Abstract
Mice and humans lacking functional caveolae are dyslipidemic and have reduced fat stores and smaller fat cells. To test the role of caveolins/caveolae in maintaining lipid stores and adipocyte integrity, we compared lipolysis in caveolin-1 (Cav1)-null fat cells to that in cells reconstituted for caveolae by caveolin-1 re-expression. We find that the Cav1-null cells have a modestly enhanced rate of lipolysis and reduced cellular integrity compared with reconstituted cells as determined by the release of lipid metabolites and lactic dehydrogenase, respectively, into the media. There are no apparent differences in the levels of lipolytic enzymes or hormonally stimulated phosphorylation events in the two cell lines. In addition, acute fasting, which dramatically raises circulating fatty acid levels in vivo, causes a significant upregulation of caveolar protein constituents. These results are consistent with the hypothesis that caveolae protect fat cells from the lipotoxic effects of elevated levels fatty acids, which are weak detergents at physiological pH, by virtue of the property of caveolae to form detergent-resistant membrane domains.
Keywords: caveolin-1, cavin, fasting, lipid rafts, lipolysis
Proper functioning of adipocytes with regard to fatty acid (FA) storage and release is an essential aspect of fuel homeostasis, and dysregulation of these processes can lead to obesity and type 2 diabetes mellitus (1). Along with their characteristically large lipid droplets, striking features of fat cells are cell surface structures called caveolae or little caves, small (50-100 nM) invaginations of the plasma membrane into the cytosol (2) that are especially abundant in this cell type, constituting as much as 50% of the surface area (3). In nonmuscle cells, caveolae are formed due to the expression of caveolin-1 (Cav1), caveolin-2 (Cav2) (4), cavin-1 (5–7), and probably, cavin-2 (8). The caveolins are small integral membrane proteins (4), and the cavins are peripheral membrane proteins without enzymatic activity that are tightly associated with caveolae (2, 9). Mice lacking caveolin-1 generated by gene knockout technology are lean, have abnormally small fat cells, and are insulin resistant (10), which is a phenotype mirrored in humans with inactivating Cav1 mutations (11, 12). Among other pathologies that they harbor, mice (6) and humans (13–16) lacking functional cavin-1 are lean, lipodystrophic, and insulin resistant. These facts suggest that caveolae serve one or more functions specific to adipocytes and their lipid metabolism.
Caveolae are a subset of so-called membrane lipid raft domains that are often experimentally defined by their resistance to solubilization by brief exposure to nonionic detergents (17). FA anions are also weak detergents at physiological pH and are trafficked in abundant amounts in and out of fat cells. Consequently, we and others have suggested that caveolae could serve to protect adipocytes (and other cells) from the possible toxic effects of FA by serving as a membrane “buffer” for these molecules (18–20). Indeed, we showed that HEK293 cells overexpressing caveolin-1 or caveolin-3 were resistant to the toxic effects of prolonged incubation with physiologically high levels of FA (20). However, compared with adipocytes, these cells lack the capacity to store large amounts of triglycerides, and they also lack the hormone sensitive lipolytic protein machinery of the fat cell (21). Thus, while the HEK293 cell line serves as a useful model system for a variety of purposes, it does not reflect the robust FA trafficking and lipid metabolism of the adipocyte, the physiologically relevant cell in this regard. Consequently, we took advantage of the caveolin-1-null mouse (5) to create an adipocyte cell line from embryonic fibroblasts, and we “rescued” these cells by expressing caveolin-1 to compare lipolysis and its effects in this context. We show that caveolae protect fat cells from the autolysis that results from lipolytic stimulation by several agents, including adrenergic hormones.
EXPERIMENTAL PROCEDURES
Antibodies
A polyclonal antibody against caveolin-1 and monoclonal anti-caveolin 2 were purchased from BD Transduction Laboratories (San Jose, CA), and anti-actin was from Sigma (St. Louis, MO). Monoclonal anti-caveolin-1 antibodies were produced in our laboratory (22). Anti-peptide antibodies directed against cavin-1, cavin-2, and cavin-3 (7, 8) were made by 21st Century Biochemicals (Marlboro, MA). Polyclonal antibodies against perilipin, hormone sensitive lipase (HSL), and adipocyte triglyceride lipase (ATGL) were kindly provided by Dr. Andrew Greenberg (Tufts University, Boston, MA). Polyclonal anti-aP2 was kindly provided by Dr. David Bernlohr (University of Minnesota, Minneapolis, MN). Anti-phospho-protein kinase A (PKA) substrate antibody was kindly provided by Dr. M. S. Gauthier (Boston University School of Medicine, Boston, MA) (23).
Reagents
Dexamethasone, 3-isobutyl-1-methylxanthine, and insulin were purchased from Sigma. Triglyceride-GPO Reagent Set was purchased from Pointe Scientific, Inc. (Canton, MI). Isoproterenol, the adenylate cyclase activator, forskolin, and O-dibutyryladenosine-3′5'-cyclic monophosphate (dbcAMP) were obtained from Sigma, and the FuGENE 6 transfection reagent was purchased from Roche Diagnostics (Indianapolis, IN).
