Abstract
To date, many studies have been conducted using 25-hydroxycholesterol, which is a potent regulator of lipid metabolism. However, the origins of this oxysterol have not been entirely elucidated. Cholesterol 25-hydroxylase is one of the enzymes responsible for the metabolism of 25-hydroxycholesterol, but the expression of this enzyme is very low in humans. This oxysterol is also synthesized by sterol 27-hydroxylase (CYP27A1) and cholesterol 24-hydroxylase(CYP46A1), but it is only a minor product of these enzymes. We now report that CYP3A synthesizes a significant amount of 25-hydroxycholesterol and may participate in the regulation of lipid metabolism. Induction of CYP3A by pregnenolone-16α-carbonitrile caused the accumulation of 25-hydroxycholesterol in a cell line derived from mouse liver. Furthermore, treatment of the cells with troleandomycin, a specific inhibitor of CYP3A, significantly reduced cellular 25-hydroxycholesterol concentrations. In cells that overexpressed human recombinant CYP3A4, the activity of cholesterol 25-hydroxylation was found to be higher than that of cholesterol 4β-hydroxylation, a known marker activity of CYP3A4. In addition, 25-hydroxycholesterol concentrations in normal human sera correlated positively with the levels of 4β-hydroxycholesterol (r = 0.650, P < 0.0001, n = 78), but did not significantly correlate with the levels of 27-hydroxycholesterol or 24S-hydroxycholesterol. These results demonstrate the significance of CYP3A on the production of 25-hydroxycholesterol.
Keywords: cerebrotendinous xanthomatosis, cholesterol 25-hydroxylase, CYP3A4, CYP27A1, CYP46A1, 4β-hydroxycholesterol, 25-hydroxycholesterol, oxysterols
Oxysterols are physiological regulators of cellular cholesterol homeostasis (1). They downregulate HMG-CoA reductase (2–4), the rate-limiting enzyme in the cholesterol biosynthetic pathway, by blocking processing of the sterol-regulatory element binding protein (SREBP) by inducing binding of SREBP cleavage-activating protein to a protein called Insig (insulin-induced gene) (5, 6). Furthermore, there is growing evidence that certain oxysterols may accelerate ubiquitination and degradation of HMG-CoA reductase protein (1, 7). On the other hand, oxysterols are endogenous ligands of the nuclear receptor liver X receptor α (LXRα; NR1H3) (8–10), which modulates immune responses and regulates various metabolic pathways, including cholesterol, bile acids, FAs, and glucose (11, 12).
In in vitro experiments, 25-hydroxycholesterol is widely used as a potent inhibitor of HMG-CoA reductase or as a ligand of LXRα, but the origins of this oxysterol are not entirely clear. Enzymatic production of 25-hydroxycholesterol has been reported by microsomal cholesterol 25-hydroxylase (CH25H) (13), and the activation of Toll-like receptors, a class of proteins that play a key role in the innate immune system, markedly induces CH25H and increases 25-hydroxycholesterol concentrations in mice macrophages and sera (14, 15). In comparison with mice, however, expression of CH25H has been reported to be very low in human tissues (13). Other enzymes involved in the production of 25-hydroxycholesterol are mitochondrial sterol 27-hydroxylase (CYP27A1) (16, 17) and brain-specific microsomal cholesterol 24S-hydroxylase (CYP46A1) (18). In addition, nonenzymatic generation of 25-hydroxycholesterol by autoxidation of cholesterol has also been described (19).
Previously, we measured hepatic concentrations of intermediates in bile acid synthesis in Cyp27−/− mice (20). In this series of analyses, we unexpectedly found that microsomal concentrations of 25-hydroxycholesterol were significantly elevated in Cyp27−/− mice (unpublished observation). This might be caused by reduced metabolism of 25-hydroxycholesterol due to inhibition of 27-hydroxylation. However, it was also possible that 25-hydroxylation of cholesterol was stimulated by enzyme upregulation in the Cyp27−/− mice. We speculated that CYP3A was the enzyme that exhibited high cholesterol 25-hydroxylation activity because CYP3A was markedly upregulated in Cyp27−/− mice and this enzyme was known to catalyze a similar reaction, i.e., 25-hydroxylation of 5β-cholestane-3α,7α,12α-triol (21).
The CYP3A subfamily consists of monooxygenases that catalyze many reactions involved in the metabolism of xenobiotics, steroid hormones, and bile acids (22). Cholesterol is also one of the substrates for CYP3A and is believed to be mainly metabolized to 4β-hydroxycholesterol (23, 24). The present study was undertaken to prove that CYP3A catalyzes not only 4β-hydroxylation but also 25-hydroxylation of cholesterol and to show the possibility that 25-hydroxycholesterol in normal human serum originates from CYP3A4.
MATERIALS AND METHODS
Chemicals
Pregnenolone-16α-carbonitrile (PCN) and troleandomycin were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Cholesterol and desmosterol were obtained from Steraloids, Inc. (Newport, RI), and cholesterol was used as substrate for the enzyme assay after purification with disposable silica cartridge columns (25) to remove contaminated oxysterols. Additional reagents and solvents were of analytical grade.
Cell culture
AML12 cells, a differentiated, nontransformed hepatocyte cell line that was derived from transforming growth factor α-overexpressing transgenic mice (26) were purchased from American Type Culture Collection (Manassas, VA). Cells were seeded in 6-well plates and cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium (Invitrogen Japan KK; Tokyo, Japan) supplemented with 0.005 mg/ml insulin, 0.005 mg/ml transferrin, 5 ng/ml selenium, 40 ng/ml dexamethasone, and 10% FBS. When the cells were subconfluent, the medium was replaced with fresh medium containing PCN, troleandomycin, or desmosterol dissolved in 1% ethanol. Although 1% ethanol in the medium had no detectable effects on cell growth, the same concentration of ethanol was also added to the control wells. Cells were incubated at 37°C in a humidified incubator containing 5% CO2 and 95% air.
