Abstract
Human data suggest that reconstituted HDL (rHDL) infusion can induce atherosclerosis regression. Studies in mice indicated that rHDL infusion adversely affects VLDL levels, but this effect is less apparent in humans. This discrepancy may be explained by the fact that humans, in contrast to mice, express cholesteryl ester transfer protein (CETP). The aim of this study was to investigate the role of CETP in the effects of rHDL on VLDL metabolism by using APOE*3-Leiden (E3L) mice, a well-established model for human-like lipoprotein metabolism. At 1 h after injection, rHDL increased plasma VLDL-C and TG in E3L mice, but not in E3L mice cross-bred onto a human CETP background (E3L.CETP mice). This initial raise in VLDL, caused by competition between rHDL and VLDL for LPL-mediated TG hydrolysis, was thus prevented by CETP. At 24 h after injection, rHDL caused a second increase in VLDL-C and TG in E3L mice, whereas rHDL had even decreased VLDL in E3L.CETP mice. This secondary raise in VLDL was due to increased hepatic VLDL-TG production. Collectively, we conclude that CETP protects against the rHDL-induced increase in VLDL. We anticipate that studies evaluating the anti-atherosclerotic efficacy of rHDL in mice that are naturally deficient for CETP should be interpreted with caution, and that treatment of atherogenic dyslipidemia by rHDL should not be combined with agents that aggressively reduce CETP activity.
Keywords: cholesterol, transgenic mice, triglycerides, very low density lipoprotein production, cholesteryl ester transfer protein, high density lipoprotein
Dyslipidemia is an important risk factor for cardiovascular disease (CVD). Current treatment mainly focuses on lowering of LDL-cholesterol (LDL-C), e.g., by statins. LDL-C-lowering treatment results in a significant reduction in the morbidity and mortality of CVD, but it cannot prevent the majority of cardiovascular events (1, 2). Prospective epidemiological studies have demonstrated a strong inverse correlation between HDL-cholesterol (HDL-C) and CVD (3), and recent studies revealed that high HDL-C levels are indeed protective against plaque progression (4). Although the exact mechanisms by which HDL protects are unclear, HDL has been shown to have antioxidant, antithrombotic and anti-inflammatory properties, and to mediate reverse cholesterol transport (RCT) via the hepatobiliary route (5). Therefore, new strategies to raise HDL-C are currently being developed to prevent and treat CVD.
Various therapeutic strategies are currently under development to raise HDL levels, including cholesteryl ester transfer protein (CETP) inhibition, niacin, upregulation of apoAI expression, and infusion of apoAI mimetics or reconstituted HDL (rHDL) (6). Although still in early stage of development, infusion of rHDL seems to be a promising strategy for the treatment of CVD. Recent reviews have demonstrated that infusion of rHDL improves atherosclerotic plaque characteristics both in animal models and humans (7–9). For example, rHDL, composed of recombinant human apoAIMilano and phosphatidylcholine, rapidly mobilized tissue cholesterol and reduced the lipid and macrophage content of atherosclerotic plaques after a single injection into apoE-deficient mice (10). Moreover, it prevented the progression of aortic atherosclerosis as well as promoted the stabilization of plaques after six weeks of administration (11). Recent clinical trials assessed the effect of rHDL that consisted of human apoAI and phosphatidylcholine (CSL-111) as a potential HDL-raising therapeutic strategy. Short-term infusion of CSL-111 significantly improved the plaque characterization index and coronary score on quantitative coronary angiography (12). In addition, a single dose of rHDL led to acute changes in plaque characteristics with a reduction in lipid content, macrophage size, and inflammatory mediators (13).
Although rHDL seems to beneficially modulate atherosclerosis in mice and humans, differences have been observed with respect to modulation of VLDL levels. Infusion of rHDL into apoE-deficient mice increased (V)LDL-C in both acute and chronic studies (10, 11), whereas rHDL did not adversely affect (V)LDL-C in clinical studies (12, 13). This discrepancy may be explained by the fact that, in contrast to mice (14), humans express CETP (15), a crucial factor involved in the metabolism of both (V)LDL and HDL by mediating the transfer of triglycerides (TG) and cholesteryl esters (CE) between these lipoproteins. Therefore, the aim of this study was to elucidate the role of CETP in the effect of rHDL on VLDL metabolism. We used APOE*3-Leiden (E3L) transgenic mice, a unique model for human-like lipoprotein metabolism, which had been crossbred with mice expressing human CETP under control of its natural flanking regions (16), resulting in E3L.CETP mice. This allows distinguishing between the effect of rHDL administration on VLDL metabolism in the absence and presence of CETP-mediated lipid transfer.
MATERIALS AND METHODS
Animals
Hemizygous human CETP transgenic (CETP) mice, expressing human CETP under the control of its natural flanking regions (16), were purchased from the Jackson Laboratory (Bar Harbor, ME) and crossbred with hemizygous E3L mice (17) at our Institutional Animal Facility to obtain E3L.CETP mice (18). In this study, female mice were used, housed under standard conditions in conventional cages with free access to food and water. At the age of 12 weeks, mice were fed a semisynthetic Western-type diet, containing 1% (w/w) corn oil and 15% (w/w) cacao butter (Hope Farms, Woerden, The Netherlands) with 0.25% (w/w) cholesterol (E3L mice) or 0.1% (w/w) cholesterol (E3L.CETP mice) for three weeks, aimed at yielding comparable VLDL levels between both mouse genotypes. Upon randomization according to total plasma cholesterol (TC) and TG levels, mice received a single intravenous injection of rHDL (CSL-111; CSL Behring AG, Bern, Switzerland) (250 mg/kg in 250 µl PBS) or vehicle. Experiments were performed after 4 h of fasting at 12:00 PM with food withdrawn at 8:00 AM. The institutional Ethical Committee on Animal Care and Experimentation approved all experiments.
Reconstituted HDL
rHDL (CSL-111) consists of apoAI isolated from human plasma and phosphatidylcholine from soybean with a molar ratio of 1:150. Before infusion, rHDL was reconstituted with 50 ml of sterile water, yielding 62.5 ml of clear, pale-yellow solution, pH 7.5, and 10% (w/v) sucrose as a stabilizing agent. The final apoAI and PL concentrations were 20 and 86 mg/ml, respectively.
Plasma lipid and lipoprotein analysis
Plasma was obtained via tail vein bleeding and assayed for TC, TG, and phospholipids using the commercially available enzymatic kits 236691, 11488872 (Roche Molecular Biochemicals, Indianapolis, IN) and phospholipids B (Wako Chemicals, Neuss, Germany), respectively. The distribution of lipids over plasma lipoproteins was determined using fast protein liquid chromatography (FPLC). Plasma was pooled per group, and then 50 μl of each pool was injected onto a Superose 6 PC 3.2/30 column (Äkta System, Amersham Pharmacia Biotech, Piscataway, NJ) and eluted at a constant flow rate of 50 μl/min in PBS, 1 mM EDTA, pH 7.4. Fractions of 50 μl were collected and assayed for TC, TG, and phospholipid as described above.