Cell culture
Primary mouse embryonic fibroblasts (MEF) were obtained from wild-type and Cav1-null mice (5). Cells were grown in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Inc., Herndon, VA) that contained a mixture of penicillin (50 units/ml) and streptomycin (50 μg/ml) (Invitrogen, Carlsbad, CA). The medium was supplemented with 10% fetal bovine serum (FBS; Invitrogen). Cells were immortalized using a 3T3 cell passaging protocol (24). Cells were passaged continuously every 3 days and plated at 6 × 105 cells per 100 mm dish until the growth rate become relatively rapid and cells had a fibroblastic morphology (∼passage 20 and beyond). Confluent cells were induced to differentiate using a cocktail of insulin (1.7 μM), isobutylmethylxanthine (0.5 mM), dexamethasone (1 μM) (25), and troglitazone (5 μM) (26). After 2-3 days, cells were maintained in FBS containing 10% FBS and 0.85 μM insulin and troglitazone (5 μM) for 3 days, then in medium containing 10% FBS and troglitazone for a total of 8-10 days.
Animals
Male Sprague Dawley rats (175 g) were obtained from Charles River Labs (Wilmington, MA). Rats were fed ad libitum or fasted prior to isolation of primary (epididymal) fat cells and Western blot analysis as previously described (27) and approved by the Institutional Animal Care and Use Committee at the Boston University Medical Center.
Retroviral vectors
The cDNA encoding for mouse caveolin-1 was subcloned into BamHI and EcoRI sites of the pBABE-puro retroviral vector. Transfection was done as previously described (28). HEK-293T cells were grown in DMEM as above, but with 10% FBS to 70% confluence in 100 mm diameter dishes, at which stage they were transfected with a DNA-FuGENE cocktail consisting of 54 µl Fugene 6 transfection reagent, 6 µg retrovirus constructs, 6 µg pVPack-VSV-G vector, 6 µg pVPack-GAG-POL vector, and DMEM without FBS to final volume of 200 µl. As a control, cells were transfected with pBabe-puro empty vector. Twenty-four h later, the medium was replaced with 6 ml fresh DMEM containing 10% FBS. After an additional 24 h, the supernatant containing high-titer retrovirus was collected and filtered through a 0.45 µm pore-sized filter. The viral filtrates were added to the target cells. After infection, cells were selected for five days in complete medium containing puromycin at a final concentration of 2.5 μg/ml. Following selection, cells were maintained in medium containing 1.25 μg/ml puromycin. Stable expression in the target cell population was confirmed by Western blot analysis.
Preparation of whole cell extracts
Cells were washed three times with PBS and incubated for 30 min on ice with iced-cold RIPA buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and a protease inhibitor cocktail (Aprotinin 10 μg/ml, Pepstatin 1 μg/ml, and Leupeptin 5 μg/ml; American Bioanalytical, Natick, MA). Lysates were vortexed and spun for 20 min at 16,000 g at 4°C. Protein concentrations were determined in the supernatant using bicinchoninic acid (BCA) reagent (Pierce, Rockford, IL).
Gel electrophoresis and immunoblotting
Proteins were separated by SDS-PAGE (acrylamide; National Diagnostics, Atlanta, GA) and electrophoretically transferred to polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The membrane was then blocked with 10% nonfat dry milk in PBS containing 0.5% Tween-20 for 1 h at room temperature. Primary antibodies were detected using secondary antibodies conjugated to horseradish peroxidase (Sigma) and chemiluminescence substrate (PerkinElmer Life Sciences, Boston, MA).
Lipolysis
Cells were grown to confluence in 6-well plates and induced to differentiate as described above. On the day of the experiment, adipocytes were preincubated for 4 h in serum-free DMEM containing 0.5% fatty acid free BSA (American Bioanalytical). Cells were washed three times with warm DMEM and then treated with 10 µM β-adrenergic agonist isoproterenol, 20 µM adenylate cyclase activator forskolin, or 1 mM dbcAMP. Agonists were prepared in serum-free culture medium containing 0.5% fatty acid free BSA, and 1 ml was added to each well. Aliquots were removed at 1, 2, and 4 h of stimulation, and the glycerol content of each sample was measured using the Triglyceride (GPO) Reagent Set and measured at 540 nm against a set of glycerol standards as in previous studies (20). Cells were then washed with cold PBS and lysed with a RIPA buffer. The protein concentration was determined and used to normalize glycerol release. Unesterified fatty acids from cells treated as above were measured in DMEM media without phenol red, and the glucose concentration was adjusted to 4.5 g/l using a kit from Waco Diagnostics (Richmond, VA). The 4 h time-point samples were used for measuring LDH release using QuantiChromTM Lactate Dehydrogenase Kit (DLDH-100; Biosystems, Hayward, CA) per manufacturer's recommendations.
Confocal laser-scanning microscopy
Cells were grown in 6-well plates to confluence and were induced to differentiate. On day 8 of differentiation, cells were washed three times with PBS, trypsinized, and plated again into 6 well plates containing coverslips pretreated with 0.1% gelatin (Millipore Billerica, MA). After 24 h, cells were fixed with 3.7% formaldehyde in PBS for 20 min at room temperature. Cells were permeabilized with solution A (0.1% saponin and 0.4% BSA in PBS) for 10 min and then blocked for 1 h at room temperature in 5% normal goat serum (Sigma) in PBS. Staining was performed with rabbit polyclonal anti-caveolin-1 antibody overnight at a dilution of 1/100 in 1% normal goat serum in PBS. Following staining, cells were washed four times with buffer A. Cells were incubated with anti-rabbit Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc.) at a dilution of 1/200 in 1% normal goat serum in PBS. The cells were covered in aluminum foil and incubated for 1 h at room temperature. The cells were washed again three times with solution A and then mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Inc., Burlingame, CA). The stained cells were observed using a Zeiss 510 confocal laser-scanning microscope (Carl Zeiss, Thornwood, NY). Images were processed using LSM 510 Image software.
