Abstract
Animal studies have suggested that angiopoietin-like 4 (Angptl4) regulates adiposity through central and peripheral mechanisms. The aim of this study was to investigate whether serum concentration and adipose tissue expression of Angptl4 are associated with obesity-related parameters in humans. Altogether, 75 dizygotic (DZ) and 46 monozygotic (MZ) twin pairs were studied, from the FinnTwin12 and FinnTwin16 cohorts. Among the MZ pairs, 21 were discordant for body mass index (BMI) (intra-pair BMI-difference >2.5 kg/m2, age 23–33 years). Serum Angptl4 (s-Angptl4) levels were measured by ELISA, and adipose tissue gene expression was analyzed by genome-wide transcript profiling. In MZ twin pairs discordant for BMI, s-Angptl4 and adipose tissue ANGPTL4 mRNA (at-ANGPTL4) levels were significantly decreased (P = 0.04 and P = 0.03, respectively) in obese twins as compared with their nonobese cotwins. In all twins, intra-pair differences in s-Angptl4 levels were inversely correlated with intra-pair differences in BMI (r = −0.27, P = 0.003). In individual MZ twins, at-ANGPTL4 expression was inversely correlated with BMI (r = −0.44, P = 0.001) and positively correlated with at-LIPE (r = 0.24, P = 0.01) and at-ABHD5 (r = 0.41, P = 0.005) expression. Our results demonstrated that variation in Angptl4 concentration was only modestly accounted for by genetic factors and suggest a role for Angptl4 in acquired obesity in humans
Keywords: Angptl4, heritability, twin pairs, obesity-discordant monozygotic twins
Angiopoietin-like 4 (Angptl4) is a multifunctional protein involved in plasma triglyceride (TG) metabolism (1), energy metabolism (2, 3), tumor survival (4), metastasis (5, 6), angiogenesis (7, 8), wound healing (9), inflammation (10), and nephrotic syndrome (11). It is expressed in a wide variety of tissues, with highest expression displayed in the liver. Angptl4 is a secretory protein, but a significant amount is retained at the cell surface attached to glycosaminoglycans and can be released after heparin treatment in vitro and in vivo (12, 13). Interaction of Angptl4 with proteoglycans protects it from proteolysis by proprotein convertases (14). Secreted Angptl4 is cleaved at a specific site, generating the N-terminal and C-terminal fragments that together with the full-length protein, can be detected in plasma associated with different lipoprotein classes (15).
The most-characterized function of Angptl4 is the inhibition of lipoprotein lipase (LPL) (16), an effect observed both in vitro and in vivo. Inhibition of LPL by Angptl4 causes elevated plasma TG levels in mice (17). These studies are supported by genetic studies in humans that have revealed mutations in ANGPTL4 to be associated with circulating TG levels (18, 19).
The role of Angptl4 in obesity has been intensely investigated in mouse models. For example, germ-free mice are protected against diet-induced obesity resulting from an increased expression of intestinal Angptl4, which is suppressed by gut microbiota in normal mice (20). Also, Angptl4 has been reported to be a powerful signal from fat and other tissues to prevent fat storage and stimulate fat mobilization. Angptl4 overexpression caused a 50% reduction in adipose tissue weight by stimulating lipolysis, FA oxidation, and uncoupling in fat (2, 15). It has further been suggested that Angptl4 has an important role in central regulation of energy metabolism (3). Intracerebroventricular administration of Angptl4 suppressed food intake and body weight gain, and enhanced energy expenditure, whereas Angptl4-null mice displayed increased body weight, but reduced energy expenditure (3).
Regarding the role of Angptl4 in human obesity, very little data are available. In a previous study from our group, we were able to show that serum Angptl4 (s-Angptl4) levels were decreased in overweight subjects as compared with normal-weight subjects (13). To further evaluate the relationship between Angptl4 and acquired human obesity, we performed a study in young twin pairs discordant for body mass index (BMI), with one twin weighing >2.5 kg/m2 more than the other. This study represents a more-detailed analysis of the role of Angptl4 in acquired human obesity, irrespective of genetic background, age, and gender. We also studied the relationship between Angptl4 and fibroblast growth factor 21 (FGF21), a fasting-induced hormone that shares similarities in gene regulation (21–23) and has been reported to correlate with s-Angptl4 levels (24), as well as Angptl3, a structurally related protein that does not correlate with s-Angptl4 levels (13).
MATERIALS AND METHODS
Participants
The study population has recently been described in detail (25). Briefly, participants for the present study were selected from two population-based longitudinal studies, FinnTwin16 (FT16) and FinnTwin12 (FT12) (26). Twin pairs were enrolled for the present study based on their BMI in the last follow-up at young adult age. Twins were selected with the aim of covering the full BMI range of both normal-weight and obese subjects and a full range of within-pair differences in BMI (ΔBMI). To best describe the effects of acquired obesity, all monozygotic (MZ) pairs from FT16 and FT12 and all dizygotic (DZ) pairs from FT16 who were discordant for obesity [intra-pair difference in BMI (ΔBMI) ≥2.5 kg/m2]; one twin obese subject (BMI ∼30) and the other cotwin nonobese subject were included in the study. In addition, random pairs were studied to obtain wide ranges of ΔBMIs within pairs. In total, the present study included 121 twin pairs (46 MZ and 75 DZ) and of them, 21 MZ pairs and 48 DZ pairs were discordant for obesity. The subjects provided written informed consent. The protocol was designed and performed according to the principles of the Helsinki Declaration and was approved by the Ethical Committee of the Helsinki University Central Hospital.