Plasma human apoAI concentration
Plasma human apoAI concentrations were determined using a sandwich ELISA. Goat anti-human apoAI antibody (Academy Biomedical Co., Inc., Houston, TX; 11A-G2b) was coated overnight onto Costar medium binding plate (Costar, Inc., New York, NY) (3 µg/ml) at 4°C and incubated with diluted mouse plasma (dilution, 1:100,000) for 2 h at 37°C. Subsequently, horseradish peroxidase-conjugated goat antihuman apoAI (Academy Biomedical; 11H-G1b) was added and incubated for 2 h at 37°C. Horseradish peroxidase was detected by incubation with tetramethylbenzidine (Organon Teknika, Boxtel, The Netherlands) for 15 min at room temperature. Human apoAI (Academy Biomedical; 11P-101) was used as a standard.
In vivo clearance of VLDL-like emulsions
Glycerol tri[3H]oleate- and [1α,2α(n)-14C]cholesteryl oleate-double labeled VLDL-like emulsion particles (80 nm) were prepared as described by Rensen et al. (19). In short, radiolabeled emulsions were obtained by adding 200 µCi of glycerol tri[3H]oleate and 20 µCi of [14C]cholesteryl oleate to 100 mg of emulsion lipids before sonication (isotopes obtained from GE Healthcare, Little Chalfont, UK). Mice were fasted for 4 h, sedated with 6.25 mg/kg acepromazine (Alfasan), 6.25 mg/kg midazolam (Roche), and 0.3125 mg/kg fentanyl (Janssen-Cilag), and injected with the radiolabeled emulsion particles (0.15 mg TG in 200 µl PBS) via the tail vein. At indicated time points after injection, blood was taken from the tail vein to determine the serum decay of glycerol tri[3H]oleate and 20 µCi of [14C]cholesteryl oleate.
In vitro LPL activity assay
The effect of rHDL on LPL activity was determined essentially as described (20). First, glycerol tri[3H]oleate-labeled VLDL-like emulsion particles (200 µg of TG, corresponding to a final concentration of 0.5 mg/ml), prepared as described above, were added to the indicated amounts of rHDL (or vehicle containing sucrose or sodium cholate only) and heat-inactivated human serum (20 µl, corresponding to a final concentration of 5% v/v) in a total volume of 75 µl of phosphate-buffered saline. Subsequently, 0.1 M Tris HCl (pH 8.5) was added to a total volume of 200 µl, and incubation mixtures were equilibrated at 37°C. At t = 0, bovine LPL (final concentration 3.5 U/ml, Sigma) in 200 µl of 120 mg/ml free fatty acid-free BSA (Sigma), corresponding with a final concentration of 60 mg/ml, was added (37°C). At t = 15, 30, 60, 90, and 120 min, [3H]oleate generated during lipolysis by LPL was extracted. Hereto, 50 µl samples were added to 1.5 ml extraction liquid (CH3OH: CHCl3: heptane: oleic acid (1,410: 1,250: 1,000: 1, v/v/v/v). Samples were mixed, and 0.5 ml of 0.2 M NaOH was added. Following vigorous mixing and centrifugation (10 min at 1,000 g), 3H radioactivity in 0.5 ml of the aqueous phase was counted. After taking the last samples, 50 µl of the incubations were also directly counted, representing the total amount of radioactivity in the assay. Lipolysis rate (i.e., LPL activity) was calculated by linear regression between incubation time and percentage of [3H]oleate released.
Hepatic VLDL-TG and VLDL-apoB production
Mice were fasted for 4 h, with food withdrawn at 8:00 AM, prior to the start of the experiment. During the experiment, mice were sedated as described above. At t = 0 min, blood was taken via tail bleeding and mice were intravenously injected with 100 µl PBS containing 100 µCi Trans35S label to measure de novo total apoB synthesis. After 30 min, the animals received 500 mg of tyloxapol (Triton WR-1339, Sigma-Aldrich) per kg body weight as a 10% (w/w) solution in sterile saline to prevent systemic lipolysis of newly secreted hepatic VLDL-TG (21). Additional blood samples were taken at 15, 30, 60, and 90 min after tyloxapol injection and used for determination of plasma TG concentration. At 120 min, the animals were euthanized, and blood was collected by orbital puncture for isolation of VLDL by density gradient ultracentrifugation. 35S-apoB was measured in the VLDL fraction, and VLDL-apoB production rate was shown as dpm.h−1 (22).