RESULTS
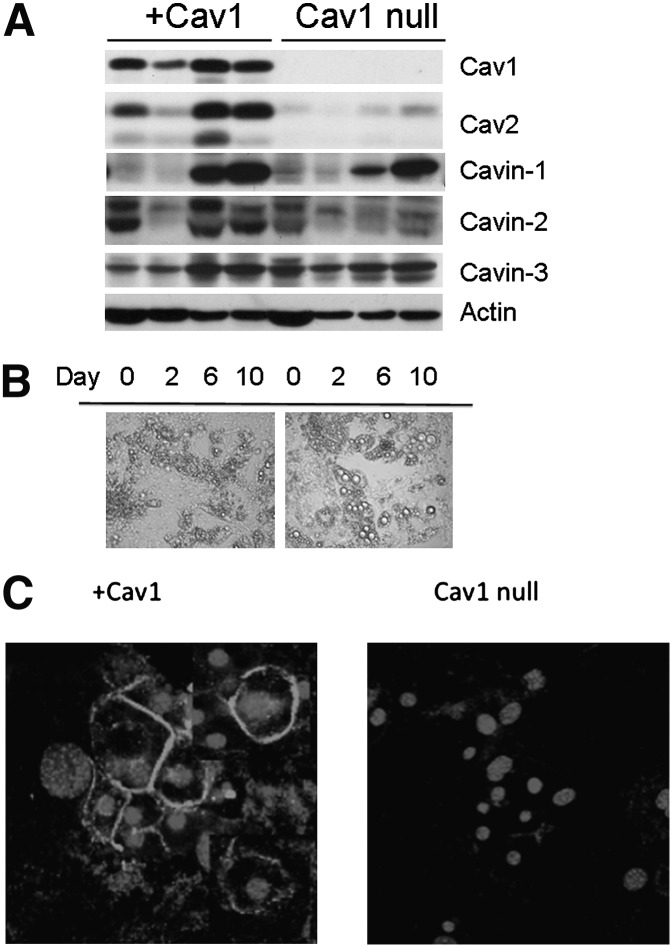
A permanent preadipocyte cell line was created from embryonic fibroblasts obtained from Cav1-null mice (5) as described in Experimental Procedures. These cells were then infected with Cav1- or empty vector-expressing retrovirus. As shown in Fig. 1A, infected cells (+Cav1) expressed Cav1, which increased somewhat during differentiation but not to the same extent as the endogenous protein in 3T3-L1 cells (29), and vector-expressing cells (Cav1-null) lacked Cav-1 expression as expected. Cav2 levels were greatly reduced in Cav1-null cultured adipocytes and were restored by Cav1 expression, as would be expected from prior data showing that Cav2 requires Cav1 expression for recruitment into caveolae (30). We also characterized the Cav1-null and wild-type cells prior to infection, and these appeared identical in appearance and caveolar protein expression to the vector-transfected and rescued cells, respectively, and had comparable levels of hormonally stimulated lipolysis (data not shown). Interestingly, cavin-1 expression is the same in Cav1-null and rescued (+Cav1) cell lines. This protein is obligatory for caveolae formation (5–7) and, along with cavin-2 and cavin-3, is a member of the cavin family of putative caveolar coat proteins in nonmuscle cells (8). All three cavin isoforms were expressed in the Cav1-null cell line (Fig. 1A), although cavin-2 expression was reduced in the absence of Cav1. The Cav1-null fat cells were larger and exhibited larger lipid droplets than cells harboring Cav1 (Fig. 1B). The Cav1 reconstituted cells closely resembled 3T3-L1 cells in their morphology and lipid droplet size (not shown). Finally, the Cav1 rescued cells target this protein to the plasma membrane as shown in Fig. 1C by immunofluorescence.
Fig. 1.
Characteristics of Cav1-null adipocytes and +Cav1 fat cells. A: On the indicated day after induction of differentiation (see Experimental Procedures), whole cell extracts (20 µg) from cells infected with vector (Cav1-null) or caveolin-1 (+Cav1) were prepared and resolved by SDS-PAGE (10% acrylamide). Western blotting was performed with the indicated antibodies, and bands were detected with horseradish peroxidase–conjugated secondary antibody and chemiluminescence. B: On day 10 of differentiation, cells were viewed in bright field on an Olympus IX70 microscope (Hamamatsu, Hamamatsu City, Japan) at 20×. C: On day 10 of differentiation, cells were fixed and subjected to immunostaining for caveolin-1 (rims) and the nucleus (center of cell) as described in Experimental Procedures prior to visualization by confocal microscopy. All results are representative of three or more independent experiments.
