the negative regulation of adipocyte differentiation, a selective inhibitor for COX-2 should mimic the facilitating effect of indomethacin. As expected, NS398, a COX-2 selective inhibitor, augmented the levels of TG content, whereas SC560, a COX-1 selective inhibitor, failed to do so (Fig. 5D). These results indicate that, in MEF cells, the differentiation-inducing stimuli induce COX-2 expression and PGE2 production, and the resultant PGE2, via acting on the EP4 receptor, negatively regulates adipocyte differentiation by reducing the peak level of Pparg2 gene induction.
Fig. 5.
COX-2-derived PGE2 suppresses adipocyte differentiation in MEF cells. A: PGE2 production of MEF cells treated with DIC for the indicated times in the presence (+Indo) or absence of indomethacin was measured. B: MEF cells were treated with DIC or control (Cont) medium. Total RNA was isolated at the indicated times and subjected to real time RT-PCR analysis. The COX-1 (Ptgs1) and COX-2 (Ptgs2) mRNA levels were normalized to the β-actin (Actb) mRNA levels. Data are represented as a fold of the value at 0 h. C: Whole cell lysate was prepared at the indicated times and subjected to SDS-PAGE followed by immunoblotting with anti-COX-1, anti-COX-2, or anti-β-actin as a control. The histogram (bottom) shows quantitative representations of COX levels normalized to β-actin levels. D: MEF cells grown to confluency were treated with DIC supplemented with vehicle, indomethacin (Indo), COX-1 selective inhibitor SC560, or COX-2 selective inhibitor NS398 (10 µM each). On day 2, the DIC was replaced with media containing insulin without COX inhibitors. On day 8, triglyceride content was measured. Values represent the means ± SEM of three independent experiments (n = 3). *P < 0.05, **P < 0.01.
DISCUSSION
PGE2-EP4 signaling endogenously suppresses adipocyte differentiation in MEF cells
PGs have long been thought to contribute to fat cell development, but the role of PGs in the regulation of adipocyte differentiation is complex and has remained unclear (12). One of the reasons for its complexity is that different classes of PG exert opposing effects on adipocyte differentiation. For instance, both PGI2 and PGE2, the two PGs predominantly synthesized by fat cells, appear to have opposing effects on early adipogenesis. PGI2 promotes adipocyte differentiation via the prostaglandin I receptor (IP) (22, 23), whereas PGE2 inhibits differentiation via the EP4 receptor (14, 15). PGF2α also suppresses differentiation via the FP receptor (11, 14). In the current study, we evaluated the contribution of each endogenous receptor signaling by using an adipocyte differentiation system from MEF cells, where pharmacological actions on the PG receptor EP4 and FP signaling were reproduced as reported previously: exogenously added PGE2 and an FP agonist suppressed adipocyte differentiation (Fig. 2D, E). Inhibition of endogenous PG synthesis by indomethacin increased the number of TG-producing cells and transcription of the Pparg2 gene (Fig. 1C, E), suggesting that suppressing PG signaling (EP4 or FP) dominates the fate of differentiation. EP4 deficiency mimicked the effect of indomethacin, and indomethacin no longer accelerated differentiation (Fig. 3A–C). On the other hand, FP deficiency failed to affect differentiation, but indomethacin was still effective (supplementary Fig. II). PGE2 treatment for two days was enough to suppress differentiation ( 4C, D). Indeed, indomethacin increased and PGE2 suppressed the peak level of Pparg2 gene transcription on day 2, which is critical for the commitment of individual cells to differentiation (Fig. 4F). These results indicate that PGE2-EP4 signaling suppresses transcription of the Pparg2 gene and, thus, the adipocyte differentiation of MEF cells (supplementary Fig. III). PGF2α-FP signaling appears to have the potential to affect adipocyte differentiation, but FP signaling is not involved in the differentiation system of MEF cells (supplementary Fig. II). Considering that treatment of MEF cells with an FP agonist for the first two days fails to alter both TG content and Pparg2 gene expression (Fig. 3C, D), PGF2α-FP signaling may work as a compensatory negative regulator of the maturation stage of adipocyte differentiation (supplementary Fig. III).