Statistical analysis
All data are presented as means ± SD. Data were analyzed using the unpaired Student's t-test. P values less than 0.05 were considered statistically significant.
RESULTS
Infusion of rHDL transiently increases plasma apoAI and phospholipid levels in both E3L and E3L.CETP mice
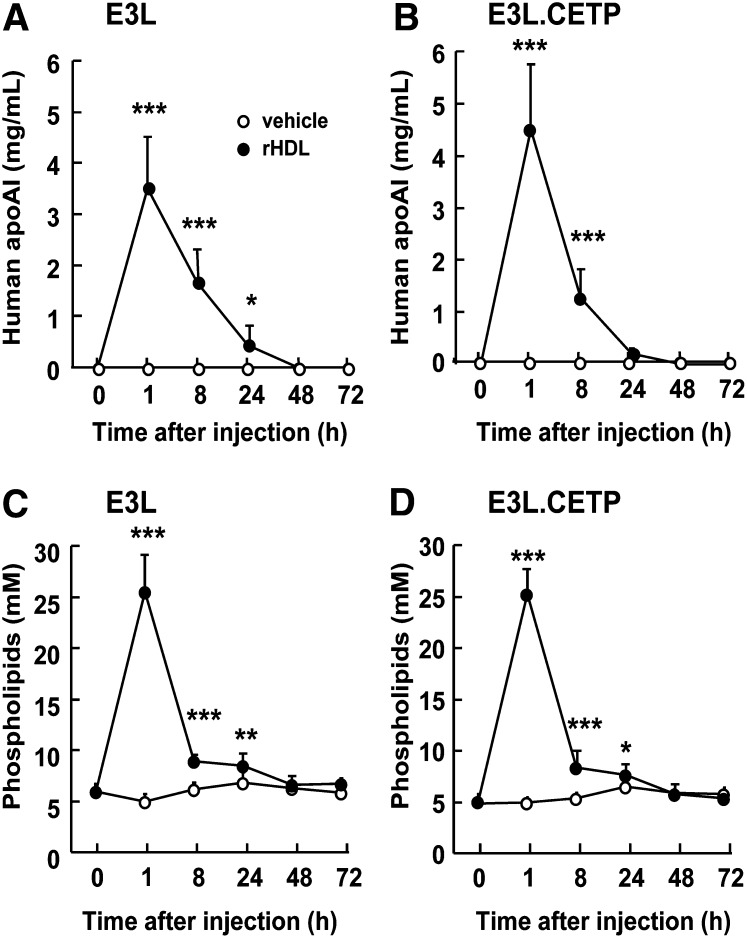
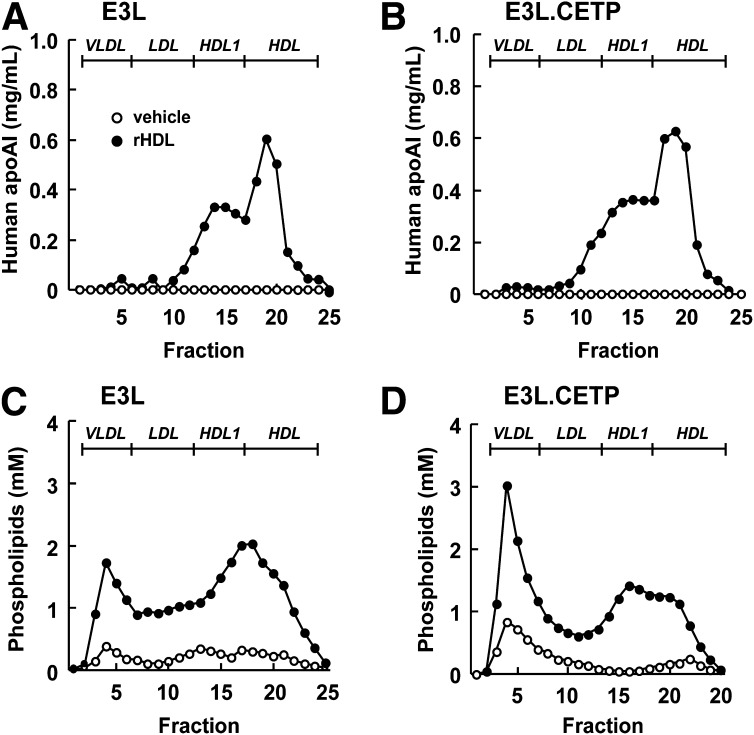
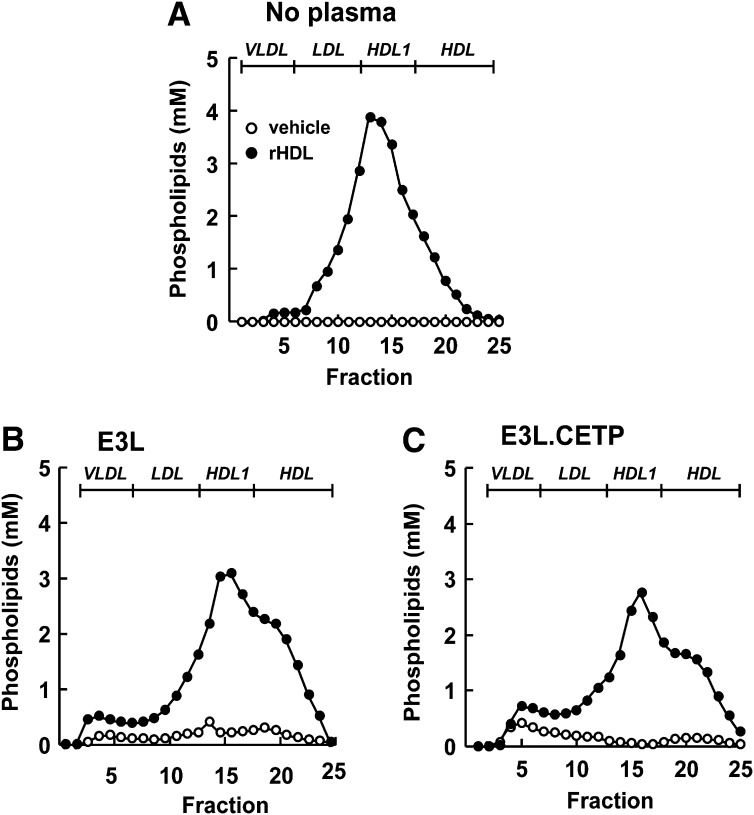
To investigate the role of CETP in the effect of rHDL infusion on VLDL metabolism, female E3L mice with or without human CETP expression received a single intravenous injection of rHDL. To assess the kinetics of rHDL that consists of human apoAI and PL, plasma levels of human apoAI and phospholipid was determined over time (Fig. 1) rHDL caused a transient increase in plasma human apoAI and phospholipid levels in both E3L mice (Fig. 1A, C) and E3L.CETP mice (Fig. 1B, D). Human apoAI and phospholipid were cleared at a similar rate and disappeared from plasma after approximately 24 h. At 1 h after injection, lipoproteins in plasma were separated, and the distribution of human apoAI and phospholipid was determined (Fig. 2). rHDL appeared to integrate into the endogenous HDL pool in both E3L and E3L.CETP mice, as both human apoAI (Fig. 2A, B) and phospholipid (Fig. 2C, D) eluted in fractions representing HDL. In addition, phospholipid derived from rHDL selectively integrated into (V)LDL fractions (Fig. 2C, D). The presence of rHDL-phospholipid in (V)LDL is not due to the presence of large rHDL aggregates that would elute in the void volume, since apoAI is not detected in the void volume (Fig. 3A); rather, it is explained by a time-dependent transfer of phospholipid to endogenous VLDL as evident from incubation of rHDL with plasma from E3L mice (Fig. 3B) and E3L.CETP mice (Fig. 3C) in vitro.
Fig. 1.
Effect of rHDL on plasma human apoAI and phospholipids in E3L and E3L.CETP mice. E3L (A, C) and E3L.CETP (B, D) mice were fed a Western-type diet for three weeks. Subsequently, they received a single intravenous injection of rHDL (250 mg/kg in 250 µl PBS) or vehicle. Blood was drawn at the indicated time points, and plasma was assayed for human apoAI (A, B) and phospholipids (C, D). Values are means ± SD (n = 8-10); *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control group.
Fig. 2.
Effect of rHDL on the lipoprotein distribution of human apoAI and phospholipids at 1 h after injection in E3L and E3L.CETP mice. E3L (A, C) and E3L.CETP (B, D) mice were fed a Western-type diet for three weeks. Subsequently, they received a single intravenous injection of rHDL (250 mg/kg in 250 µl PBS) or vehicle. After 1 h, blood was drawn, and plasma was pooled per group (n = 8-10). Pooled plasma was fractionated using FPLC on a Superose 6 column, and the individual fractions were assayed for human apoAI (A, B) and phospholipids (C, D).
Fig. 3.
Effect of in vitro incubation of rHDL with E3L and E3L.CETP mouse plasma on phospholipid distribution. E3L and E3L.CETP mice were fed a Western-type diet for three weeks, and fresh plasma was collected. rHDL was incubated (1 h at 37°C) without mouse plasma (A) or with plasma of E3L mice (B) or E3L.CETP mice (C). Samples were pooled per group (n = 8-10) and fractionated using FPLC on a Superose 6 column, and the individual fractions were assayed for phospholipids.