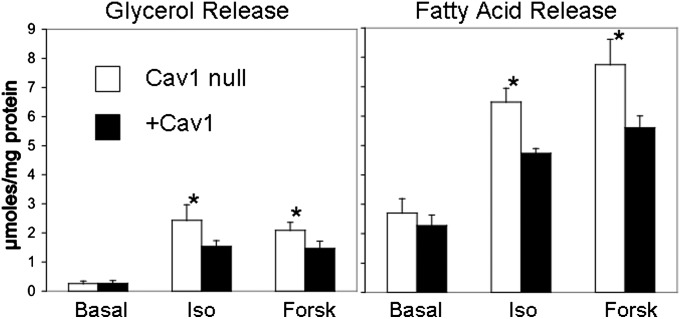
The dyslipidemia seen in mice lacking adipocyte caveolae is accompanied by reduced adiposity and higher levels of circulating FA and triglycerides (6, 10); consequently, we assessed how the Cav1-null adipocytes in vitro respond to lipolytic stimuli by measuring FA and glycerol release under basal and stimulated conditions. As shown in Fig. 2, after exposure to the lipolytic agents forskolin and isoproterenol, the Cav1-null cells hydrolyzed triglycerides to a slightly but significantly higher extent than the Cav1 expressing cells, and they showed equivalent basal lipolysis.
Fig. 2.
Lipolysis is enhanced in Cav1-null adipocytes. Glycerol and fatty acid release of fully differentiated Cav1-null and +Cav1 adipocytes were measured after treatment with the lipolytic stimuli, 10 μM isoproterenol (Iso), and 20 μM forskolin (Forsk), for 4 h. *P ≤ 0.05 ± SD for four independent determinations carried out in duplicate or triplicate.
The differences in lipolysis could be explained by a number of factors, including differences in lipolytic enzyme expression and/or lipolytic signaling. Accordingly, we measured expression levels for a number of proteins involved in adipocyte FA lipolysis in Cav1 reconstituted and Cav1-null cell lines as a function of time of differentiation, with day 0 being the fibroblast state and day 10 being the fully differentiated state (Fig. 3). There were no differences in the two cell lines in expression levels for the lipid droplet-associated proteins adipocyte triglyceride lipase (ATGL) and perilipin, which are involved in lipolysis, or in HSL or the fatty acid binding protein aP2. As a further control, the Glut4 storage vesicle (GSV) (31) proteins, insulin-responsive aminopeptidase, and Glut4, as well as the mitochondrial proteins, uncoupling protein 1 and cytochrome oxidase IV, were measured and found to be unchanged (data not shown).
Fig. 3.
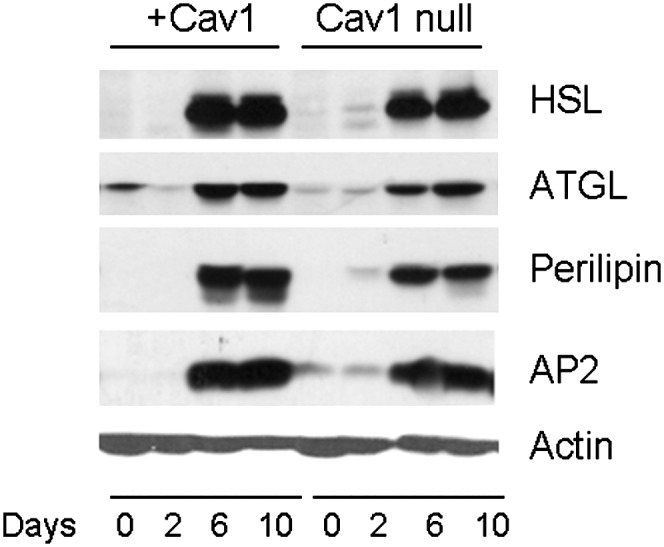
Lipolytic enzyme levels are the same in Cav1-null and +Cav1 adipocytes. Cells differentiated as in Fig. 1 were lysed, and equal proteins amounts were subjected to SDS-PAGE and Western blotting with the antibodies indicated. Shown is a blot representative of three independent experiments. Statistical analysis of scanned gels revealed no significant differences in day 10 levels for the proteins shown.
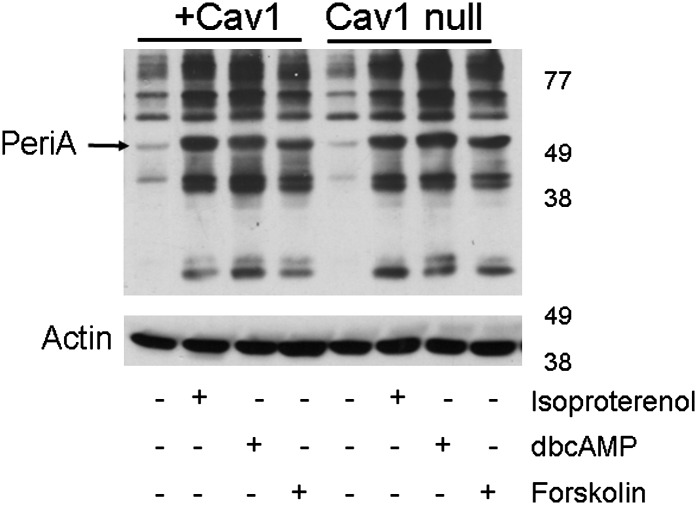
We then determined if hormone-dependent lipolytic signaling was altered in the Cav1-null adipocytes. As shown in Fig. 4, there were no apparent differences between the two cell lines in the extent of phosphorylation as assayed by a Western blot for PKA phosphorylation sites. Perilipin phosphorylation (Fig. 4, arrowhead), the rate limiting step in lipolysis (32, 33), was the same in response to the three lipolytic stimuli, namely, isoproterenol, dbcAMP, and forskolin, indicated in Fig. 4. Thus, the presence or absence of caveolae seems to be the major and possibly only factor that affected the rate of fatty acid release in these two adipocyte cell lines.