RNA measurements
Total RNA was extracted from the cells using an AllPrep RNA/protein kit (QIAGEN KK; Tokyo, Japan). Reverse transcription was performed on 1 μg of total RNA using a first-strand cDNA synthesis kit for RT-PCR (Roche Diagnostics; Mannheim, Germany). Real-time quantitative PCR was performed on cDNA aliquots with FastStart DNA Master SYBR Green I and a LightCycler (Roche). The sequences of the oligonucleotide primer pairs used to amplify mouse mRNAs are 5′-GGCAGCATTGATCCTTATG-3′ and 5′-AAGAACTCCTTGAGGGAGAC-3′ for Cyp3a11 (NM_007818), 5′-ACACCTACTTTGAAGACCCAT-3′ and 5′-TGACAACTTTCACCTCCAT-3′ for Cyp46a1 (NM_010010), 5′-CTTCCTGCTGACCAATGAAT-3′ and 5′-AGCTTTTAGCAGAGGCATGT-3′ for Cyp27a1 (NM_024264), 5′-CCAGCTCCTAAGTCACGTC-3′ and 5′-CACGTCGAAGAAGGTCAG-3′ for Ch25h (NM_009890) and 5′-CCTGTATGCCTCTGGTCGTA-3′, and 5′-CCATCTCCTGCTCGAAGTCT-3′ for β-actin (X03672). PCR amplification began with a 10 min preincubation step at 95°C, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 62°C for 10 s, and elongation at 72°C for 16 s. The relative concentration of the PCR product derived from the target gene was calculated using LightCycler System software. A standard curve for each run was constructed by plotting the crossover point against the log concentration. The concentration of target molecules in each sample was then calculated automatically by reference to this curve (r = −1.00), and results were standardized to the expression of β-actin. The specificity of each PCR product was assessed by melting curve analysis.
SDS-PAGE and immunoblot analysis
Cell homogenate was resolved by SDS-PAGE on a 5–20% gradient gel (e-PAGEL; ATTO Corporation, Tokyo, Japan) and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA). Immunoblot analyses of mouse CYP3A, CH25H, and β-actin were conducted with goat polyclonal antibody against mouse CYP3A, goat polyclonal antibodies against human CH25H (Santa Cruz Biotechnology; Santa Cruz, CA), and mouse monoclonal anti-β-actin antibody (Sigma), respectively. The membrane was blocked for 1 h in 5% fat-free milk in TBS-T (Tris-buffered saline/0.1% Tween-20) and incubated with the primary antibody against either CYP3A (1:200 dilution), CH25H (1:200 dilution), or β-actin (1:1,000 dilution) in 5% fat-free milk in TBS-T overnight at 4°C. The blot was washed three times for 10 min in TBS-T and incubated with an HRP-conjugated donkey anti-goat IgG antibody (Santa Cruz Biotechnology) for CYP3A and CH25H or with an HRP-conjugated sheep anti-mouse IgG antibody (Amersham; Buckinghamshire, UK) for β-actin. After washing, the bands were visualized by exposure to film (Hyperfilm ECL; Amersham) with an ECL Western blotting analysis system (Amersham) according to the manufacturer's instructions. The gradient gel was calibrated with prestained molecular-weight markers (Bio-Rad Japan; Tokyo, Japan).
Sample collection from human subjects
Blood samples were collected from 78 healthy adults. After coagulation and centrifugation at 1,500 g for 10 min, serum samples were stored at −20°C until analysis. Informed consent was obtained from all subjects, and the experimental procedures were approved by the Teikyo University Institutional Review Board.
Determination of sterol concentrations
Sterol concentrations in cell homogenate and serum were measured using our previously described HPLC-ESI-MS/MS method (27, 28). In brief, 5 μl aliquots of serum or cell homogenate (approximately 1 × 104 cells) were incubated with stable isotope-labeled oxysterols as internal standards in 1 N ethanolic KOH at 37°C for 1 h. Sterols were extracted with n-hexane, derivatized to picolinyl esters, and analyzed by HPLC-ESI-MS/MS. Conventional derivatization was conducted at 80°C for 60 min, but room temperature for 30 min was chosen for the specific monopicolinyl ester formation of 25-hydroxycholesterol. Monopicolinyl 25-hydroxycholesterol exhibited [M+Na+CH3CN]+ ion as the base peak, and [picolinic acid (C6H5NO2)+Na]+ ion was observed as the most-abundant product ion under various levels of collision energy. Therefore, m/z 571 → 146 (25 V) and m/z 574 → 146 (25 V) were used as the monitoring ions and optimal collision energy for authentic and deuterated 25-hydroxycholesterol monopicolinate, respectively. Essentially, the Hypersil GOLD column (150 mm × 2.1 mm ID, 3 μm; Thermo Fisher Scientific, San Jose, CA) was employed for the HPLC separation of sterols, and the Hypersil GOLD aQ column (150 mm × 2.1 mm ID, 3 μm) was also used to obtain better separation of the stereoisomers (29).