Infusion of rHDL affects plasma levels of endogenous lipids differentially in E3L and E3L.CETP mice
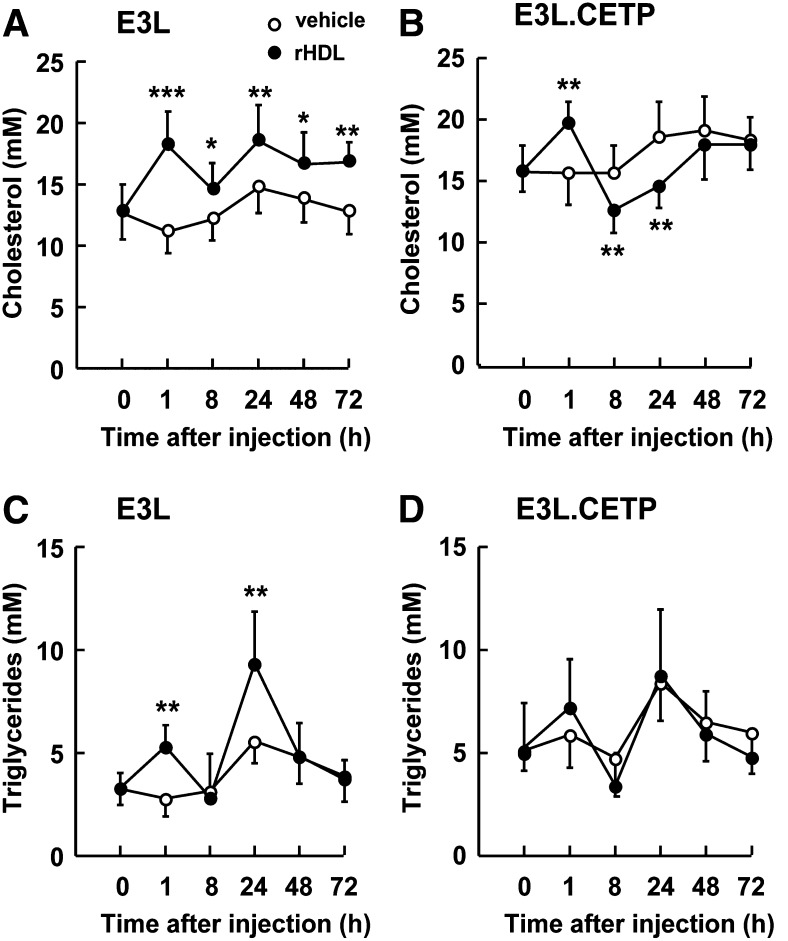
Although rHDL was cleared at a similar rate in E3L and E3L.CETP mice, its effects on endogenous plasma levels of cholesterol and TG were clearly different in both mouse types (Fig. 4). At 1 h after injection, rHDL significantly increased plasma cholesterol in both E3L mice (+63%; P < 0.001) (Fig. 4A) and E3L.CETP mice (+28%; P < 0.01) (Fig. 4B). However, at 24 h after injection, rHDL still significantly increased plasma cholesterol in E3L mice (+26%, P < 0.01) (Fig. 4A) but actually decreased plasma cholesterol in E3L.CETP mice (−22%, P < 0.01) (Fig. 4B). In addition, whereas rHDL caused a significant increase in plasma TG levels in E3L mice at both 1 h (+89%; P < 0.01) and 24 h after injection (+67%; P < 0.01) (Fig. 4C), rHDL did not significantly increase plasma TG at any time point in E3L.CETP mice (Fig. 4D).
Fig. 4.
Effect of rHDL on plasma cholesterol and triglycerides in E3L and E3L.CETP mice. E3L (A, C) and E3L.CETP (B, D) mice were fed a Western-type diet for three weeks. Subsequently, they received a single intravenous injection of rHDL (250 mg/kg in 250 µl PBS) or vehicle. Blood was drawn at the indicated time points, and plasma was assayed for total cholesterol (A, B) and triglycerides (C, D). Values are means ± SD (n = 8-10); *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control group.
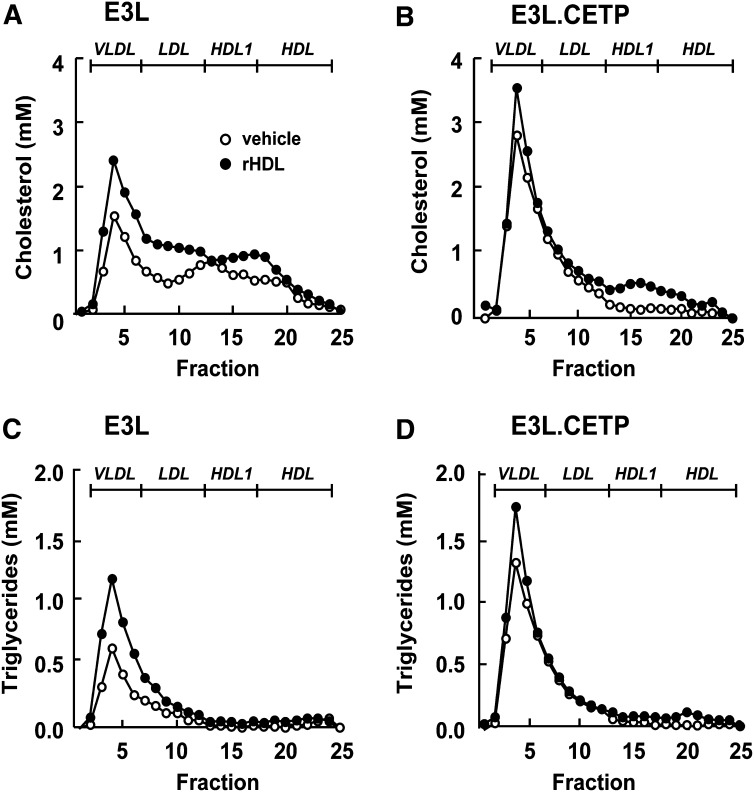
At short term, rHDL raises HDL-C in E3L and E3L.CETP mice, and increases VLDL mainly in E3L mice
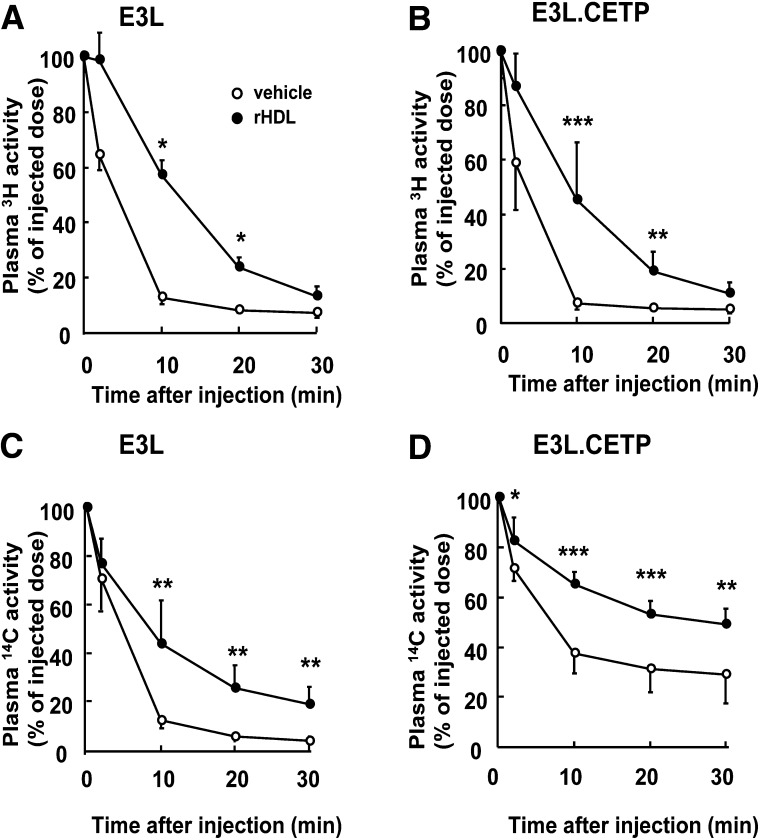
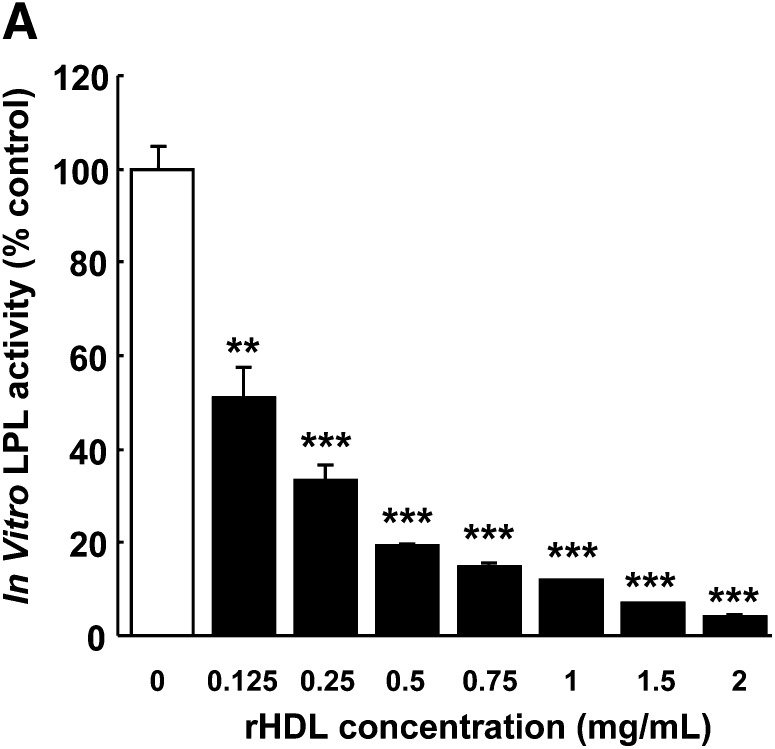
To investigate the mechanism underlying the early effects of rHDL infusion on plasma lipids, plasma was obtained at 1 h after injection, and lipoproteins were fractionated by FPLC (Fig. 5). rHDL increased HDL-C in both E3L mice (Fig. 5A) and E3L.CETP mice (Fig. 5B), indicating that rHDL induces a rapid cholesterol efflux from peripheral tissues into plasma. In addition, rHDL markedly increased VLDL-C (Fig. 5A) and VLDL-TG (Fig. 5C) in E3L mice, while its VLDL-increasing effect was only modest in E3L.CETP mice (Fig. 5B, D). To investigate whether the raise in VLDL was due to competition between rHDL and VLDL for binding and subsequent TG hydrolysis by LPL, we assessed the effect of rHDL on the plasma kinetics of intravenously injected glycerol tri[3H]oleate [14C]cholesteryl oleate double-labeled VLDL-like emulsion particles (Fig. 6). Indeed, rHDL decreased the plasma clearance of the VLDL-like emulsion particles, including glycerol tri[3H]oleate and [14C]cholesteryl oleate, in both E3L mice (Fig. 6A, C) and E3L.CETP mice (Fig. 6B, D). An in vitro LPL activity assay confirmed that rHDL dose-dependently decreased LPL-mediated lipolysis of VLDL-like emulsion particles (Fig. 7), whereas sucrose and sodium cholate at amounts present at the various rHDL concentrations did not (data not shown). These data indicate that rHDL competes for the binding of VLDL-like emulsion particles with LPL in both E3L and E3L.CETP mice, resulting in delayed clearance of TG-derived fatty acids (i.e., 3H-activity) as well as the resulting core remnants (i.e., 14C-activity). The fact that rHDL does not substantially raise VLDL levels in E3L.CETP mice is probably related to rapid remodeling of VLDL by CETP.
Fig. 5.
Effect of rHDL on lipoprotein distribution of cholesterol and triglycerides at 1 h after injection in E3L and E3L.CETP mice. E3L (A, C) and E3L.CETP (B, D) mice were fed a Western-type diet for three weeks. Subsequently, they received a single intravenous injection of rHDL (250 mg/kg in 250 µl PBS) or vehicle. Blood was drawn, and plasma was pooled per group (n = 8-10). Pooled plasma was fractionated using FPLC on a Superose 6 column, and the individual fractions were assayed for total cholesterol (A, B) and triglycerides (C, D).
Fig. 6.