Fig. 4.
The absence of Cav1 does not affect PKA signaling. Differentiated adipocytes (day 8) were incubated with serum-free DMEM containing 0.5% fatty acid free BSA for 3 h, followed by treatment with or without isoproterenol (10 µM), dbcAMP (1 mM), or forskolin (20 µM) for 4 h. Then cell lysates were prepared, subjected to SDS-PAGE, and immunoblotted with antibodies recognizing phosphorylated serine and threonine residues of PKA substrate. The immunoblot shown is representative of three independent experiments.
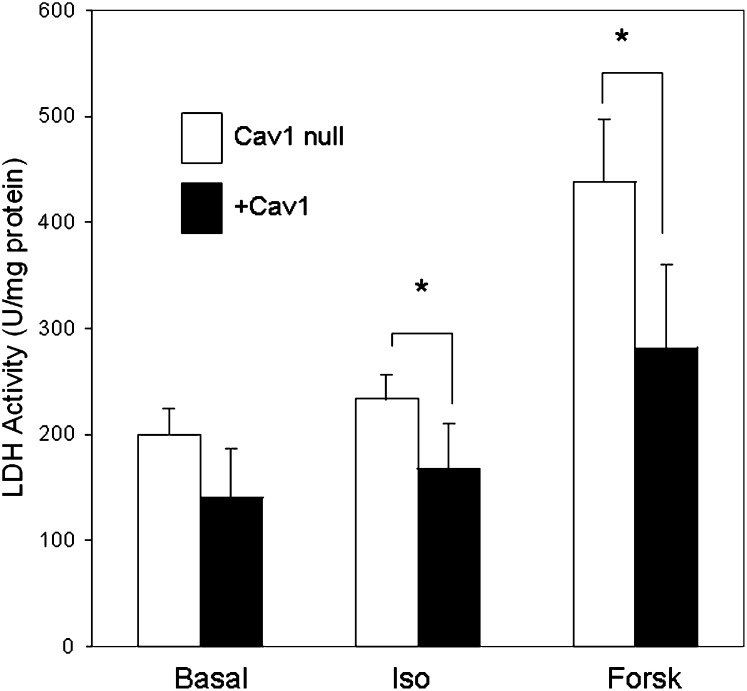
We previously hypothesized that the detergent-resistant nature of caveolae and caveolin-dependent lipid rafts would protect cells against the cytotoxic effects of high FA concentrations. We confirmed this hypothesis in HEK293 cells transfected with Cav1 or Cav3 by showing they were protected from the toxic effects of prolonged exposure to high levels of exogenous FA (20). HEK cells do not significantly express enzymes for FA storage nor do they possess hormonally sensitive lipolytic machinery. We wished to test the ability of adipocytes to resist lipotoxicity produced by endogenous FA, as these are the most physiologically relevant cells that would see the highest levels of FA upon lipolytic stimulation in vivo. Indeed, as shown in Fig. 5, the release of lactic dehydrogenase (LDH), a measure of plasma membrane integrity (34), is greater in adipocytes lacking caveolae (Cav1-null) than in rescued cells (+Cav1), which is consistent with our hypothesis concerning the cytoprotective effects of caveolins and caveolae.
Fig. 5.
Caveolae protect adipocytes from autolysis. On day 10 of differentiation, vector-transfected cells (Cav1-null, open bars) and Cav1-transfected cells (+Cav1, closed bars) were incubated in DMEM media without phenol red and with a glucose concentration of 25 mM. After 4 h incubation in the presence or absence of isoproterenol (Iso) and forskolin (Forsk), lactic dehydrogenase (LDH) released into the media was measured as described in Experimental Procedures. The data are the mean + SEM from three or four independent experiments carried out in triplicate, *P ≤ 0.03 for Iso; *P ≤ 0.003 for Forsk.
A corollary regarding this protective effect is that the expression of caveolae-related proteins might be acutely increased in vivo under conditions of high circulating FA (e.g., fasting), as had been shown for caveolin-1 in mice (35) and, more recently, for cavin-1 (36), in both cases measured separately from other caveolar proteins. Accordingly, we subjected rats to a fast for three days and measured Cav1, cavin-1, cavin-2, and cavin-3 levels as well as Glut4 and Glut1 protein expression. As we showed previously, fasting causes a loss of Glut4 and an increase in Glut1 (27), and we show in this study that Cav1, cavin-1, cavin-2, and cavin-3 are increased after three days of fasting (Fig. 6), indicating that the known caveolar protein constituents are coordinately regulated under these conditions. Given the interdependence of caveolins and cavin-1 for caveolae formation (2), these data suggest that the number of caveolae increases under these conditions.
Fig. 6.