Enzyme assay
Microsomes (baculosomes) prepared from insect cells that were infected with a baculovirus containing the cDNA for rabbit cytochrome P450 reductase and human CYP1A2, CYP2C9, CYP2D6, or CYP3A4 were purchased from Invitrogen. The microsomes (10 pmol of P450) were incubated for 30 min at 37°C with various amounts of cholesterol (dissolved in 12 μl of a 33% aqueous solution of 2-hydroxypropyl-β-cyclodextrin), NADPH (1.2 mM), glucose-6-phosphate (3.6 mM), 2 U glucose-6-phosphate dehydrogenase, and 100 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM EDTA in a total volume of 0.5 ml. The incubation was stopped by the addition of 1 ml ethanol. After the addition of the internal standards and 5 μg butylated hydroxytoluene to the mixture, oxysterols were extracted twice with 2 ml n-hexane, derivatized to picolinyl esters, and analyzed by HPLC-ESI-MS/MS, as described above. To exclude the possible effects of contaminated oxysterols in substrate (cholesterol) and cholesterol autoxidation, incubations without adding NADPH generating system were conducted simultaneously, as a control, and the data were subtracted from those obtained using complete assay mixtures. An assay using boiled CYP3A4 was also conducted to exclude the direct effects of the NADPH generating system on cholesterol oxidation.
Statistics
Data are expressed as the mean ± SD. The statistical significance of differences between the results in the different groups was evaluated using the Student's two-tailed t-test. Correlation was tested by calculating Pearson's correlation coefficient, r. For all analyses, significance was accepted at the level of P < 0.05.
RESULTS
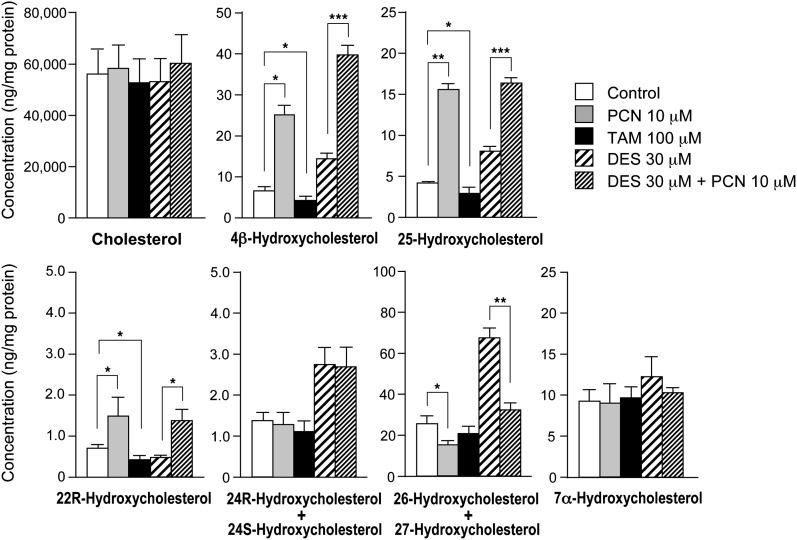
The effects of PCN, troleandomycin, and desmosterol on sterol concentrations in AML12 cells are shown in Fig. 1. The concentrations of 4β-hydroxycholesterol, 25-hydroxycholesterol, and 22R-hydroxycholesterol were significantly increased by treatment with PCN, a classical inducer of CYP3A by the activation of pregnane X receptor (NR1I2) (22). In contrast, these oxysterol concentrations were significantly decreased by treatment with troleandomycin, a specific inhibitor of CYP3A activity (30). Furthermore, the increase of 25-hydroxycholesterol by PCN treatment was not suppressed by the addition of desmosterol, a potent inhibitor of CH25H (13). On the other hand, significant increase by PCN was not observed regarding the other oxysterol concentrations.
Fig. 1.
Effects of PCN, troleandomycin (TAM), and desmosterol (DES) on sterol concentrations in AML12 cells. Cells were incubated with PCN (10 μM), TAM (100 μM), DES (30 μM), or DES (30 μM) plus PCN (10 μM) for 7 days. A Hypersil GOLD column was used for HPLC separation of oxysterols. This column cannot distinguish between 26- and 27-hydroxycholesterol and between 24R- and 24S-hydroxycholesterol. Each column and error bar represents the mean and SD obtained in triplicate assay. ***P < 0.001, **P < 0.01, *P < 0.05.
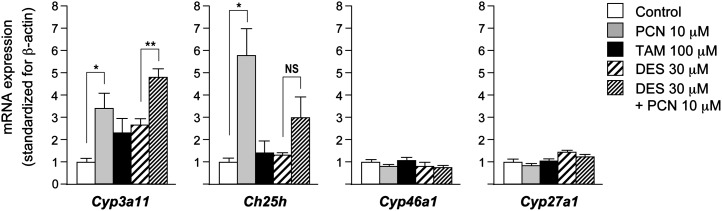
The effects of PCN, troleandomycin, and desmosterol on mRNA expressions of Cyp3a11, Ch25h, Cyp46a1, and Cyp27a1 in AML12 cells are shown in Fig. 2. Treatment with PCN significantly upregulated Cyp3a11 expression. Marked upregulation of Ch25h expression was also observed in the PCN-treated cells, but the absolute mRNA expression of Ch25h in untreated AML12 cells was more than 50 times lower than that of Cyp3a11 (data not shown). Troleandomycin tended to upregulate the mRNA expression of Cyp3a11, but the difference was not statistically significant. The addition of desmosterol to cell culture medium did not affect the induction of Cyp3a11 by PCN. However, desmosterol seemed to inhibit the induction of Ch25h by PCN.
Fig. 2.
Effects of PCN, troleandomycin (TAM), and desmosterol (DES) on relative mRNA expression of Cyp3a11, Ch25h, Cyp46a1, and Cyp27a1 in AML12 cells. Cells were incubated with PCN (10 μM), TAM (100 μM), DES (30 μM), or DES (30 μM) plus PCN (10 μM) for 72 h. Each column and error bar represents the mean and SD obtained in triplicate assay. **P < 0.01, *P < 0.05. NS, not significant.