Metabolic measurements
Plasma glucose was measured using the spectrophotometric hexokinase and glucose-6-phosphate dehydrogenase assay and serum insulin with time-resolved immunofluorometric assay (Perkin Elmer). Serum HDL cholesterol (HDL-C) and TG concentrations were determined using enzymatic methods (Roche Diagnostics Hitachi), and LDL-cholesterol (LDL-C) was calculated using the Friedewald formula (27). Serum free fatty acids (FFAs) were quantified using a kit from Zen-Bio, Inc.
Serum samples for Angptl4, Angptl3, and FGF21 analysis were stored at −80°C until analyzed. S-Angptl4, Angptl3, and FGF21 levels were measured using ELISAs, as previously described (13, 25). The Angptl4 ELISA recognizes full-length Angptl4 in human plasma (28).
Adipose tissue transcriptomics
Subcutaneous adipose tissue biopsies were obtained from 22 MZ twin pairs, 17 of which were discordant for BMI. Fat biopsies were collected under local anesthesia using a surgical technique and snap-frozen in liquid nitrogen for later processing. Total RNA was extracted using the RNeasy Lipid Mini Kit (Qiagen) according to the manufacturer's instructions. The quality of the RNA was analyzed using the 2100 Bioanalyzer platform (Agilent Technologies) prior to proceeding to array. Two micrograms of total RNA were treated according to conventional Affymetrix eukaryotic RNA labeling protocols (Affymetrix; Santa Clara, CA), followed by biotin labeling and fragmentation of the cRNA according to standard protocol. Hybridization, staining, and washing of the Affymetrix U133 Plus 2.0 chips were performed using the Affymetrics Fluidics Station 450 and Hybridization Oven 640 under standard conditions. The scanning of the chips was done using the Affymetrix GeneChip Scanner 3000. Raw expression values were normalized using the Robust Multi-array Average (RMA) with the help of probe sequence and with GC-content background correction (GCRMA algorithm) and analyzed using the GeneSpring GX 7.3 software (Agilent Technologies).
Statistical analyses
Statistical analyses were performed using SPSS version 17.0 for Windows (SPSS, Inc.), Stata statistical software (release 11.0; Stata Corporation, College Station, TX) and GraphPad Prism 4.03 (GraphPad Software, Inc.). All parameters were log10 transformed before they were used in statistical analyses. In individual twins, the relationships between serum concentrations or adipose tissue mRNA levels of Angptl4 with other measured parameters, Pearson correlations with P values corrected for clustered sampling of cotwins within-pairs were used. Comparisons between the cotwins were made by paired t-test. Within-pair differences of the measures were calculated by subtracting the leaner cotwin's value from the heavier cotwin's value. Pearson correlations of the within-pair differences were calculated to assess the relationships of the measures fully (in MZ) or partially (in DZ) adjusted for genetic influences.
To estimate the heritability of Angptl4 and FGF21, we assessed twin similarity within each zygosity group using intra-class correlations (ICCs) and biometric methods using the Mx statistical package (6th ed., Richmond, VA), as previously described (25). Preliminary information on the potential influence of factors underlying Angptl4 and FGF21 protein levels was obtained by comparing the ICC between MZ and DZ twins. A higher within-pair resemblance in MZ twins with respect to DZ twins would be indicative of potential genetic influences in the traits. This suggestive evidence was confirmed in subsequent biometric modeling. Accordingly, the variance in every trait was estimated to derive from the following different sources: additive genetic (labeled as A), reflecting the summed effects of individual alleles over the loci; dominance genetic (D), reflecting interactions between alleles at the same or different loci; common environmental (C), reflecting the influence of factors that are shared by both cotwins; and individual-specific environmental (E) factors, which reflect exposures that are not shared by the cotwins as well as measurement error (a random effect uncorrelated between twins). Hence, the overall genetic influence on the trait, also named heritability (broad sense), can be obtained by adding the estimates from A and D.
As a first step, different univariate models were computed for FGF21, Angptl3, and Angplt4 to estimate the proportion of the total variance in a trait due to A, D, C, and E. Because only data from MZ and DZ twins reared together were available for this study, C and D influences could not be modeled together, and the possible hypothetical combinations of influences did not include them simultaneously (e.g., they were ADE, ACE, AE, CE, and E). Subsequently, a bivariate Cholesky decomposition was calculated entering both Angptl4 and FGF21 protein data, to estimate the extent to which influences identified for Angptl4 were also present for FGF21. A full-information maximum likelihood method was applied in these procedures using individual-based observations. The simplest bivariate model that fit the data best was chosen by comparing the fit statistics [likelihood ratio test (LRT), as well as Akaike's information criterion (AIC)] of the full model against those of the hierarchically nested models (those with fewer parameters estimated).