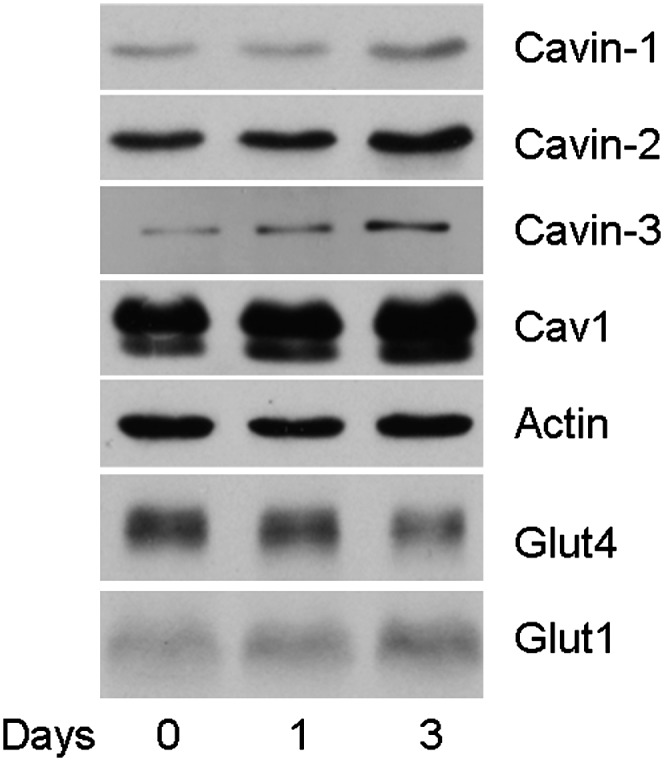
Fasting induces caveolae formation. Rats were fasted for no, one, or three days. Primary fat cells were isolated and lysed. Lysates (10 μg protein) were subjected to SDS-PAGE and Western blotting as in previous figures. Shown is a representative of three experiments (one rat per time point) with actin, cavin-1, Cav1, and Glut4 and two experiments with cavin-2 and cavin-3. The gels were scanned for Cav1, cavin-1, Glut4, and actin using NIH Image 1.63 software. The day 0 value was normalized to actin and set to 1.0. The proteins levels for Cav1, cavin-1, and Glut4 were changed by 2.5-fold ± 0.3, 2.9-fold ± 0.1, and 0.4-fold ± 0.2, respectively. P ≤ 0.05 for day 0 versus day 3 for the three indicated proteins.
DISCUSSION
The role of caveolae in adipocyte fatty acid metabolism is underscored most dramatically by the phenotype of caveolae-deficient mice (6, 10) and humans (11–13, 15). Both species exhibit reduced fat mass and hyperlipidemia, and the mice have smaller adipocytes and a blunted hormone-induced lipolytic response when isolated as primary cells (35) (S. Y. Ding, S. Fried, and P. F. Pilch, unpublished observations). The latter result might be surprising, as one could imagine that an elevated rate of lipolysis could account for the hyperlipidemia. The in vivo situation is complicated by the fact that the hyperlipidemia likely precedes the elevated insulin levels and insulin resistance observed in the knockout mice, and these conditions likely develop shortly after birth and well before the 8-12 week time when metabolic parameters were first assessed. In addition these mice have reduced leptin and adiponectin levels (6, 10) and, most likely, alterations in other hormones, all of which could complicate interpretation of the in vivo analysis.
As a consequence, we used cultured murine adipocytes obtained from Cav-1-null MEFs to study lipolysis, and we compared them with the same cells into which Cav-1 had been reintroduced. Compared with controls, the Cav1-null fat cells showed a small but significant enhancement of lipid release (Fig. 2). Moreover, we saw neither differences in any of the enzymatic machinery for lipolysis nor alteration of lipolytic signaling (Figs. 3 and 4). The products of lipolysis, glycerol and FAs, are measured in the extracellular milieu and need to cross the plasma membrane. Previously, we have shown that, in HEK293 cells transfected with caveolin-1 or caveolin-3, the transfected cells had the property of modulating transmembrane FA movement by sequestering FAs in the inner leaflet of the plasma membrane where caveolins are inserted (18, 20). Because the HEK cells lack hormonally sensitive lipolytic protein, we can measure significant FA movement only in the direction of FA uptake; however, in fat cells, we can measure FA release, one of the most physiologically relevant situations where autolysis might be a problem. Our results (Fig. 2), together with the data of Figs. 3 and 4, are consistent with the interpretation that the reduced extent of lipid release measured extracellularly in the +Cav1 versus Cav1-null cells can be attributed to the enhanced FA sequestration in caveolae in the former cell line. Importantly, the condition under which these activities would likely be most physiologically relevant is prolonged hormone-stimulated lipolysis, which was shown to result in autolysis in primary rat adipocytes as measured by LDH release (34). We used the same assay as in Ref. 34 to show that caveolae do indeed protect against this form of lipotoxicity in cultured cells (Fig. 5).
Note, however, that the extent of lipid release from the Cav1-null fat cells was roughly proportional to the LDH release as normalized to total protein. However, the Cav1-null cells were significantly bigger, nearly twice the diameter of the Cav1-expressing cells. Thus, although it is not readily feasible to normalize lipid release to total cell surface area due to the irregular shape of the cultured fat cell, FA release normalized to cell surface would actually be less per a given surface area in the Cav1-null cells compared with the Cav1-expressing cells. Thus these data are consistent with our interpretation that caveolins/caveolae protect the cells from lipotoxicity.