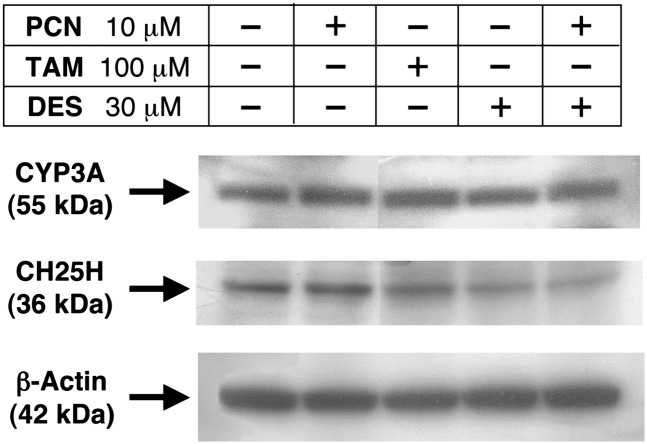
Figure 3 shows the effects of PCN, troleandomycin, and desmosterol on protein levels of CYP3A and CH25H. PCN increased CYP3A protein level, which was associated with the upregulated transcription of Cyp3a11 (Fig. 2). However, although the transcription of Ch25h was also upregulated by the addition of PCN, the protein level of CH25H was not elevated. In addition, desmosterol did not affect the expression of cellular CYP3A protein, but CH25H protein level was obviously decreased by desmosterol treatment.
Fig.
Effects of PCN, troleandomycin (TAM), and desmosterol (DES) on CYP3A and CH25H protein in AML12 cells. Cells were incubated with PCN (10 μM), TAM (100 μM), DES (30 μM), or DES (30 μM) plus PCN (10 μM) for 72 h. Cell homogenates (10 μg protein per lane) were subjected to SDS-PAGE analysis.
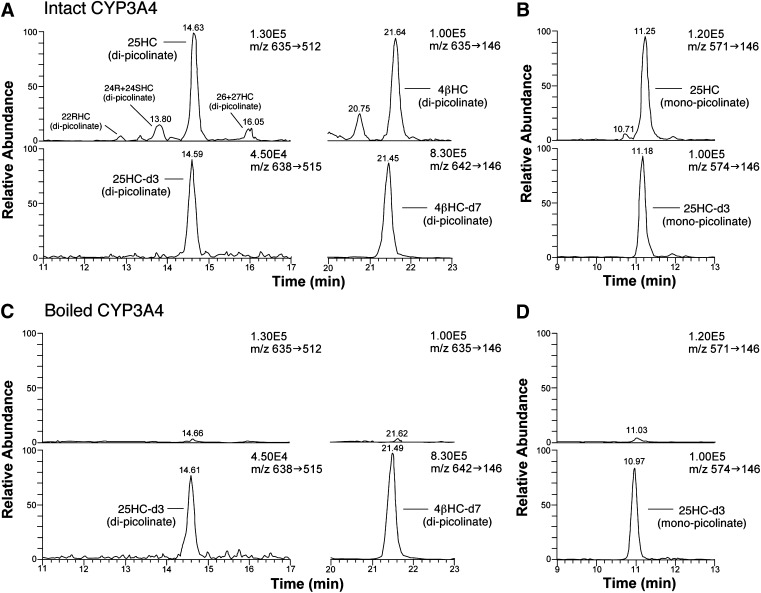
Intact or boiled aliquots of insect cell microsomes overexpressing recombinant human CYP3A4 (10 pmol of P450) were incubated at 37°C for 30 min with 200 μM cholesterol and an NADPH generating system, and the sterol fraction was derivatized to picolinyl esters by two different methods. Figures 4A, C represent selected reaction monitoring (SRM) of samples that were derivatized at 80°C for 60 min. This derivatizing method generally produced di-picolinyl esters of oxysterols, and the SRM data indicated that 25-hydroxycholesterol was a major product of intact CYP3A4, as well as 4β-hydroxycholesterol. We also derivatized the sample at room temperature for 30 min, which produced mono-picolinyl ester of 25-hydroxycholesterol (Fig. 4B, D). The mass spectrum and retention time of mono-picolinyl 25-hydroxycholesterol are completely distinct from those of di-picolinyl 25-hydroxycholesterol. The production of 25-hydroxycholesterol by intact CYP3A4 was confirmed using this specific derivatization technique.
Fig. 4.
SRM chromatograms obtained during HPLC-ESI-MS/MS analysis of the oxysterol fraction from an incubation mixture of overexpressed recombinant human CYP3A4 (A, B) or boiled CYP3A4 (C, D) with 200 μM cholesterol. The oxysterol fraction was derivatized to picolinyl esters by two different methods, 80°C for 60 min (A, C) and room temperature for 30 min (B, D). The former produces di-picolinyl esters of 25-hydroxycholesterol (25HC) and 4β-hydroxycholesterol (4βHC), whereas the latter produces the mono-picolinyl ester of 25HC. 25HC-d3 (1 ng) and 4βHC-d7 (5 ng) were added to each incubated mixture as internal standards. The same Hypersil GOLD column and the same mobile phase were used for HPLC separation of both di- and mono-picolinyl esters of 25HC. The numbers on the right upper side of each chromatogram represent the full scale of the chromatogram.