RESULTS
S-Angptl4 levels in all study subjects
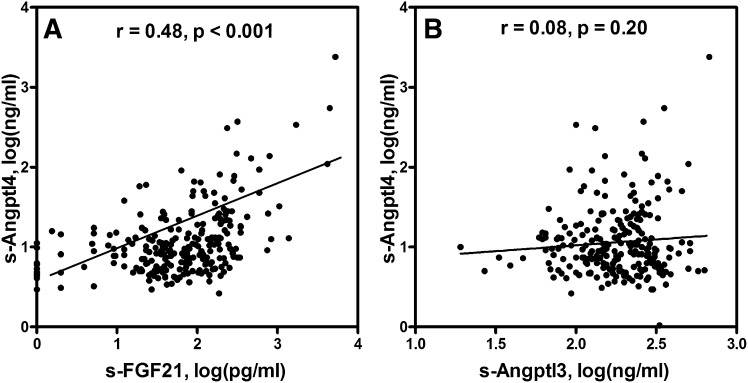
In the total study population, s-Angptl4 levels ranged from 1 to 373 ng/ml, and the distribution was skewed to the left. Two clear outliers were identified, having s-Angptl4 concentration of 549 and 2,391 ng/ml. Although the values were reproduced in several measurements, no explanation could be identified for these high values. In the whole-study population, s-Angptl4 levels were significantly correlated only with serum FFAs (r = 0.15, P = 0.01), and this correlation was independent of gender, zygosity, or BMI. As expected, s-Angptl4 levels were strongly correlated with s-FGF21 levels (r = 0.48, P < 0.001) (Fig. 1A), but not with s-Angptl3 levels (r = 0.08, P = 0.20) (Fig. 1B). Angptl3 and FGF21 serum levels showed no correlation with each other (P > 0.68).
Fig. 1.
Correlation between serum concentration of Angptl4 and FGF21 (A), and Angptl4 and Angptl3 (B) in all twins (n = 242). Concentrations were measured by specific ELISAs and analyzed by Pearson correlations after logarithmic transformation and corrected for clustered sampling.
Angptl4 serum levels in MZ twin pairs
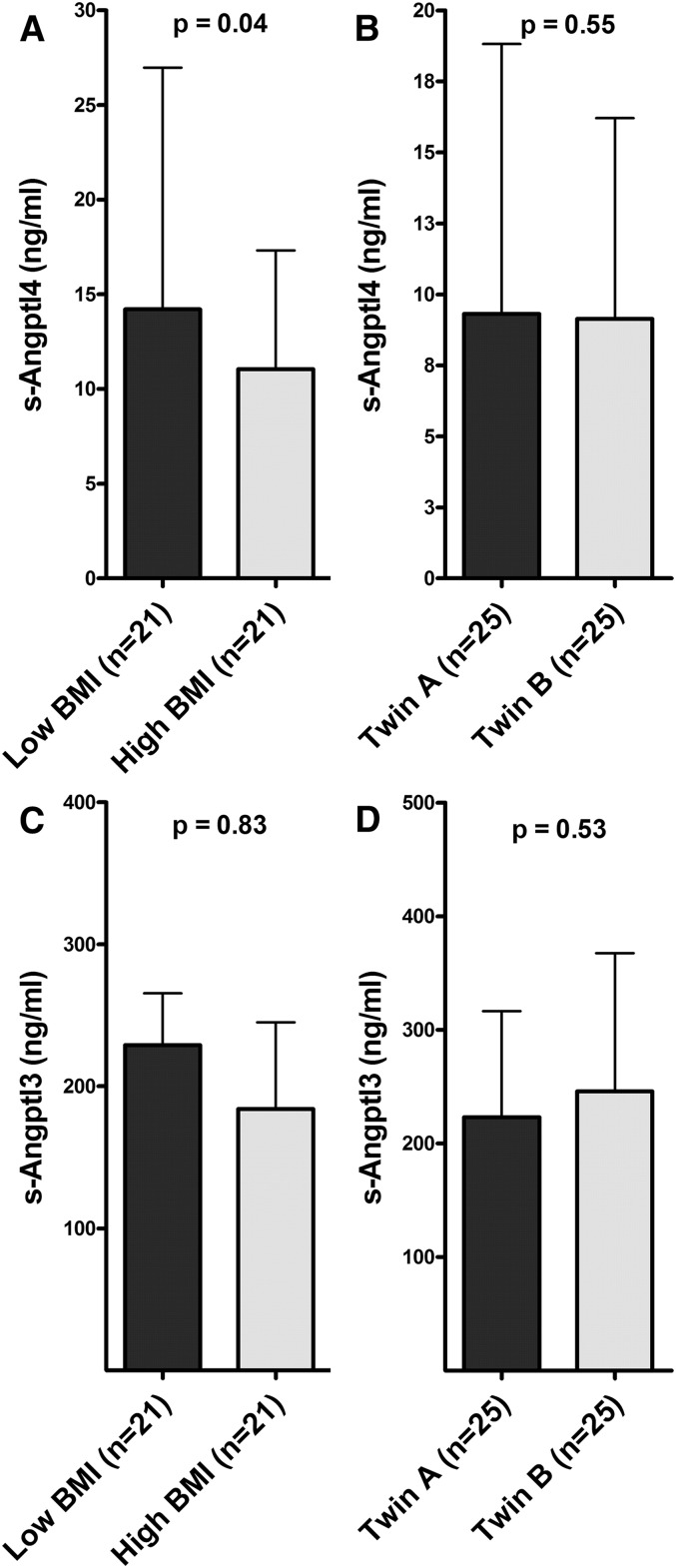
To assess the effect of acquired obesity on s-Angptl4 levels, we compared the concentrations in MZ twins concordant and discordant for BMI. Twins with an intra-pair difference in BMI greater than 2.5 kg/m2 were considered discordant, and those with an intra-pair difference in BMI less than 2.5 kg/m2 were considered concordant. Among discordant pairs, s-Angptl4 levels were significantly decreased (P = 0.04) in heavier twins (11 ng/ml, median; 5–17 ng/ml, 25%–75% percentile; n = 21) compared with leaner twins (15 ng/ml, median; 7–27 ng/ml, 25%–75% percentile; n = 21) (Fig. 2A). No differences were observed in concordant MZ twins (Fig. 2B). In contrast, s-Angptl3 levels were not significantly different in MZ twins discordant or concordant for BMI (P = 0.83 and 0.54, respectively) (Fig. 2C, D).