Finally, we showed that in vivo conditions of high circulating FA (namely, fasting) led to upregulation of the structural components of caveolae (Fig. 6), including recently characterized cavin family members, cavin-1, cavin-2, and cavin-3 (8, 37, 38). These results are consistent with a cytoprotective effect of caveolae in adipocytes that may also apply to other cells, such as endothelial cells and the lung epithelium (39), in the latter case, as a protection against endogenously produced surfactant. These possibilities can be experimentally addressed in a fashion similar to ours.
Are there roles for caveolae and their structural components in adipocyte lipid metabolism other than to modulate FA flux and protect cell integrity? Several investigators have reported that caveolin-1 is associated with the lipid droplet, although its function is not known (35, 40). It has also been shown that lipogenesis can occur in a subset of membranes enriched in caveolae (19). More recently, cavin-1 has been implicated in playing a regulatory role in hormonally stimulated lipolysis (36). Our data demonstrated that caveolae are not obligatory for lipid droplet formation/function in adipocytes. At first glance, our data seem contradictory to the results obtained for hormonally stimulated lipolysis from primary Cav1-null adipocytes, where expression was markedly reduced (35), a result we have also observed with cavin-1-null primary fat cells (data not shown). However, the stable cell lines retain cavin-1 expression, whereas it is obviously absent from the cavin-1 knockout animal (6) and the Cav1-null mouse (5). Thus, cavin-1 may play a critical and direct role in regulation of adipocyte lipolysis apart from or as part of the caveolae structure. The use of primary cells, along with suitable cell lines, should allow us to determine the mechanism by which primary adipocytes from caveolae-null mice (and presumably humans) are refractory to hormonally stimulated lipolysis. It has also recently been shown that there is an increase in autophagy in Cav1-null adipocytes (41), and while this does not seem likely to have a direct effect on lipolysis, particularly ex vivo, the phenomenon could certainly contribute to the lipoatrophic phenotype of the mouse. Finally, the cavin-1-null mouse is profoundly insulin resistant (6), so another likely contributor to this phenotype is the imbalance of lipid metabolism, where lipolysis is decreased but insulin-dependent lipid storage is even more decreased, as we recently discussed along with providing a possible model for these processes (42).
Acknowledgments
The authors wish to thank Gino Vallega for excellent technical assistance.
Footnotes
Abbreviations:
- ATGL
- adipocyte triglyceride lipase
- Cav1(2)
- caveolin-1(2)
- HSL
- hormone sensitive lipase
- Iso
- Isoproterenol
- LDH
- lactic dehydrogenase
- MEF
- mouse embryonic fibroblast
- PKA
- protein kinase A
REFERENCES
- 1.Guilherme A., Virbasius J. V., Puri V., Czech M. P. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastiani M., Parton R. G. 2010. Caveolae at a glance. J. Cell Sci. 123: 3831–3836. [DOI] [PubMed] [Google Scholar]
- 3.Thorn H., Stenkula K. G., Karlsson M., Ortegren U., Nystrom F. H., Gustavsson J., Stralfors P. 2003. Cell surface orifices of caveolae and localization of caveolin to the necks of caveolae in adipocytes. Mol. Biol. Cell. 14: 3967–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams T. M., Lisanti M. P. 2004. The caveolin proteins. Genome Biol. 5: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill M. M., Bastiani M., Luetterforst R., Kirkham M., Kirkham A., Nixon S. J., Walser P., Abankwa D., Oorschot V. M., Martin S., et al. 2008. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 132: 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L., Brown D., McKee M., Lebrasseur N. K., Yang D., Albrecht K. H., Ravid K., Pilch P. F. 2008. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 8: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L., Pilch P. F. 2008. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem. 283: 4314–4322. [DOI] [PubMed] [Google Scholar]
- 8.Bastiani M., Liu L., Hill M. M., Jedrychowski M. P., Nixon S. J., Lo H. P., Abankwa D., Luetterforst R., Fernandez-Rojo M., Breen M. R., et al. 2009. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J. Cell Biol. 185: 1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen C. G., Nichols B. J. 2010. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 20: 177–186. [DOI] [PubMed] [Google Scholar]
- 10.Cohen A. W., Razani B., Wang X. B., Combs T. P., Williams T. M., Scherer P. E., Lisanti M. P. 2003. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am. J. Physiol. Cell Physiol. 285: C222–C235. [DOI] [PubMed] [Google Scholar]
- 11.Cao H., Alston L., Ruschman J., Hegele R. A. 2008. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis. 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim C. A., Delepine M., Boutet E., El Mourabit H., Le Lay S., Meier M., Nemani M., Bridel E., Leite C. C., Bertola D. R., et al. 2008. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J. Clin. Endocrinol. Metab. 93: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi Y. K., Matsuda C., Ogawa M., Goto K., Tominaga K., Mitsuhashi S., Park Y. E., Nonaka I., Hino-Fukuyo N., Haginoya K., et al. 2009. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J. Clin. Invest. 119: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwianingsih E. K., Takeshima Y., Itoh K., Yamauchi Y., Awano H., Malueka R. G., Nishida A., Ota M., Yagi M., Matsuo M. 2010. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol. Genet. Metab. 101: 233–237. [DOI] [PubMed] [Google Scholar]