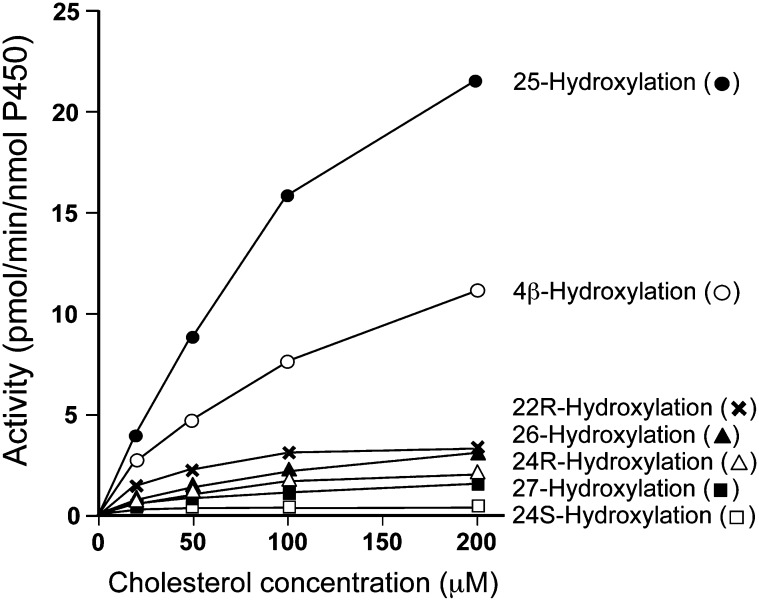
The effects of substrate (cholesterol) concentrations on various hydroxylase activities in recombinant human CYP3A4 are presented in Fig. 5. The most significant activity was 25-hydroxylation, which was higher than that of 4β-hydroxylation, a marker activity of CYP3A4. Other hydroxylation activities, i.e., 22R-, 24R-, 24S-, 26-, and 27-hydroxylation were also observed, but the activities were much lower than that of 4β-hydroxylation. Apparent Vmax and Km were calculated by Lineweaver-Burk plots. Vmax of 25-, 4β-, 22R-, 24R-, 24S-, 26-, and 27-hydroxylation were 7.0 × 10−4, 2.0 × 10−4, 5.7 × 10−5, 5.8 × 10−5, 3.4 × 10−6, 5.3 × 10−5, and 2.3 × 10−5 mol/s/mol P450, respectively, and Km of those hydroxylations were 182, 62, 37, 161, 15, 80, and 45 μM, respectively.
Fig. 5.
Effects of cholesterol (substrate) concentrations on 25-, 4β-, 22R-, 24R-, 24S-, 26-, and 27-hydroxylase activities in overexpressed recombinant human CYP3A4. A Hypersil GOLD aQ column was used for HPLC separation of oxysterols. Data points represent the mean of duplicate determinations.
In Table 1, cholesterol 25- and 4β-hydroxylase activities are compared among four different insect cell microsomes containing recombinant human CYP1A2, CYP2C9, CYP2D6, or CYP3A4. Not only CYP3A4 but also the other three P450 enzymes significantly catalyzed 25-hydroxylation of cholesterol, but these activities were lower than that by CYP3A4. In contrast, 4β-hydroxylation of cholesterol was exclusively observed in microsomes containing CYP3A4. Control microsomes without expressed human P450 enzymes did not convert cholesterol into 25-hydroxycholesterol or 4β-hydroxycholesterol.
TABLE 1.
Cholesterol 25- and 4β-hydroxylation activities in recombinant overexpressed human cytochrome P450 (baculosomesa)
| Baculosomes | P450 concentration | 25-Hydroxylationb | 4β-Hydroxylation | ||
| pmol P450/mg protein | pmol/min/mg protein | pmol/min/nmol P450 | pmol/min/mg protein | pmol/min/nmol P450 | |
| WT controlc | 0 | 0.06 (0.07, 0.05) | — | 0.01 (0.00, 0.01) | — |
| CYP1A2 | 98 | 0.58 (0.54. 0.62) | 5.95 (5.54, 6.35) | 0.12 (0.13, 0.11) | 1.21 (1.30, 1.12) |
| CYP2C9 | 313 | 1.36 (1.50, 1.21) | 4.34 (4.79, 3.89) | 0.25 (0.28, 0.21) | 0.79 (0.90, 0.68) |
| CYP2D6 | 252 | 0.59 (0.64, 0.54) | 2.36 (2.56, 2.15) | 0.14 (0.17, 0.10) | 0.54 (0.69, 0.39) |
| CYP3A4 | 96 | 1.86 (2.07, 1.64) | 19.4 (21.6, 17.1) | 0.99 (1.08, 0.89) | 10.3 (11.2, 9.31) |
WT, wild type.
Microsomes prepared from insect cells that were infected with baculovirus containing the cDNAs for human cytochrome P450 and rabbit cytochrome P450 reductase.
Average of two assays. Individual values in parentheses.
Control microsomes prepared from insect cells that were infected with a wild-type baculovirus.
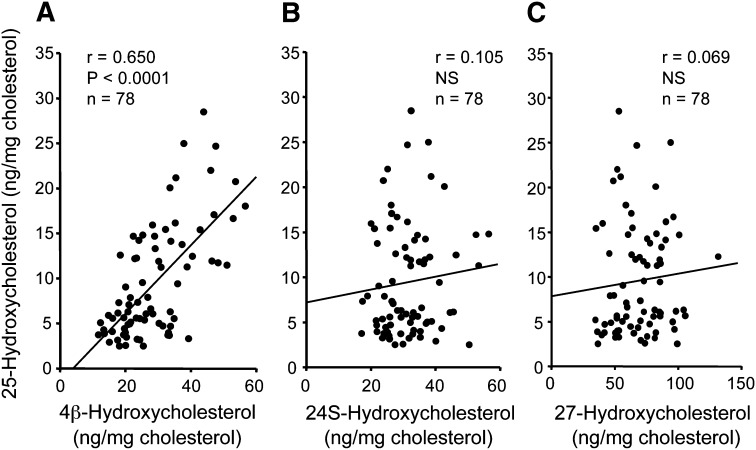
The relationships between serum 25-hydroxycholesterol concentrations and serum 4β-, 24S-, and 27-hydroxycholesterol concentrations in 78 normal Japanese subjects are shown in Fig. 6. Serum 25-hydroxycholesterol concentrations correlated significantly with 4β-hydroxycholesterol concentrations (Fig. 6A), but did not correlate significantly with the concentrations of 24S-hydroxycholesterol (Fig. 6B) or 27-hydroxycholesterol (Fig. 6C). On the other hand, serum 24S-hydroxycholesterol and 27-hydroxycholesterol concentrations correlated significantly (r = 0.408, P < 0.0005, n = 78) in the group of normal subjects.