Effect of rHDL on the plasma clearance of VLDL-like emulsion particles in E3L and E3L.CETP mice. E3L (A, C) and E3L.CETP (B, D) mice were fed a Western-type diet for three weeks, and they received a single intravenous injection of rHDL (250 mg/kg in 200 µl PBS) or vehicle. After 1 min, mice were intravenously injected with glycerol tri[3H]oleate- and [14C]cholesteryl oleate-double labeled VLDL-like emulsion particles (0.15 mg TG in 200 µl PBS). Blood was drawn at the indicated time points, and 3H and 14C-activity was determined. Values are means ± SD (n = 8); *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control group.
Fig. 7.
Effect of rHDL on in vitro LPL activity. Glycerol tri[3H]oleate-labeled VLDL-like emulsion particles were incubated at 37°C with bovine LPL (3.5 U/ml) in 0.1 M Tris HCl (pH 8.5) in the presence of heat-inactivated human serum (5%, v/v) and free fatty acid-free BSA (60 mg/ml). [3H]oleate generated during lipolysis was extracted after 15, 30, 60, 90, and 120 min of incubation. The lipolysis rate (i.e., LPL activity) was calculated by the linear regression between incubation time and percentage of [3H]oleate generated. Values are means ± SD (n = 3); **P < 0.01, ***P < 0.001 compared with control incubations containing vehicle.
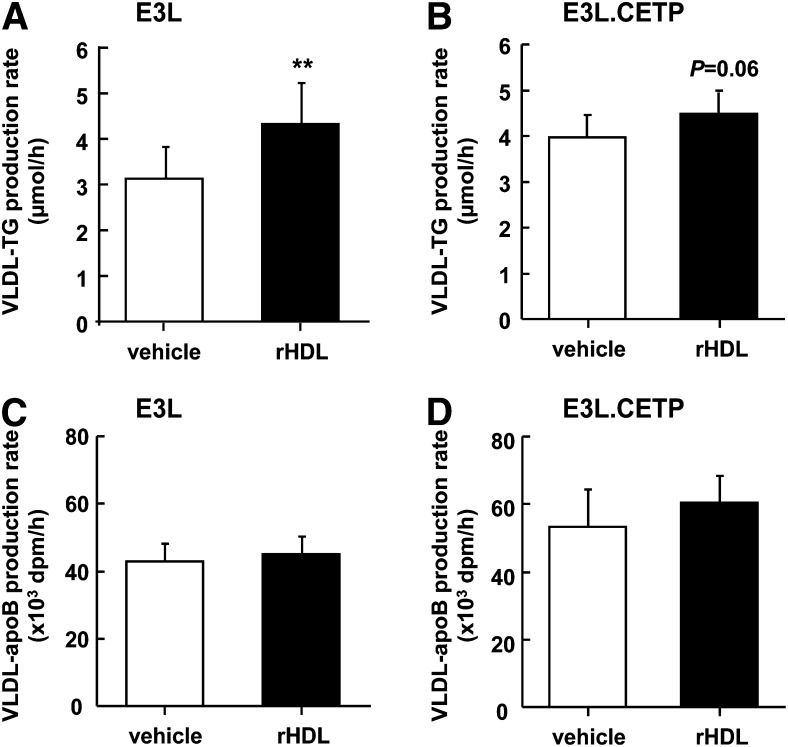
At long term, rHDL raises VLDL in E3L mice and decreases VLDL in E3L.CETP mice
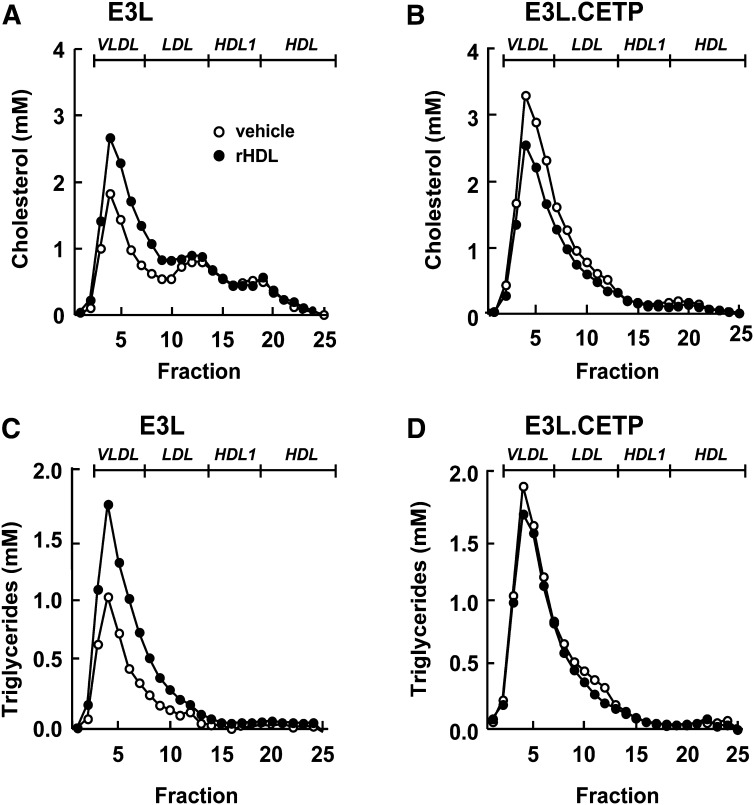
To determine the mechanism underlying the divergent long-term effects of rHDL, infusion on plasma was also obtained 24 h after administration, and lipoproteins were fractionated by FPLC (Fig. 8). In both E3L and E3L.CETP mice, the effect of rHDL on increasing HDL-C levels had disappeared (Fig. 8A, B). However, whereas rHDL still significantly raised VLDL-C (+60%) (Fig. 8A) and VLDL-TG (+86%) (Fig. 8C) in E3L mice, rHDL actually decreased VLDL-C (−25%) in E3L.CETP mice (Fig. 8B). Since it has been shown that increasing the flux of HDL to the liver can increase the availability of substrate for hepatic VLDL synthesis and subsequently VLDL-TG secretion (23), we speculated that rHDL may have increased the VLDL production. Therefore, the effect of rHDL on VLDL production was evaluated after injection of Triton WR1339 (tyloxapol) to block LPL-mediated lipolysis (Fig. 9). Indeed, at 24 h after administration of rHDL, the VLDL-TG production rate was increased in E3L mice (+36%; P < 0.01) (Fig. 9A). ApoB production was not affected (Fig. 9C), indicating that rHDL increases lipidation of VLDL particles rather than increasing the VLDL particle secretion rate. Likewise, rHDL tended to increase the VLDL-TG production rate (Fig. 9B) without affecting the apoB production rate (Fig. 9D) in E3L.CETP mice.
Fig. 8.
Effect of rHDL on lipoprotein distribution of cholesterol and triglycerides at 24 h after injection in E3L and E3L.CETP mice. E3L (A, C) and E3L.CETP (B, D) mice were fed a Western-type diet for three weeks. Subsequently, they received a single intravenous injection of rHDL (250 mg/kg in 250 µl PBS) or vehicle. Blood was drawn, and plasma was pooled per group (n = 8-10). Pooled plasma was fractionated using FPLC on a Superose 6 column, and the individual fractions were assayed for total cholesterol (A, B) and triglycerides (C, D).
Fig. 9.
Effect of rHDL on the hepatic VLDL-TG production at 24 h after injection in E3L and E3L.CETP mice. E3L (A, C) and E3L.CETP (B, D) mice were fed a Western-type diet for three weeks, and they received a single intravenous injection of rHDL (250 mg/kg in 250 µl PBS) or vehicle. At 24 h after rHDL or vehicle injection, mice were injected with Trans35S label and tyloxapol to block VLDL-TG clearance. Blood was drawn at the indicated time points, and plasma TG concentrations were determined. VLDL-TG production rate was calculated from the slopes of the TG-time curves from the individual mice (A, B). At 120 after tyloxapol injection, mice were exsanguinated, and VLDL was isolated by ultracentrifugation. 35S-activity was determined, and VLDL-apoB production rate was calculated as dpm.h−1 (C, D). Values are means ± SD (n = 7-11); **P < 0.01 compared with the control group.