- 15.Rajab A., Straub V., McCann L. J., Seelow D., Varon R., Barresi R., Schulze A., Lucke B., Lutzkendorf S., Karbasiyan M., et al. 2010. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 6: e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shastry S., Delgado M. R., Dirik E., Turkmen M., Agarwal A. K., Garg A. 2010. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am. J. Med. Genet. A. 152A: 2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons K., Gerl M. J. 2010. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 11: 688–699. [DOI] [PubMed] [Google Scholar]
- 18.Meshulam T., Simard J. R., Wharton J., Hamilton J. A., Pilch P. F. 2006. Role of caveolin-1 and cholesterol in transmembrane fatty acid movement. Biochemistry. 45: 2882–2893. [DOI] [PubMed] [Google Scholar]
- 19.Ost A., Ortegren U., Gustavsson J., Nystrom F. H., Stralfors P. 2005. Triacylglycerol is synthesized in a specific subclass of caveolae in primary adipocytes. J. Biol. Chem. 280: 5–8. [DOI] [PubMed] [Google Scholar]
- 20.Simard J. R., Meshulam T., Pillai B. K., Kirber M. T., Brunaldi K., Xu S., Pilch P. F., Hamilton J. A. 2010. Caveolins sequester FA on the cytoplasmic leaflet of the plasma membrane, augment triglyceride formation, and protect cells from lipotoxicity. J. Lipid Res. 51: 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zechner R., Kienesberger P. C., Haemmerle G., Zimmermann R., Lass A. 2009. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 50: 3–21. [DOI] [PubMed] [Google Scholar]
- 22.Souto R. P., Vallega G., Wharton J., Vinten J., Tranum-Jensen J., Pilch P. F. 2003. Immunopurification and characterization of rat adipocyte caveolae suggest their dissociation from insulin signaling. J. Biol. Chem. 278: 18321–18329. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier M. S., Miyoshi H., Souza S. C., Cacicedo J. M., Saha A. K., Greenberg A. S., Ruderman N. B. 2008. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 283: 16514–16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todaro G. J., Green H. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17: 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green H., Kehinde O. 1975. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 5: 19–27. [DOI] [PubMed] [Google Scholar]
- 26.El-Jack A. K., Hamm J. K., Pilch P. F., Farmer S. R. 1999. Reconstitution of insulin-sensitive glucose transport in fibroblasts requires expression of both PPARgamma and C/EBPalpha. J. Biol. Chem. 274: 7946–7951. [DOI] [PubMed] [Google Scholar]
- 27.Berger J., Biswas C., Vicario P. P., Strout H. V., Saperstein R., Pilch P. F. 1989. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 340: 70–72. [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Qiang L., Farmer S. R. 2008. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol. Cell. Biol. 28: 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandror K. V., Stephens J. M., Pilch P. F. 1995. Expression and compartmentalization of caveolin in adipose cells: coordinate regulation with and structural segregation from GLUT4. J. Cell Biol. 129: 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parolini I., Sargiacomo M., Galbiati F., Rizzo G., Grignani F., Engelman J. A., Okamoto T., Ikezu T., Scherer P. E., Mora R., et al. 1999. Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the golgi complex. J. Biol. Chem. 274: 25718–25725. [DOI] [PubMed] [Google Scholar]
- 31.Pilch P. F. 2008. The mass action hypothesis: formation of Glut4 storage vesicles, a tissue-specific, regulated exocytic compartment. Acta Physiol. (Oxf.). 192: 89–101. [DOI] [PubMed] [Google Scholar]
- 32.Brasaemle D. L. 2007. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559. [DOI] [PubMed] [Google Scholar]
- 33.Ducharme N. A., Bickel P. E. 2008. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 149: 942–949. [DOI] [PubMed] [Google Scholar]
- 34.Stralfors P. 1990. Autolysis of isolated adipocytes by endogenously produced fatty acids. FEBS Lett. 263: 153–154. [DOI] [PubMed] [Google Scholar]
- 35.Cohen A. W., Razani B., Schubert W., Williams T. M., Wang X. B., Iyengar P., Brasaemle D. L., Scherer P. E., Lisanti M. P. 2004. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 53: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 36.Aboulaich N., Chui P. C., Asara J. M., Flier J. S., Maratos-Flier E. 2011. Polymerase I and transcript release factor regulates lipolysis via a phosphorylation-dependent mechanism. Diabetes. 60: 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen C. G., Bright N. A., Howard G., Nichols B. J. 2009. SDPR induces membrane curvature and functions in the formation of caveolae. Nat. Cell Biol. 11: 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon K. A., Zajicek H., Li W. P., Peyton M. J., Minna J. D., Hernandez V. J., Luby-Phelps K., Anderson R. G. 2009. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 28: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietl P., Haller T. 2005. Exocytosis of lung surfactant: from the secretory vesicle to the air-liquid interface. Annu. Rev. Physiol. 67: 595–621. [DOI] [PubMed] [Google Scholar]
- 40.Brasaemle D. L., Dolios G., Shapiro L., Wang R. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279: 46835–46842. [DOI] [PubMed] [Google Scholar]
- 41.Le Lay S., Briand N., Blouin C. M., Chateau D., Prado C., Lasnier F., Le Liepvre X., Hajduch E., Dugail I. 2010. The lipoatrophic caveolin-1 deficient mouse model reveals autophagy in mature adipocytes. Autophagy. 6: 754–763. [DOI] [PubMed] [Google Scholar]
- 42.Pilch P.F., Liu L. Fat caves: caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol. Metab. Epub ahead of print. May 16, 2011; doi:10.1016/j.tem.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]