Fig. 2.
Serum Angptl4 (A, B) and Angptl3 (C, D) levels in MZ twins discordant (A, C) and concordant (B, D) for obesity. Angptl4 and Angptl3 concentration in serum was measured in 21 discordant and 25 concordant MZ pairs using established ELISAs. Data are presented as median ± interquartile range; P values were calculated using paired t-test after logarithmic transformation of the values.
Within-pair associations
Pearson correlations for the intra-pair differences (Δ) in s-Angptl4 levels were calculated for a number of relevant phenotypes. Utilizing this procedure allowed excluding genetic influences in MZ twin pairs and partially controlling for them in DZ twin pairs, while controlling fully for shared familial (nongenetic) effects in both types of twins. As presented in Table 1, Δs-Angptl4 was negatively correlated with ΔBMI (r = −0.27, P = 0.003) and ΔWaist (r = −0.21, P = 0.01) and positively correlated with ΔFGF21 (r = 0.497, P < 0.001). The observed correlations were independent of zygosity.
TABLE 1.
Correlation of intra-pair differences in Angptl4 serum levels with intra-pair differences in clinical and biochemical parameters of MZ and DZ twins
| ΔAngptl4 |
|||
| Parameter (intra-pair difference) | Pearson correlation | Sig. (2-tailed) | N |
| ΔBMI | −0.270 | 0.003 | 121 |
| ΔWaist | −0.214 | 0.019 | 119 |
| ΔFFAs | 0.140 | 0.14 | 112 |
| ΔTriglycerides | −0.054 | 0.56 | 121 |
| ΔCholesterol | 0.004 | 0.96 | 121 |
| ΔLDL-C | 0.005 | 0.96 | 117 |
| ΔHDL-C | 0.038 | 0.68 | 121 |
| ΔGlucose | −0.079 | 0.39 | 120 |
| ΔInsulin | −0.134 | 0.16 | 114 |
Genetic and environmental effects on Angptl4
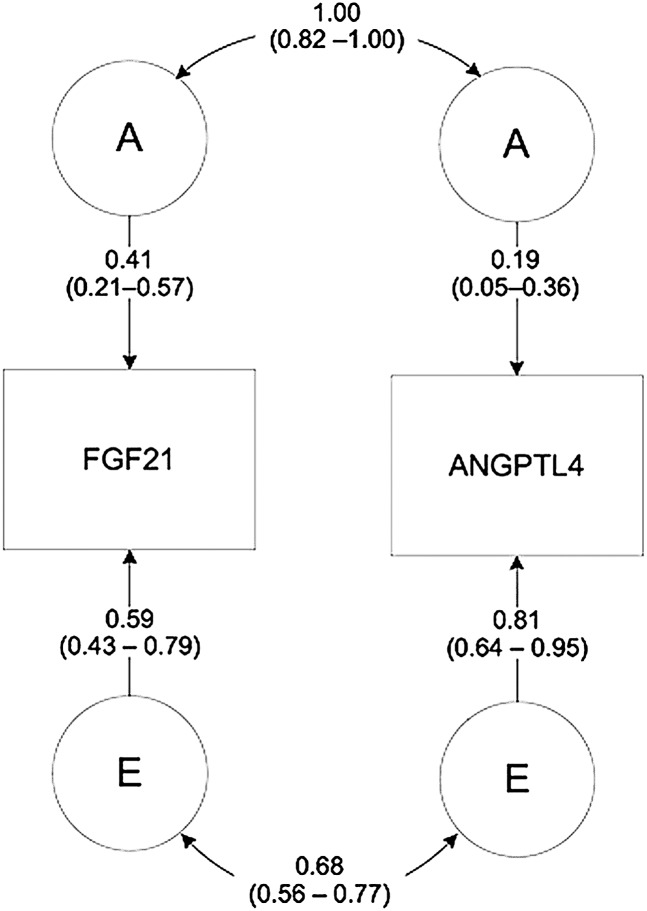
Preliminary evidence for genetic influences on s-Angptl4 levels was found in the slightly greater within-pair similarity between MZ than between DZ cotwins. The corresponding ICCs were: ICC-MZ = 0.21 (95% CI: −0.09–0.47), ICC-DZ = 0.17 (95% CI: −0.06–0.38). This initial estimation was further confirmed in the quantitative genetic analyses. Supported by preliminary univariate modeling, the bivariate Cholesky decomposition including s-FGF21 and s-Angptl4 suggested that an AE model including additive genetic (A) and specific environmental (E) effects best fit the data. The heritability was 0.41 (95% CI: 0.21–0.57) for s-FGF21 and 0.19 (95% CI: 0.05–0.36) for s-Angptl4 (Fig. 3). Importantly, these genetic influences on s-FGF21 and s-Angptl4 levels were due to a common set of genes (rg = 1.0, 95% CI: 0.82–1.00), a phenomenon accounting for 37.2% (95% CI: 14.7–57.1) of the phenotypic correlation between the traits (rp = 0.75, 95% CI: 0.68–0.80). Environmental factors were also highly shared by the traits (re = 0.68, 95% CI: 0.56–0.77).
Fig. 3.
Summary model of the best-fitting bivariate Cholesky decomposition representing the genetic and environmental architecture between s-Angptl4 and FGF21. A indicates additive genetic influences, whereas E indicates specific environmental influences. Numbers on double-headed arrows correspond to the estimated genetic (top) and environmental correlations (bottom) between phenotypes.