Fig. 6.
Correlations between serum concentrations of 25-hydroxycholesterol and 4β-hydroxycholesterol (A), 24S-hydroxycholesterol (B), or 27-hydroxycholesterol (C) in 78 normal subjects. NS, not significant.
DISCUSSION
Our results provide strong evidence that 25-hydroxylation of cholesterol is catalyzed by CYP3A. First, CYP3A induction caused the accumulation of 25-hydroxycholesterol in a cell line derived from mouse liver. The addition of desmosterol downregulated CH25H protein in the cells, but did not reduce the concentration of cellular 25-hydroxycholesterol. Second, the presence of significant cholesterol 25-hydroxylation activity was proven by using recombinant human CYP3A4. Third, 25-hydroxycholesterol concentrations in normal human sera correlated positively with the 4β-hydroxycholesterol level; a known marker of CYP3A4 activity (23, 24).
In this study, we paid close attention to identifying 25-hydroxycholesterol by using two different derivatization methods, i.e., 80°C for 60 min and room temperature for 30 min. The former method synthesizes the usual di-picolinyl derivative of 25-hydroxycholesterol, whereas the latter method produces the mono-picolinyl derivative, because the C-25 position of 25-hydroxycholesterol is resistant to picolinyl ester formation at room temperature (28). The identification of 25-hydroxycholesterol by our conventional HPLC-MS/MS method was confirmed using this specific derivatization technique. Furthermore, we quantified 25-hydroxycholesterol with great care because this oxysterol may be a normal contaminant of the substrate (cholesterol) and could be generated by cholesterol autoxidation. Therefore, in the recombinant cytochrome P450 experiments, control assays without adding the NADPH generating system were conducted simultaneously and the data were subtracted from those obtained using the complete assay system.
It was surprising that recombinant CYP3A4 produced much more 25-hydroxycholesterol than 4β-hydroxycholesterol, which is used as a marker of CYP3A4 activity (23, 24). However, serum concentrations of 25-hydroxycholesterol were low compared with those of 4β-hydroxycholesterol (Fig. 6A), which may be explained by the fact that the metabolism of 25-hydroxycholesterol is faster than that of 4β-hydroxycholesterol (31). Whereas 4β-hydroxycholesterol is metabolized slowly by CYP7A1 and CYP27A1 (31), 25-hydroxycholesterol is metabolized faster by CYP7A1 (32) and CYP7B1 (33).
It has been reported that 25-hydroxycholesterol is synthesized not only by CH25H (13) but also by CYP27A1 (16, 17) and CYP46A1 (18). Because only very low levels of CH25H are expressed in normal human tissues (13), the roles of CYP27A1 and CYP46A1 in the formation of 25-hydroxycholesterol may be relatively important in humans. However, our results showed that the serum concentrations of 25-hydroxycholesterol did not correlate with the concentrations of either 27-hydroxycholesterol, a product of CYP27A1, or 24S-hydroxycholesterol, a product of CYP46A1. In contrast, 25-hydroxycholesterol levels were significantly correlated with 4β-hydroxycholesterol concentrations in normal human subjects. The results lend support to the hypothesis that CYP3A4 synthesizes 25-hydroxycholesterol, as well as 4β-hydroxycholesterol.
Our results showed that not only CYP3A4 but also CYP1A2, CYP2C9, and CYP2D6 catalyzed 25-hydroxylation of cholesterol to some extent (Table 1). However, CYP3A4 is the most abundantly expressed form of P450 in human liver (as much as 60% of all hepatic P450) (34). In addition, because cholesterol 4β-hydroxylase activities by CYP1A2, CYP2C9, and CYP2D6 were negligible, the positive correlation between serum concentrations of 25-hydroxycholesterol and 4β-hydroxycholesterol cannot be explained by these P450 activities. Thus, at least in normal human subjects, most of the serum 25-hydroxycholesterol appears to originate from CYP3A4.
Under abnormal conditions, however, serum 25-hydroxycholesterol concentrations may not change with 4β-hydroxycholesterol levels. For example, in a patient with cerebrotendinous xanthomatosis (CTX), CYP27A1 deficiency, serum 25-hydroxycholesterol concentration was low but 4β-hydroxycholesterol concentration was high compared with those in a normal subject (28). Because CYP3A4 activity is not significantly altered in CTX (21), it is likely that these oxysterol concentrations were affected by the activities of other enzymes, i.e., impaired CYP27A1 and upregulated CYP7A1 (21) that metabolize 4β-hydroxycholesterol and 25-hydroxycholesterol, respectively. A recent report by Diczfalusy et al. (15) showed that intravenous injection of lipopolysaccharide (endotoxin) in healthy volunteers resulted in an increase in plasma 25-hydroxycholesterol concentration. Although CH25H activity was not determined in these subjects, the increase might be due to the induction of CH25H, as suggested by their experiments using mouse macrophage.
The biochemical role of the production of 25-hydroxycholesterol by CYP3A remains unclear. However, this oxysterol appears to be further metabolized to bile acids (35), which may be one of the important alternative pathways for bile acid biosynthesis. In addition, this oxysterol is a potent inhibitor of HMG-CoA reductase and a ligand of LXRα, so that it may participate in the regulation of lipid metabolism. It should be noted that CYP3A4 catalyzes not only 25-hydroxylation but also 4β-hydroxylation, 22R-hydroxylation, and other nonstereospecific hydroxylations of cholesterol, including 24R-, 24S-, 26-, and 27-hydroxylation (Fig. 5). Because 4β-hydroxycholesterol, 22R-hydroxycholesterol, and 24S-hydroxycholesterol have been reported to be more potent activators of LXRα compared with 25-hydroxycholesterol (8, 9), the influence of CYP3A induction on LXRα activity is not explained by the effects of 25-hydroxycholesterol alone.