Differentiation stimuli-induced COX-2 gene expression and PGE2 production in MEF cells
The current study demonstrates that COX-2 is responsible for PGE2-elicited suppression of adipocyte differentiation in MEF cells. There have been a number of reports regarding the contribution of COX isozymes to the regulation of adipocyte differentiation (24–26). Yan et al. reported that both COX-1- and COX-2-inhibitors enhanced differentiation of 3T3-L1 cells, indicating that both COX isozymes participate in the negative regulation of adipogenesis (25). Interestingly, Yan et al. also demonstrated that COX-2 inhibitors, but not a COX-1 inhibitor, reversed TNF-α-induced inhibition of differentiation. A similar modulating effect of COX-2 has been shown in adiponectin-elicited inhibition of adipocyte differentiation from BMS2 cells (27). Chu et al. recently established COX-2-knocked down 3T3-L1 cell lines; they found that these cell lines show augmented levels of adipocyte differentiation and that this phenotype was reversed by the addition of PGE2 (26). Thus, the COX-2-PGE2-EP4 pathway may work as a conserved negative regulator of adipocyte differentiation in broad types of preadipocytes.
EP3 receptor gene expression is induced upon adipocyte differentiation
One of the interesting findings in this study is differentiation-dependent induction of EP3 gene expression; the expression of three isoforms (α, β and γ) of EP3 mRNA was equally induced when MEF cells were differentiated into mature adipocytes. Similar results were previously reported: mRNAs of the three isoforms of EP3 were expressed exclusively in mature adipocytes isolated from mouse adipose tissue (28). Although the EP3γ isoform may have the potential to activate the Gs/adenylyl cyclase pathway (21), activation of the EP3 receptor resulted in inhibition of cAMP production in adipocyte-differentiated MEF cells (Fig. 2C). Indeed, PGE2-EP3 signaling has been shown to inhibit lipolysis by suppression of cAMP production (29). Since the adipocyte-specific phospholipase A2 (Pla2g16 product) works as a trigger for PGE2 production in mature adipocytes, and since the Pla2g16 gene expression also depends on adipocyte differentiation (30), it is possible that the Pla2g16 and Ptger3 genes share common mechanisms in their regulation of expression during adipocyte differentiation.
PGE2-EP4 signaling may elicit reciprocal actions on adipogenesis and osteogenesis in MSCs
Adipocytes and osteoblasts represent two distinct cell types that develop from a common progenitor cell: bone marrow-derived mesenchymal stem cells (31). The number of osteoblasts in bone marrow is known to decrease during age-related bone loss (osteoporosis), and the number of adipocytes has been found to increase in negative association with that of osteoblasts (32, 33). Importantly, a reciprocal relationship exists between the number of adipocytes and osteoblasts generated from the marrow MSC pool (34, 35). Factors that enhance osteogenic differentiation are suspected to negatively impact adipogenesis. In this respect, PGE2-EP4 signaling may not only suppress an early stage of adipocyte differentiation but may also promote osteoblast formation. Indeed, we demonstrated that exogenous PGE2 suppression, as well as an EP4 agonist, shows bone-forming activity by sensitizing Runx2 (Cbfa1) gene expression both in vivo and in vitro (36). Thus, PGE2-EP4 signaling may play a physiological role in the reciprocal regulation of osteogenesis and adipogenesis within bone marrow. Such a possibility that EP4 signaling plays a role in the commitment of MSCs to adipocytes and osteoblasts is an interesting issue to be examined in the future.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. H. Akiko Popiel for careful reading of the manuscript.
Footnotes
Abbreviations:
- COX
- cyclooxygenase
- DIC
- differentiation-inducing cocktail
- EP
- prostaglandin E receptor
- FP
- prostaglandin F receptor
- IBMX
- isobutylmethylxanthine
- MEF
- mouse embryonic fibroblast
- MSC
- mesenchymal stem cell
- PG
- prostaglandin
- TG
- triglyceride
- WT
- wild-type
This work was supported by grants from the Suzuken Memorial Foundation, Naito Foundation, and Mochida Memorial Foundation for Medical and Pharmaceutical Research; and by Grants-In-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Ministry of Health and Labor of Japan.
The online version of this article (available at http://www.jlr.org)contains supplementary data in the form of three figures.