We also evaluated the impact of genetic and environmental factors on s-Angptl3 levels. Higher ICC for MZ (0.40, 95% CI: 0.13–0.62) compared with DZ (0.13, 95% CI: −0.10–0.35) cotwins suggests that s-Angptl3 levels are in part genetically controlled. Heritability counted for 35% of s-Angptl3 levels, with the remaining 65% attributed to specific environmental factors. Because of the lack of phenotypic correlation of s-Angptl3 with s-Angptl4 or s-FGF21, no bivariate models were conducted for s-Angpltl3.
Adipose tissue Angptl4 mRNA expression levels and obesity in MZ twins
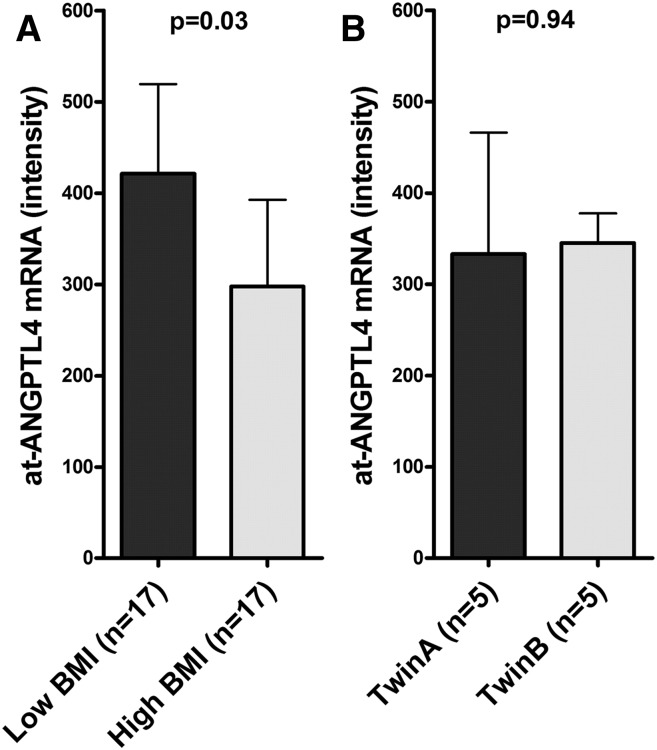
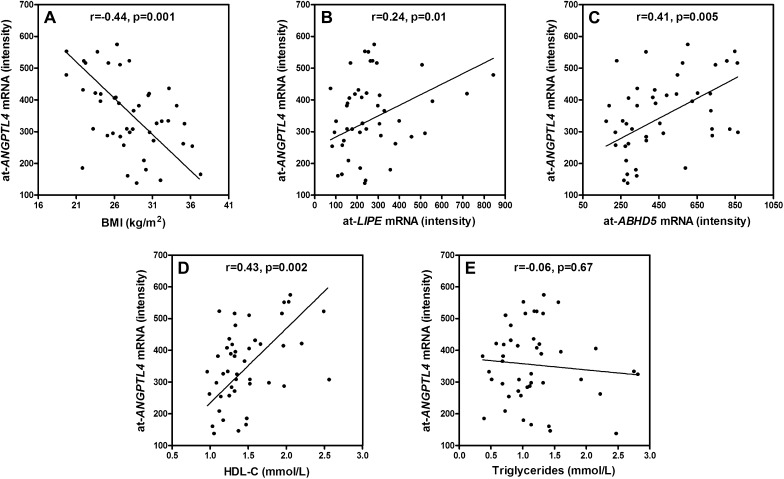
Adipose tissue Angptl4 (at-ANGPTL4) mRNA expression levels were obtained from genome-wide transcriptome analysis performed in fat biopsies obtained from 44 individual MZ twins. Affymetrix U133 Plus 2.0 chips contain two probes for Angptl4 that gave almost-identical results (r2 = 0.92). We next compared the at-ANGPTL4 expression levels in discordant and concordant MZ twins. In the discordant pairs, heavier twins exhibited significantly (P = 0.03) lower at-ANGPTL4 expression levels, as compared with their leaner cotwins (Fig. 4A); whereas in concordant twins, similar expression levels were observed (Fig. 4B). Pearson correlation analyses revealed that at-ANGPTL4 is negatively correlated with BMI (r = −0.44, P = 0.001; Fig. 5A), LDL-C (r = −0.29, P = 0.017), and fasting plasma glucose (r = −0.25, P = 0.044) and positively correlated with FFAs (r = 0.36, P = 0.016) and HDL-C (r = 0.43, P = 0.002; Fig. 5D) in individual twins. No significant correlations were observed between at-ANGPTL4 and fasting TG (P = 0.67; Fig. 5E) or s-Angptl4 levels (P = 0.94). In multivariate linear regression analyses including BMI, FFAs, LDL-C, HDL-C, and fasting plasma glucose, only BMI significantly explains the at-ANGPTL4 (Table 2).
Fig. 4.
At-ANGPTL4 mRNA expression levels in discordant (A) and concordant (B) MZ twins. At-ANGPTL4 mRNA expression levels were obtained from genome-wide transcriptome analysis performed in fat biopsies from 17 discordant and 5 concordant MZ twins. Data are presented as median ± interquartile range; P values were calculated using paired t-test after logarithmic transformation of the values.
Fig. 5.