Fatty liver and hypertriglyceridemia are characteristic features in Cyp27−/− mice (36) but not in CTX patients. Because CYP3A is markedly upregulated in Cyp27−/− mice but not in CTX patients (21), oxysterols synthesized by CYP3A may induce fatty liver in Cyp27−/− mice. In fact, SREBP1, a target gene of LXRα, and SREBP1-regulated FA biosynthetic enzymes were upregulated in Cyp27−/− mice (36), whereas SREBP1 was not upregulated in CTX patients (37).
In summary, 25-hydroxycholesterol was quantified using the latest HPLC-ESI-MS/MS technique in a mouse liver cell line, in microsomes overexpressing recombinant human cytochrome P450 enzymes and in normal human sera. All data support the idea that CYP3A was one of the responsible enzymes that catalyzed the 25-hydroxylation of cholesterol.
Footnotes
Abbreviations:
- CH25H
- cholesterol 25-hydroxylase
- CTX
- cerebrotendinous xanthomatosis
- CYP27A1
- sterol 27-hydroxylase
- CYP46A1
- cholesterol 24S-hydroxylase
- Insig
- insulin-induced gene
- LXR
- liver X receptor
- PCN
- pregnenolone-16α-carbonitrile
- SREBP
- sterol-regulatory element binding protein
- SRM
- selected reaction monitoring
This work was supported in part by Kakenhi grants (20591309, 21790633, and 22590747) from the Japan Society for the Promotion of Science and by a grant from Health Labour Sciences Research (the intractable hepato-biliary disease study group in Japan).
REFERENCES
- 1.Gill S., Chow R., Brown A. J. 2008. Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog. Lipid Res. 47: 391–404. [DOI] [PubMed] [Google Scholar]
- 2.Krieger M., Goldstein J. L., Brown M. S. 1978. Receptor-mediated uptake of low density lipoprotein reconstituted with 25-hydroxycholesteryl oleate suppresses 3-hydroxy-3-methylglutaryl-coenzyme A reductase and inhibits growth of human fibroblasts. Proc. Natl. Acad. Sci. USA. 75: 5052–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer T. A., Gayen A. K., Phirwa S., Nelson J. A., Taylor F. R., Kandutsch A. A., Erickson S. K. 1985. 24(S),25-epoxycholesterol. Evidence consistent with a role in the regulation of hepatic cholesterogenesis. J. Biol. Chem. 260: 13391–13394. [PubMed] [Google Scholar]
- 4.Axelson M., Larsson O. 1995. Low density lipoprotein (LDL) cholesterol is converted to 27-hydroxycholesterol in human fibroblasts. J. Biol. Chem. 270: 15102–15110. [DOI] [PubMed] [Google Scholar]
- 5.Adams C. M., Reitz J., De Brabander J. K., Feramisco J. D., Li L., Brown M. S., Goldstein J. L. 2004. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 279: 52772–52780. [DOI] [PubMed] [Google Scholar]
- 6.Radhakrishnan A., Ikeda Y., Kwon H. J., Brown M. S., Goldstein J. L. 2007. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. USA. 104: 6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein J. L., DeBose-Boyd R. A., Brown M. S. 2006. Protein sensors for membrane sterols. Cell. 124: 35–46. [DOI] [PubMed] [Google Scholar]
- 8.Janowski B. A., Willy P. J., Devi T. R., Falck J. R., Mangelsdorf D. J. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature. 383: 728–731. [DOI] [PubMed] [Google Scholar]
- 9.Janowski B. A., Grogan M. J., Jones S. A., Wisely G. B., Kliewer S. A., Corey E. J., Mangelsdorf D. J. 1999. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl. Acad. Sci. USA. 96: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reschly E. J., Ai N., Welsh W. J., Ekins S., Hagey L. R., Krasowski M. D. 2008. Ligand specificity and evolution of liver X receptors. J. Steroid Biochem. Mol. Biol. 110: 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojcicka G., Jamroz-Wisniewska A., Horoszewicz K., Beltowski J. 2007. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig. Med. Dosw. (Online). 61: 736–759. [PubMed] [Google Scholar]
- 12.Jamroz-Wisniewska A., Wojcicka G., Horoszewicz K., Beltowski J. 2007. Liver X receptors (LXRs). Part II: non-lipid effects, role in pathology, and therapeutic implications. Postepy Hig. Med. Dosw. (Online). 61: 760–785. [PubMed] [Google Scholar]
- 13.Lund E. G., Kerr T. A., Sakai J., Li W. P., Russell D. W. 1998. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J. Biol. Chem. 273: 34316–34327. [DOI] [PubMed] [Google Scholar]
- 14.Bauman D. R., Bitmansour A. D., McDonald J. G., Thompson B. M., Liang G., Russell D. W. 2009. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. USA. 106: 16764–16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diczfalusy U., Olofsson K. E., Carlsson A. M., Gong M., Golenbock D. T., Rooyackers O., Flaring U., Bjorkbacka H. 2009. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 50: 2258–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lund E., Björkhem I., Furster C., Wikvall K. 1993. 24-, 25- and 27-hydroxylation of cholesterol by a purified preparation of 27-hydroxylase from pig liver. Biochim. Biophys. Acta. 1166: 177–182. [DOI] [PubMed] [Google Scholar]