DISCUSSION
In this study, we investigated the role of CETP in the effect of rHDL on VLDL metabolism by using E3L mice with or without human CETP expression. In both E3L and E3L.CETP mice, rHDL caused a similar transient increase in plasma human apoAI and phospholipid levels, and it induced a transient increase in the endogenous HDL-C pool. However, rHDL caused an increase in VLDL in E3L mice, at both 1 h and 24 h after injection, which was prevented by CETP expression in E3L.CETP mice.
We observed that rHDL caused a rapid increase in VLDL-TC and VLDL-TG in E3L mice within 1 h after administration. This finding is in line with previous observations showing that rHDL, composed of human apoAIMilano and PL, also increased the VLDL-TC pool in apoE-deficient mice at 1 h after injection (10). These effects cannot be explained simply by transfer of lipid compounds from rHDL to VLDL, since rHDL does not contain cholesterol or triglycerides. Rather, we speculated that infusion of a substantial amount of rHDL may interfere with endogenous VLDL catabolism. Indeed, rHDL decreased the plasma clearance of VLDL-like emulsion particles, including the clearance of both glycerol tri[3H]oleate and [14C]cholesteryl oleate. Apparently, rHDL competes with endogenous VLDL for binding to triacylglycerol hydrolases, resulting in impaired hydrolysis of TG within VLDL. Indeed, rHDL dose-dependently inhibited LPL activity in an in vitro assay. At an rHDL concentration of 0.625 mg/ml, resulting in an rHDL:TG ratio similar to the in vivo situation, rHDL inhibited LPL activity by as much as 80%. Sucrose and sodium cholate, both present in rHDL, did not inhibit LPL activity in vitro and are thus unlikely to inhibit LPL in vivo. As a consequence of LPL inhibition by rHDL in vivo, the clearance of core remnants is attenuated, and plasma VLDL levels are increased. Our finding that normalization of elevated phospholipid levels at 8 h after injection also normalized plasma TG levels in E3L mice is consistent with such a mechanism.
In addition to increasing VLDL at 1 h after injection, rHDL caused a second raise in VLDL-TC and VLDL-TG in E3L mice at 24 h after administration. Because competition of rHDL for VLDL clearance mechanisms can be excluded at this time point because rHDL has been cleared from the circulation, we hypothesized that rHDL may have caused an increase in hepatic VLDL production. Indeed, we observed that rHDL increased the production rate of VLDL-TG without affecting the production rate of VLDL-apoB. Since each VLDL particle contains a single molecule of apoB, this indicates that rHDL infusion increases the lipidation of hepatic apoB rather than increasing the number of VLDL particles produced. Interestingly, it has been observed previously that increasing the flux of HDL to the liver by hepatic overexpression of scavenger receptor class B member 1 also increases the VLDL-TG production rate, and that HDL-derived cholesterol can be resecreted from the liver within VLDL particles (23). Therefore, we postulate that, in a similar manner, infusion of rHDL causes an increased net flux of lipids to the liver. We showed that rHDL transiently enhanced HDL-C, which may at least partly be attributed by efflux of cholesterol from peripheral tissues. This increased HDL-C may subsequently be taken up by the liver via SR-BI, which could then be resecreted within VLDL. However, it is even more likely that a high flux of rHDL-associated phospholipid to the liver enhances the amount of hepatic TG available for secretion as VLDL-TG. Indeed, it has been demonstrated that HDL-associated phosphatidylcholine that has been taken up by hepatocytes is converted to TG after phosphatidylcholine-phospholipase C-mediated hydrolysis of phosphatidylcholine, resulting in diacylglycerol that is subsequently converted into TG by DGAT2 (24).