Correlation between at-ANGPTL4 mRNA expression levels and obesity-related parameters: BMI (A), at-LIPE (B), and at-ABHD5 (C). Correlations between at-ANGPTL4 levels and plasma lipid parameters: HDL (D) and triglycerides (E); in MZ twins, n = 44. ANGPTL4, LIPE, and ABHD5 mRNA expression levels in adipose tissue (at-ANGPTL4, at-LIPE, and at-ABHD5, respectively) were measured from adipose tissue biopsies using Affymetrix U133 Plus 2.0 array chips and normalized using GCRMA algorithm. Relationships between variables were analyzed by Pearson correlations after logarithmic transformation and corrected for clustered sampling. P < 0.05 was considered significant.
TABLE 2.
Multivariate linear regression analyses between at-ANGPTL4 expression (dependent variable) and BMI, FFA, LDL-C, HDL-C, and glucose in MZ twins, n = 44
| Dependent variable: at-ANGPTL4 expression |
||
| Independent variable | Regression coefficient | Pa |
| BMI | −32.6 ± 15 | 0.03 |
| FFAs | 0.57 ± 0.4 | 0.19 |
| LDL-C | −193.2 ± 100 | 0.06 |
| HDL-C | −1.75 ± 2.15 | 0.99 |
| Glucose | −12.7 ± 127 | 0.92 |
P values are corrected for correlations of trait values of twins within pairs.
Because Angptl4 was suggested to induce adipose tissue lipolysis, we investigated the association between at-ANGPTL4 and expression levels of several key players involved in this process (29). Although the Affymetrix U133 Plus 2.0 chip has one probe for PNPLA2 [adipose triglyceride lipase (ATGL)], for unknown reasons, the readouts were under the detection capacity of the Affymetrix system used and could not be analyzed. Expression levels for hormone-sensitive lipase (LIPE), monoglyceride lipase (MGLL), G0/G1 switch gene 2 (G0S2), an ATGL inhibitor, and abhydrolase domain containing 5/gene identification-58 (ABHD5/CGI-58), an ATGL activator, were available and thus were analyzed against at-ANGPTL4. Pearson correlations in individual twins showed that at-ANGPTL4 expression levels are positively correlated with the mRNA expression levels of LIPE (r = 0.24, P = 0.01; Fig. 5B) and ABHD5/CGI-58, (r = 0.41, P = 0.005; Fig. 5C) but not with MGLL or G0S2 (P < 0.3).
DISCUSSION
Primed by our previous observation that s-Angptl4 is inversely correlated with obesity in a normal Finnish population sample (13), in this study, we evaluated in more detail the relationship between Angptl4 and obesity-related parameters in a collection of young MZ and DZ twin pairs.
Most genetic studies have not identified any association between ANGPTL4 polymorphisms and obesity-related traits (18, 19, 30), with the exception of one study (31). Our results suggested that this may be partially due to the strong influence of environmental factors on Angptl4 protein and the relatively weak correlation between Angptl4 and adiposity parameters. Furthermore, s-Angptl4 levels showed no correlation with BMI in all individual twins. However, when controlling for genetic influences, we observed a significant inverse correlation between s-Angptl4 levels and BMI. Furthermore, in MZ twins, adipose tissue ANGPTL4 (at-ANGPTL4) expression levels were also inversely correlated with BMI. Finally, both s-Angptl4 and at-ANGPTL4 expression levels were significantly decreased in heavier twins compared with their leaner counterparts.
Functional studies in mice suggested two mechanisms for the influence of Angptl4 on measures of adiposity. First, Angptl4 seems to stimulate adipose tissue lipolysis (2, 15) and second, Angptl4 appears to have an important role in the central regulation of energy metabolism by modulating AMPK activity in hypothalamus (3). Interestingly, here we showed that at-ANGPTL4 expression levels are positively correlated with the adipose tissue hormone-sensitive lipase (LIPE) and CGI-58 gene expression, supporting the notion that Angptl4 could promote adipose tissue lipolysis also in humans. However, definite conclusions based on LIPE mRNA levels must be made with caution, because it is well documented that hormone-sensitive lipase is regulated at the posttranslational level by phosphorylation (29). It is possible that reduction in at-Angptl4 would promote white adipose tissue triglyceride storage, whereas increasing its expression would favor the lipolytic catabolism of cellular fat stores. Similar suggestions have recently been proposed by Koliwad et al. (32), who identified Angptl4 as a potential key mediator of the effects of glucocorticoids on adipose tissue triglyceride homeostasis.
The relationship between Angptl4 and obesity seems to be very complex both in humans and in mice. Although we observed an inverse association between Angptl4 and body weight in young individuals, in an older population, the opposite relationship could be observed (33). Furthermore, Angptl4-null mice had impaired satiety generation and gained more weight when fed a normal diet but were resistant to diet-induced obesity (3). Other functions of Angptl4, such as the regulation of angiogenesis, could explain these discrepancies. Interestingly, the interplay between VEGFA and ANGPTL4 appears to mediate the increase in vascularization necessary for adipose tissue expansion (34). With current knowledge, it is difficult to draw a clear picture, and further studies are necessary to better understand the role of Angptl4 in obesity.
Although inhibition of LPL activity by Angptl4 and consequent hypertriglyceridemia in mice is well established (17), in our previous study in humans, we were not able to detect a correlation between s-Angptl4 and fasting TGs, whereas we successfully found that s-Angptl4 levels were positively correlated with HDL-C (33). In the present study, we observed as well that at-ANGPTL4 expression correlates with HDL-C but not with triglycerides. These results seem to be in line with a recent genome-wide association study performed in more than 100,000 individuals that revealed an association of variants in ANGPTL4 with HDL-C but not with TGs (35). All these results support the hypothesis that the main lipoprotein parameter related to Angptl4 is HDL-C, not TG. How this observation can be reconciled with the inhibitory effect on LPL activity remains to be clarified.