REFERENCES
- 1.Gregoire F. M., Smas C. M., Sul H. S. 1998. Understanding adipocyte differentiation. Physiol. Rev. 78: 783–809. [DOI] [PubMed] [Google Scholar]
- 2.Rosen E. D., Spiegelman B. M. 2000. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 16: 145–171. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzawa Y. 2006. The metabolic syndrome and adipocytokines. FEBS Lett. 580: 2917–2921. [DOI] [PubMed] [Google Scholar]
- 4.Karastergiou K., Mohamed-Ali V. 2010. The autocrine and paracrine roles of adipokines. Mol. Cell. Endocrinol. 318: 69–78. [DOI] [PubMed] [Google Scholar]
- 5.Coleman R. A., Smith W. L., Narumiya S. 1994. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 46: 205–229. [PubMed] [Google Scholar]
- 6.Narumiya S., Sugimoto Y., Ushikubi F. 1999. Prostanoid receptors; structures, properties and functions. Physiol. Rev. 79: 1193–1226. [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto Y., Hasumoto K., Namba T., Irie A., Katsuyama M., Negishi M., Kakizuka A., Narumiya S., Ichikawa A. 1994. Cloning and expression of a cDNA for mouse PGF receptor. J. Biol. Chem. 269: 1356–1360. [PubMed] [Google Scholar]
- 8.Yao C., Sakata D., Esaki Y., Li Y., Matsuoka T., Kuroiwa K., Sugimoto Y., Narumiya S. 2009. Prostaglandin E2-EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat. Med. 15: 633–640. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto Y., Narumiya S. 2007. Prostaglandin E receptors. J. Biol. Chem. 282: 11613–11617. [DOI] [PubMed] [Google Scholar]
- 10.Curtis-Prior P. B. 1975. Prostaglandins and obesity. Lancet. 1: 897–899. [DOI] [PubMed] [Google Scholar]
- 11.Casimir D. A., Miller C. W., Ntambi J. M. 1996. Preadipocyte differentiation blocked by prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3–L1 cells. Differentiation. 60: 203–210. [DOI] [PubMed] [Google Scholar]
- 12.Kim S., Moustaid-Moussa N. 2000. Secretory, endocrine and autocrine/paracrine function of the adipocyte. J. Nutr. 130: 3110S–3115S. [DOI] [PubMed] [Google Scholar]
- 13.Fain J. N., Ballou L. R., Bahouth S. W. 2001. Obesity is induced in mice heterozygous for cyclooxygenase-2. Prostaglandins Other Lipid Mediat. 65: 199–209. [DOI] [PubMed] [Google Scholar]
- 14.Tsuboi H., Sugimoto Y., Kainoh T., Ichikawa A. 2004. Prostanoid EP4 receptor is involved in suppression of 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 322: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto Y., Tsuboi H., Okuno Y., Tamba S., Tsuchiya S., Tsujimoto G., Ichikawa A. 2004. Microarray evaluation of EP4 receptor mediated prostaglandin E2 suppression of 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 322: 911–917. [DOI] [PubMed] [Google Scholar]
- 16.Segi E., Sugimoto Y., Yamasaki A., Aze Y., Oida H., Nishimura T., Murata T., Matsuoka T., Ushikubi F., Hirose M., et al. 1998. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem. Biophys. Res. Commun. 246: 7–12. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto Y., Yamasaki A., Segi E., Tsuboi K., Aze Y., Nishimura T., Oida H., Yoshida N., Tanaka T., Katsuyama M., et al. 1997. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 277: 681–684. [DOI] [PubMed] [Google Scholar]
- 18.Kabashima K., Saji T., Murata T., Nagamachi M., Matsuoka T., Segi E., Tsuboi K., Sugimoto Y., Kobayashi T., Miyachi Y., et al. 2002. The prostaglandin E receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J. Clin. Invest. 109: 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segi E., Haraguchi K., Sugimoto Y., Tsuji M., Tsunekawa H., Tamba S., Tsuboi K., Tanaka S., Ichikawa A. 2003. Expression of messenger RNA for prostaglandin E receptor subtypes EP4/EP2 and cyclooxygenase isozymes in mouse periovulatory follicles and oviducts during superovulation. Biol. Reprod. 68: 804–811. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto Y., Negishi M., Hayashi Y., Namba T., Honda A., Watabe A., Hirata M., Narumiya S., Ichikawa A. 1993. Two isoforms of EP3 receptor with different C-terminal domains; identical ligand binding properties and different coupling properties with Gi proteins. J. Biol. Chem. 268: 2712–2718. [PubMed] [Google Scholar]