Although rHDL infusion caused a clear hyperlipidemic side effect in E3L mice, there was no increase in VLDL in E3L.CETP mice at any time point, although rHDL induced a significant decrease of VLDL clearance at short term and tended to increase VLDL production at long term in E3L.CETP mice, similar to E3L mice. Expression of CETP thus clearly prevents the adverse effects of rHDL on VLDL levels. These data are in line with various human studies in which infusion of rHDL was used as an experimental treatment of CVD (12), diabetes (25), and inflammation (26). In these clinical studies, no specific adverse VLDL-increasing effects were reported, which is in line with our data that human CETP may protect against rHDL-induced elevation of VLDL.
It is interesting to speculate on the mechanism(s) underlying the protective effect of CETP on the rHDL-induced raise in VLDL. It is well known that CETP mediates the transfer of CE from HDL particles to LDL and VLDL particles in exchange for TG, and that this reciprocal neutral lipid transfer approaches equilibrium under physiological conditions. However, under conditions of increased VLDL levels as observed at 1 and 24 h after administration of rHDL to E3L mice, the increase in VLDL results in elevated acceptor activity for CETP, which would result in an increased net rate of TG transfer from VLDL particles to both HDL and LDL particles (27). Both TG-enriched HDL and LDL particles are avidly bound to hepatic lipase (HL) that effectively hydrolyzes TG (as well as phospholipid) to form small, dense LDL and HDL, respectively (28), thereby effectively eliminating TG from plasma.
On the basis of our data and literature studies, we propose the following mechanism by which CETP has a protective effect on rHDL-induced increase in VLDL. Infusion of rHDL initially decreases VLDL clearance via blocking of LPL-mediated lipolysis and, at a later stage, increases VLDL production via HDL-mediated delivery of lipids to the liver, both of which increase plasma VLDL levels. The transient accumulation of VLDL particles leads to an increase of CETP activity, resulting in an accelerated transfer of TG from VLDL to HDL and LDL, in which TG is hydrolyzed quickly through the action of HL.
Since our primary research question was to examine the effect of CETP on the rHDL-induced increase on VLDL metabolism, we used saline as a control for rHDL treatment as similarly applied in clinical trials. Note that such a study setup does not allow evaluating the individual contributions of apoAI versus lipids. As the cholesterol-efflux properties of rHDL could largely depend on the apoAI moiety, it would be interesting to investigate in future studies the effect of rHDL compared with apoAI-free lipid micelles on cholesterol mobilization into plasma as well as VLDL metabolism.
In addition to rHDL infusion therapy, other strategies to raise HDL-C are currently being developed to prevent and treat CVD, alone or combined with LDL-lowering drugs, among which are CETP inhibitors. The first CETP inhibitor, torcetrapib, increased HDL-C levels by approximately 60% (29), but it failed to demonstrate any effect on the primary atherosclerotic burden as assessed by carotid intima-media thickness and coronary intravascular ultrasound imaging (30, 31), and it even increased cardiovascular events and mortality, accompanied by off-target effects (32). However, other CETP inhibitors, such as dalcetrapib (33) and anacetrapib (34), have now progressed into phase III clinical trials without showing any off-target toxicity. Additionally, niacin effectively increases HDL by 25-30% (35) and improves carotid intima-media thickness (36). We recently showed that the HDL-raising effect of niacin is caused by reducing the hepatic CETP expression and plasma CETP protein (37). Since the HDL-raising effects of CETP inhibitors and niacin both depend on reducing CETP activity in plasma, whereas CETP activity now appears crucial to prevent the raise in VLDL as induced by rHDL infusion, combining rHDL with these HDL-raising agents could reveal adverse VLDL effects and, as a consequence, counteract the potentially protective effect of rHDL in CVD.
In conclusion, our results show that rHDL infusion induces an increase in VLDL levels, which is prevented by CETP expression. Therefore, studies that evaluate the anti-atherosclerotic efficacy of rHDL in mouse models that are naturally deficient for CETP should be interpreted with caution, and treatment of atherogenic dyslipidemia as a risk factor for CVD by rHDL should not be combined with agents that aggressively reduce CETP activity.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- CVD
- cardiovascular disease
- E3L
- APOE*3-Leiden
- HDL-C
- HDL-cholesterol
- rHDL
- reconstituted HDL
- HL
- hepatic lipase
- LDL-C
- LDL-cholesterol
- LXR
- liver X receptor
- PL
- phosphatidylcholine
- RTC
- reverse cholesterol transport
- TC
- total cholesterol
- TG
- triglyceride
REFERENCES
- 1.Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 2.LaRosa J. C., Deedwania P. C., Shepherd J., Wenger N. K., Greten H., DeMicco D. A., Breazna A. 2010. Comparison of 80 versus 10 mg of atorvastatin on occurrence of cardiovascular events after the first event (from the Treating to New Targets [TNT] trial). Am. J. Cardiol. 105: 283–287. [DOI] [PubMed] [Google Scholar]
- 3.Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Jr, Bangdiwala S., Tyroler H. A. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79: 8–15. [DOI] [PubMed] [Google Scholar]
- 4.Johnsen S. H., Mathiesen E. B., Fosse E., Joakimsen O., Stensland-Bugge E., Njolstad I., Arnesen E. 2005. Elevated high-density lipoprotein cholesterol levels are protective against plaque progression: a follow-up study of 1952 persons with carotid atherosclerosis the Tromso study. Circulation. 112: 498–504. [DOI] [PubMed] [Google Scholar]
- 5.Rader D. J., Alexander E. T., Weibel G. L., Billheimer J., Rothblat G. H. 2009. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 50 (Suppl.): S189–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy D., Rader D. J. 2009. Update on strategies to increase HDL quantity and function. Nat. Rev. Cardiol. 6: 455–463. [DOI] [PubMed] [Google Scholar]
- 7.Tardif J. C., Heinonen T., Noble S. 2009. High-density lipoprotein/apolipoprotein A-I infusion therapy. Curr. Atheroscler. Rep. 11: 58–63. [DOI] [PubMed] [Google Scholar]
- 8.Shah P. K. 2007. Apolipoprotein A-I/HDL infusion therapy for plaque stabilization-regression: a novel therapeutic approach. Curr. Pharm. Des. 13: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 9.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290: 2292–2300. [DOI] [PubMed] [Google Scholar]
- 10.Shah P. K., Yano J., Reyes O., Chyu K. Y., Kaul S., Bisgaier C. L., Drake S., Cercek B. 2001. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 103: 3047–3050. [DOI] [PubMed] [Google Scholar]