Human Angptl4 expression and secretion were previously shown to be increased by FFAs (28), and we observed that both of these parameters are significantly correlated in serum samples derived from a general population (13). Also in this study, s-Angptl4 and FFAs were positively correlated. Furthermore, we observed a strong positive correlation between s-Angptl4 and FGF21, another factor known to be regulated by FFAs through peroxisome proliferator-activated receptor-dependent pathways (36). The strong relationship between Angptl4 and FGF21 is interesting, inasmuch as both factors are potent regulators of energy metabolism and their concerted action could play an important role in adaptation to fasting (37). This notion is also supported by our observation that both serum proteins share most genetic and environmental factors.
In summary, we have demonstrated that s-Angptl4 levels are modestly modulated by genetic factors and inversely correlated with measures of adiposity in humans. Our data support a role for Angptl4 in body fat regulation and suggest that many of the observed effects in animal models are relevant in the context of human obesity.
Acknowledgments
The authors are thankful for the important contributions of all of the participants in the study and for the excellent technical assistance of Sari Nuutinen.
Footnotes
Abbreviations:
- AIC
- Akaike's information criterion
- Angptl4
- angiopoietin-like 4
- at-ANGPTL4
- adipose tissue ANGPTL4
- ATGL
- adipose triglyceride lipase
- BMI
- body mass index
- DZ
- dizygotic
- FFA
- free fatty acid
- FGF21
- fibroblast growth factor 21
- FT16
- FinnTwin16
- HDL-C
- HDL cholesterol
- ICC
- intra-class correlation
- LDL-C
- LDL cholesterol
- LPL
- lipoprotein lipase
- LRT
- likelihood ratio test
- MZ
- monozygotic
- s-Angptl4
- serum Angptl4
- TG
- triglyceride
This work was supported by National Institute for Health and Welfare, Finnish Foundation for Cardiovascular Research and Magnus Ehrnrooth Foundation (M.R.R., M.J., C.E.), Finska Läkaresällskapet (C.E., M.R.R.), Paulo Foundation, Oskar Öflunds Stiftelse (M.R.R.), Helsinki University Central Hospital grants (A.R., K.H.P.), the Centre of Excellence in Complex Disease Genetics grant by the Academy of Finland (J.K.), Jalmari and Rauha Ahokas, Yrjö Jahnsson, Gyllenberg, Biomedicum Helsinki, Finnish Foundation for Cardiovascular Research, and Novo Nordisk Foundations (K.H.P.).
REFERENCES
- 1.Yoshida K., Shimizugawa T., Ono M., Furukawa H. 2002. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 43: 1770–1772. [DOI] [PubMed] [Google Scholar]
- 2.Sanderson L. M., Degenhardt T., Koppen A., Kalkhoven E., Desvergne B., Muller M., Kersten S. 2009. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Mol. Cell. Biol. 29: 6257–6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H. K., Youn B. S., Shin M. S., Namkoong C., Park K. H., Baik J. H., Kim J. B., Park J. Y., Lee K. U., Kim Y. B., et al. 2010. Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes. 59: 2772–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu P., Tan M. J., Huang R. L., Tan C. K., Chong H. C., Pal M., Lam C. R., Boukamp P., Pan J. Y., Tan S. H., et al. 2011. Angiopoietin-like 4 protein elevates the prosurvival intracellular O(2)(-):H(2)O(2) ratio and confers anoikis resistance to tumors. Cancer Cell. 19: 401–415. [DOI] [PubMed] [Google Scholar]
- 5.Galaup A., Cazes A., Le Jan S., Philippe J., Connault E., Le Coz E., Mekid H., Mir L. M., Opolon P., Corvol P., et al. 2006. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc. Natl. Acad. Sci. USA. 103: 18721–18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padua D., Zhang X. H., Wang Q., Nadal C., Gerald W. L., Gomis R. R., Massague J. 2008. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 133: 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y., Oike Y., Yasunaga K., Hamada K., Miyata K., Matsumoto S., Sugano S., Tanihara H., Masuho Y., Suda T. 2003. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 63: 6651–6657. [PubMed] [Google Scholar]
- 8.Le Jan S., Amy C., Cazes A., Monnot C., Lamande N., Favier J., Philippe J., Sibony M., Gasc J. M., Corvol P., et al. 2003. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am. J. Pathol. 162: 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goh Y. Y., Pal M., Chong H. C., Zhu P., Tan M. J., Punugu L., Tan C. K., Huang R. L., Sze S. K., Tang M. B., et al. 2010. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J. Biol. Chem. 285: 32999–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtenstein L., Mattijssen F., de Wit N. J., Georgiadi A., Hooiveld G. J., van der Meer R., He Y., Qi L., Koster A., Tamsma J. T., et al. 2010.;Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 12: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement L. C., Avila-Casado C., Mace C., Soria E., Bakker W. W., Kersten S., Chugh S. S. 2011. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med. 17: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazes A., Galaup A., Chomel C., Bignon M., Brechot N., Le Jan S., Weber H., Corvol P., Muller L., Germain S., et al. 2006. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ. Res. 99: 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robciuc M. R., Tahvanainen E., Jauhiainen M., Ehnholm C. 2010. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. J. Lipid Res. 51: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomel C., Cazes A., Faye C., Bignon M., Gomez E., Ardidie-Robouant C., Barret A., Ricard-Blum S., Muller L., Germain S., et al. 2009. Interaction of the coiled-coil domain with glycosaminoglycans protects angiopoietin-like 4 from proteolysis and regulates its antiangiogenic activity. FASEB J. 23: 940–949. [DOI] [PubMed] [Google Scholar]