- 21.Irie A., Sugimoto Y., Namba A., Harazono A., Honda A., Watabe A., Negishi M., Narumiya S., Ichikawa A. 1993. Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. Eur. J. Biochem. 217: 313–318. [DOI] [PubMed] [Google Scholar]
- 22.Vassaux G., Gaillard D., Darimont C., Ailhaud G., Negrel R. 1992. Differential response of preadipocytes and adipocytes to prostacyclin and prostaglandin E2: physiological implications. Endocrinology. 131: 2393–2398. [DOI] [PubMed] [Google Scholar]
- 23.Vassaux G., Gaillard D., Ailhaud G., Negrel R. 1992. Prostacyclin is a specific effector of adipose cell differentiation. Its dual role as a cAMP- and Ca2+-elevating agent. J. Biol. Chem. 267: 11092–11097. [PubMed] [Google Scholar]
- 24.Fajas L., Miard S., Briggs M. R., Auwerx J. 2003. Selective cyclo-oxygenase-2 inhibitors impair adipocyte differentiation through inhibition of the clonal expansion phase. J. Lipid Res. 44: 1652–1659. [DOI] [PubMed] [Google Scholar]
- 25.Yan H., Kermouni A., Abdel-Hafez M., Lau D. C. 2003. Role of cyclooxygenases COX-1 and COX-2 in modulating adipogenesis in 3T3-L1 cells. J. Lipid Res. 44: 424–429. [DOI] [PubMed] [Google Scholar]
- 26.Chu X., Nishimura K., Jisaka M., Nagaya T., Shono F., Yokota K. 2010. Up-regulation of adipogenesis in adipocytes expressing stably cyclooxygenase-2 in the antisense direction. Prostaglandins Other Lipid Mediat. 91: 1–9. [DOI] [PubMed] [Google Scholar]
- 27.Yokota T., Meka C. S., Medina K. L., Igarashi H., Comp P. C., Takahashi M., Nishida M., Oritani K., Miyagawa J., Funahashi T., et al. 2002. Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins. J. Clin. Invest. 109: 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Börglum J. D., Pedersen S. B., Ailhaud G., Negrel R., Richelsen B. 1999. Differential expression of prostaglandin receptor mRNAs during adipose cell differentiation. Prostaglandins Other Lipid Mediat. 57: 305–317. [DOI] [PubMed] [Google Scholar]
- 29.Jaworski K., Ahmadian M., Duncan R. E., Sarkadi-Nagy E., Varady K. A., Hellerstein M. K., Lee H. Y., Samuel V. T., Shulman G. I., Kim K. H., et al. 2009. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat. Med. 15: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan R. E., Sarkadi-Nagy E., Jaworski K., Ahmadian M., Sul H. S. 2008. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA). J. Biol. Chem. 283: 25428–25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caplan A. I., Bruder S. P. 2001. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol. Med. 7: 259–264. [DOI] [PubMed] [Google Scholar]
- 32.Gimble J. M., Zvonic S., Floyd Z. E., Kassem M., Nuttall M. E. 2006. Playing with bone and fat. J. Cell. Biochem. 98: 251–266. [DOI] [PubMed] [Google Scholar]
- 33.Muruganandan S., Roman A. A., Sinal C. J. 2009. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 66: 236–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akune T., Ohba S., Kamekura S., Yamaguchi M., Chung U. I., Kubota N., Terauchi Y., Harada Y., Azuma Y., Nakamura K., et al. 2004. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 113: 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen B., Xie Z., Case N., Ma M., Rubin C., Rubin J. 2008. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable β-catenin signal. Endocrinology. 149: 6065–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida K., Oida H., Kobayashi T., Maruyama T., Tanaka M., Katayama T., Yamaguchi K., Segi E., Tsuboyama T., Matsushita M., et al. 2002. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc. Natl. Acad. Sci. USA. 99: 4580–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.