- 11.Shah P. K., Nilsson J., Kaul S., Fishbein M. C., Ageland H., Hamsten A., Johansson J., Karpe F., Cercek B. 1998. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 97: 780–785. [DOI] [PubMed] [Google Scholar]
- 12.Tardif J. C., Gregoire J., L'Allier P. L., Ibrahim R., Lesperance J., Heinonen T. M., Kouz S., Berry C., Basser R., Lavoie M. A., et al. 2007. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 297: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 13.Shaw J. A., Bobik A., Murphy A., Kanellakis P., Blombery P., Mukhamedova N., Woollard K., Lyon S., Sviridov D., Dart A. M. 2008. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ. Res. 103: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 14.Jiao S., Cole T. G., Kitchens R. T., Pfleger B., Schonfeld G. 1990. Genetic heterogeneity of lipoproteins in inbred strains of mice: analysis by gel-permeation chromatography. Metabolism. 39: 155–160. [DOI] [PubMed] [Google Scholar]
- 15.Ha Y. C., Barter P. J. 1982. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp. Biochem. Physiol. B. 71: 265–269. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X. C., Agellon L. B., Walsh A., Breslow J. L., Tall A. 1992. Dietary cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice. Dependence on natural flanking sequences. J. Clin. Invest. 90: 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Maagdenberg A. M., Hofker M. H., Krimpenfort P. J., de Bruijn I., van Vlijmen B., van der Boom H., Havekes L. M., Frants R. R. 1993. Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J. Biol. Chem. 268: 10540–10545. [PubMed] [Google Scholar]
- 18.Westerterp M., van der Hoogt C. C., de Haan W., Offerman E. H., Dallinga-Thie G. M., Jukema J. W., Havekes L. M., Rensen P. C. 2006. Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden mice. Arterioscler. Thromb. Vasc. Biol. 26: 2552–2559. [DOI] [PubMed] [Google Scholar]
- 19.Rensen P. C., Herijgers N., Netscher M. H., Meskers S. C., van Eck M., van Berkel T. J. 1997. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J. Lipid Res. 38: 1070–1084. [PubMed] [Google Scholar]
- 20.Schaap F. G., Rensen P. C., Voshol P. J., Vrins C., van der Vliet H. N., Chamuleau R. A., Havekes L. M., Groen A. K., van Dijk K. W. 2004. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 279: 27941–27947. [DOI] [PubMed] [Google Scholar]
- 21.Aalto-Setala K., Fisher E. A., Chen X., Chajek-Shaul T., Hayek T., Zechner R., Walsh A., Ramakrishnan R., Ginsberg H. N., Breslow J. L. 1992. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J. Clin. Invest. 90: 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Catalina F., Grundy S. M., Patel S. 1996. Method to measure apolipoprotein B-48 and B-100 secretion rates in an individual mouse: evidence for a very rapid turnover of VLDL and preferential removal of B-48- relative to B-100-containing lipoproteins. J. Lipid Res. 37: 210–220. [PubMed] [Google Scholar]
- 23.Wiersma H., Nijstad N., Gautier T., Iqbal J., Kuipers F., Hussain M. M., Tietge U. J. 2010. Scavenger receptor BI facilitates hepatic very low density lipoprotein production in mice. J. Lipid Res. 51: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robichaud J. C., van der Veen J. N., Yao Z., Trigatti B., Vance D. E. 2009. Hepatic uptake and metabolism of phosphatidylcholine associated with high density lipoproteins. Biochim. Biophys. Acta. 1790: 538–551. [DOI] [PubMed] [Google Scholar]
- 25.Drew B. G., Duffy S. J., Formosa M. F., Natoli A. K., Henstridge D. C., Penfold S. A., Thomas W. G., Mukhamedova N., de Courten B., Forbes J. M., et al. 2009. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 119: 2103–2111. [DOI] [PubMed] [Google Scholar]
- 26.Patel S., Drew B. G., Nakhla S., Duffy S. J., Murphy A. J., Barter P. J., Rye K. A., Chin-Dusting J., Hoang A., Sviridov D., et al. 2009. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J. Am. Coll. Cardiol. 53: 962–971. [DOI] [PubMed] [Google Scholar]
- 27.Guerin M., Egger P., Le G. W., Soudant C., Dupuis R., Chapman M. J. 2002. Atorvastatin reduces postprandial accumulation and cholesteryl ester transfer protein-mediated remodeling of triglyceride-rich lipoprotein subspecies in type IIb hyperlipidemia. J. Clin. Endocrinol. Metab. 87: 4991–5000. [DOI] [PubMed] [Google Scholar]
- 28.Barter P. J., Brewer H. B., Jr, Chapman M. J., Hennekens C. H., Rader D. J., Tall A. R. 2003. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23: 160–167. [DOI] [PubMed] [Google Scholar]
- 29.Nissen S. E., Tardif J. C., Nicholls S. J., Revkin J. H., Shear C. L., Duggan W. T., Ruzyllo W., Bachinsky W. B., Lasala G. P., Tuzcu E. M. 2007. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 356: 1304–1316. [DOI] [PubMed] [Google Scholar]
- 30.Kastelein J. J., van Leuven S. I., Burgess L., Evans G. W., Kuivenhoven J. A., Barter P. J., Revkin J. H., Grobbee D. E., Riley W. A., Shear C. L., et al. 2007. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N. Engl. J. Med. 356: 1620–1630. [DOI] [PubMed] [Google Scholar]
- 31.Vergeer M., Bots M. L., van Leuven S. I., Basart D. C., Sijbrands E. J., Evans G. W., Grobbee D. E., Visseren F. L., Stalenhoef A. F., Stroes E. S., et al. 2008. Cholesteryl ester transfer protein inhibitor torcetrapib and off-target toxicity: a pooled analysis of the rating atherosclerotic disease change by imaging with a new CETP inhibitor (RADIANCE) trials. Circulation. 118: 2515–2522. [DOI] [PubMed] [Google Scholar]
- 32.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 33.Kuivenhoven J. A., de Grooth G. J., Kawamura H., Klerkx A. H., Wilhelm F., Trip M. D., Kastelein J. J. 2005. Effectiveness of inhibition of cholesteryl ester transfer protein by JTT-705 in combination with pravastatin in type II dyslipidemia. Am. J. Cardiol. 95: 1085–1088. [DOI] [PubMed] [Google Scholar]
- 34.Bloomfield D., Carlson G. L., Sapre A., Tribble D., McKenney J. M., Littlejohn T. W., III, Sisk C. M., Mitchel Y., Pasternak R. C. 2009. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am. Heart J. 157: 352–360. [DOI] [PubMed] [Google Scholar]
- 35.Brown B. G., Zhao X. Q. 2008. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am. J. Cardiol. 101: 58B–62B. [DOI] [PubMed] [Google Scholar]
- 36.Thoenes M., Oguchi A., Nagamia S., Vaccari C. S., Hammoud R., Umpierrez G. E., Khan B. V. 2007. The effects of extended-release niacin on carotid intimal media thickness, endothelial function and inflammatory markers in patients with the metabolic syndrome. Int. J. Clin. Pract. 61: 1942–1948. [DOI] [PubMed] [Google Scholar]
- 37.van der Hoorn J. W., de Haan W., Berbee J. F., Havekes L. M., Jukema J. W., Rensen P. C., Princen H. M. 2008. Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arterioscler. Thromb. Vasc. Biol. 28: 2016–2022. [DOI] [PubMed] [Google Scholar]