- 15.Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Muller M., Kersten S. 2006. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281: 934–944. [DOI] [PubMed] [Google Scholar]
- 16.Kersten S. 2009. Angiopoietin-like proteins and lipid metabolism. Cellular Lipid Metabolism. Ehnholm C., editor Springer, Heidelberg, Germany: 237–250. [Google Scholar]
- 17.Lichtenstein L., Kersten S. 2010. Modulation of plasma TG lipolysis by Angiopoietin-like proteins and GPIHBP1. Biochim. Biophys. Acta. 1801: 415–420. [DOI] [PubMed] [Google Scholar]
- 18.Romeo S., Pennacchio L. A., Fu Y., Boerwinkle E., Tybjaerg-Hansen A., Hobbs H. H., Cohen J. C. 2007. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 39: 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romeo S., Yin W., Kozlitina J., Pennacchio L. A., Boerwinkle E., Hobbs H. H., Cohen J. C. 2009. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 119: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backhed F., Manchester J. K., Semenkovich C. F., Gordon J. I. 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 104: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kersten S., Mandard S., Tan N. S., Escher P., Metzger D., Chambon P., Gonzalez F. J., Desvergne B., Wahli W. 2000. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 275: 28488–28493. [DOI] [PubMed] [Google Scholar]
- 22.Yoon J. C., Chickering T. W., Rosen E. D., Dussault B., Qin Y., Soukas A., Friedman J. M., Holmes W. E., Spiegelman B. M. 2000. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 20: 5343–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badman M. K., Pissios P., Kennedy A. R., Koukos G., Flier J. S., Maratos-Flier E. 2007. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5: 426–437. [DOI] [PubMed] [Google Scholar]
- 24.Stejskal D., Karpisek M., Reutova H., Humenanska V., Petzel M., Kusnierova P., Vareka I., Varekova R., Stejskal P. 2008. Angiopoietin-like protein 4: development, analytical characterization, and clinical testing of a new ELISA. Gen. Physiol. Biophys. 27: 59–63. [PubMed] [Google Scholar]
- 25.Tyynismaa H., Raivio T., Hakkarainen A., Ortega-Alonso A., Lundbom N., Kaprio J., Rissanen A., Suomalainen A., Pietilainen K. H. 2010. Liver fat but not other adiposity measures influence circulating FGF21 levels in healthy young adult twins. J. Clin. Endocrinol. Metab. 96: E351–E355. [DOI] [PubMed] [Google Scholar]
- 26.Kaprio J. 2006. Twin studies in Finland 2006. Twin Res. Hum. Genet. 9: 772–777. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 28.Kersten S., Lichtenstein L., Steenbergen E., Mudde K., Hendriks H. F., Hesselink M. K., Schrauwen P., Muller M. 2009. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 29: 969–974. [DOI] [PubMed] [Google Scholar]
- 29.Kolditz C. I., Langin D. 2010. Adipose tissue lipolysis. Curr. Opin. Clin. Nutr. Metab. Care. 13: 377–381. [DOI] [PubMed] [Google Scholar]
- 30.Talmud P. J., Smart M., Presswood E., Cooper J. A., Nicaud V., Drenos F., Palmen J., Marmot M. G., Boekholdt S. M., Wareham N. J., et al. 2008. ANGPTL4 E40K and T266M: effects on plasma triglyceride and HDL levels, postprandial responses, and CHD risk. Arterioscler. Thromb. Vasc. Biol. 28: 2319–2325. [DOI] [PubMed] [Google Scholar]
- 31.Legry V., Bokor S., Cottel D., Beghin L., Catasta G., Nagy E., Gonzalez-Gross M., Spinneker A., Stehle P., Molnar D., et al. 2009. Associations between common genetic polymorphisms in angiopoietin-like proteins 3 and 4 and lipid metabolism and adiposity in European adolescents and adults. J. Clin. Endocrinol. Metab. 94: 5070–5077. [DOI] [PubMed] [Google Scholar]
- 32.Koliwad S. K., Kuo T., Shipp L. E., Gray N. E., Backhed F., So A. Y., Farese R. V., Jr, Wang J. C. 2009. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J. Biol. Chem. 284: 25593–25601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smart-Halajko M. C., Robciuc M. R., Cooper J. A., Jauhiainen M., Kumari M., Kivimaki M., Khaw K. T., Boekholdt S. M., Wareham N. J., Gaunt T. R., et al. 2010. The relationship between plasma angiopoietin-like protein 4 levels, angiopoietin-like protein 4 genotype, and coronary heart disease risk. Arterioscler. Thromb. Vasc. Biol. 30: 2277–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gealekman O., Burkart A., Chouinard M., Nicoloro S. M., Straubhaar J., Corvera S. 2008. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am. J. Physiol. Endocrinol. Metab. 295: E1056–E1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teslovich T. M., Musunuru K., Smith A. V., Edmondson A. C., Stylianou I. M., Koseki M., Pirruccello J. P., Ripatti S., Chasman D. I., Willer C. J., et al. 2010. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 466: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mai K., Andres J., Biedasek K., Weicht J., Bobbert T., Sabath M., Meinus S., Reinecke F., Mohlig M., Weickert M. O., et al. 2009. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes. 58: 1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kharitonenkov A., Shanafelt A. B. 2009. FGF21: a novel prospect for the treatment of metabolic diseases. Curr. Opin. Investig. Drugs. 10: 359–364. [PubMed] [Google Scholar]