- 17.Li X., Pandak W. M., Erickson S. K., Ma Y., Yin L., Hylemon P., Ren S. 2007. Biosynthesis of the regulatory oxysterol, 5-cholesten-3β,25-diol 3-sulfate, in hepatocytes. J. Lipid Res. 48: 2587–2596. [DOI] [PubMed] [Google Scholar]
- 18.Lund E. G., Guileyardo J. M., Russell D. W. 1999. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA. 96: 7238–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith L. L. 1981. Cholesterol Autoxidation. Plenum Press, New York. [Google Scholar]
- 20.Honda A., Salen G., Matsuzaki Y., Batta A. K., Xu G., Leitersdorf E., Tint G. S., Erickson S. K., Tanaka N., Shefer S. 2001. Differences in hepatic levels of intermediates in bile acid biosynthesis between Cyp27–/– mice and CTX. J. Lipid Res. 42: 291–300. [PubMed] [Google Scholar]
- 21.Honda A., Salen G., Matsuzaki Y., Batta A. K., Xu G., Leitersdorf E., Tint G. S., Erickson S. K., Tanaka N., Shefer S. 2001. Side chain hydroxylations in bile acid biosynthesis catalyzed by CYP3A are markedly up-regulated in Cyp27–/– mice but not in cerebrotendinous xanthomatosis. J. Biol. Chem. 276: 34579–34585. [DOI] [PubMed] [Google Scholar]
- 22.Kliewer S. A., Willson T. M. 2002. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J. Lipid Res. 43: 359–364. [PubMed] [Google Scholar]
- 23.Bodin K., Bretillon L., Aden Y., Bertilsson L., Broome U., Einarsson C., Diczfalusy U. 2001. Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterol in humans: evidence for involvement of cytochrome p450 3A4. J. Biol. Chem. 276: 38685–38689. [DOI] [PubMed] [Google Scholar]
- 24.Diczfalusy U., Kanebratt K. P., Bredberg E., Andersson T. B., Bottiger Y., Bertilsson L. 2008. 4β-Hydroxycholesterol as an endogenous marker for CYP3A4/5 activity. Stability and half-life of elimination after induction with rifampicin. Br. J. Clin. Pharmacol. 67: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225: 73–80. [DOI] [PubMed] [Google Scholar]
- 26.Wu J. C., Merlino G., Fausto N. 1994. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor α. Proc. Natl. Acad. Sci. USA. 91: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda A., Yamashita K., Miyazaki H., Shirai M., Ikegami T., Xu G., Numazawa M., Hara T., Matsuzaki Y. 2008. Highly sensitive analysis of sterol profiles in human serum by LC-ESI-MS/MS. J. Lipid Res. 49: 2063–2073. [DOI] [PubMed] [Google Scholar]
- 28.Honda A., Yamashita K., Hara T., Ikegami T., Miyazaki T., Shirai M., Xu G., Numazawa M., Matsuzaki Y. 2009. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J. Lipid Res. 50: 350–357. [DOI] [PubMed] [Google Scholar]
- 29.Honda A., Miyazaki T., Ikegami T., Iwamoto J., Yamashita K., Numazawa M., Matsuzaki Y. 2010. Highly sensitive and specific analysis of sterol profiles in biological samples by HPLC-ESI-MS/MS. J. Steroid Biochem. Mol. Biol. 121: 556–564. [DOI] [PubMed] [Google Scholar]
- 30.Ono S., Hatanaka T., Hotta H., Satoh T., Gonzalez F. J., Tsutsui M. 1996. Specificity of substrate and inhibitor probes for cytochrome P450s: evaluation of in vitro metabolism using cDNA-expressed human P450s and human liver microsomes. Xenobiotica. 26: 681–693. [DOI] [PubMed] [Google Scholar]
- 31.Bodin K., Andersson U., Rystedt E., Ellis E., Norlin M., Pikuleva I., Eggertsen G., Bjorkhem I., Diczfalusy U. 2002. Metabolism of 4β-hydroxycholesterol in humans. J. Biol. Chem. 277: 31534–31540. [DOI] [PubMed] [Google Scholar]
- 32.Norlin M., Andersson U., Bjorkhem I., Wikvall K. 2000. Oxysterol 7α-hydroxylase activity by cholesterol 7α-hydroxylase (CYP7A). J. Biol. Chem. 275: 34046–34053. [DOI] [PubMed] [Google Scholar]
- 33.Li-Hawkins J., Lund E. G., Turley S. D., Russell D. W. 2000. Disruption of the oxysterol 7α-hydroxylase gene in mice. J. Biol. Chem. 275: 16536–16542. [DOI] [PubMed] [Google Scholar]
- 34.Guengerich F. P. 1990. Mechanism-based inactivation of human liver microsomal cytochrome P-450 IIIA4 by gestodene. Chem. Res. Toxicol. 3: 363–371. [DOI] [PubMed] [Google Scholar]
- 35.Swell L., Schwartz C. C., Gustafsson J., Danielsson H., Vlahcevic Z. R. 1981. A quantitative evaluation of the conversion of 25-hydroxycholesterol to bile acids in man. Biochim. Biophys. Acta. 663: 163–168. [DOI] [PubMed] [Google Scholar]
- 36.Repa J. J., Lund E. G., Horton J. D., Leitersdorf E., Russell D. W., Dietschy J. M., Turley S. D. 2000. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. J. Biol. Chem. 275: 39685–39692. [DOI] [PubMed] [Google Scholar]
- 37.Honda A., Salen G., Matsuzaki Y., Batta A. K., Xu G., Hirayama T., Tint G. S., Doy M., Shefer S. 2005. Disrupted coordinate regulation of farnesoid X receptor target genes in a patient with cerebrotendinous xanthomatosis. J. Lipid Res. 46: 287–296. [DOI] [PubMed] [Google Scholar]