Abstract
The nonenzymatic cofactor high molecular weight kininogen (HK) is a precursor of bradykinin (BK). The production of BK from HK by plasma kallikrein has been implicated in the pathogenesis of inflammation and vascular injury. However, the functional role of HK in the absence of prekallikrein (PK), the proenzyme of plasma kallikrein, on vascular endothelial cells is not fully defined. In addition, no clinical abnormality is seen in PK-deficient patients. Therefore, an investigation into the effect of HK, in the absence of PK, on human pulmonary artery endothelial cell (HPAEC) function was performed. HK caused a marked and dose-dependent increase in the intracellular calcium [Ca2+]i level in HPAEC. Gd3+ and verapamil potentiated the HK-induced increase in [Ca2+]i. HK-induced Ca2+ increase stimulated endothelial nitric oxide (NO) and prostacyclin (PGI2) production. The inhibitors of B2 receptor-dependent signaling pathway impaired HK-mediated signal transduction in HPAEC. HK had no effect on endothelial permeability at physiological concentration. This study demonstrated that HK regulates endothelial cell function. HK could play an important role in maintaining normal endothelial function and blood flow and serve as a cardioprotective peptide.
Keywords: Blood Coagulation Factors, Cell Surface Receptor, Cellular Regulation, Inflammation, Kallikrein, Kininogen
Introduction
The plasma kallikrein-kinin system (KKS)2 consists of three proenzymes; factor XII (FXII, Hageman factor), prekallikrein (PK, Fletcher factor), and factor XI (FXI, plasma thromboplastin antecedent) as well as one cofactor; high molecular weight kininogen (HK, Fitzgerald factor). KKS is involved in the regulation of hemodynamics, inflammation, complement activation, angiogenesis, thrombosis, and fibrinolysis. Basically, all these proposed roles represent a range of overlapping effects that contribute to various extents toward vasodilation and healing. Therefore, the plasma KKS can be considered to have a spectrum of physiological effects, ranging at one extreme from a hemostatic state of vasodilation and promotion of smooth blood flow, all the way to a prothrombotic state. It is conceivable to suggest that other mechanisms proposed about respiratory, retinal, and renal systems can fit into this spectrum of physiological effects (1–3). There is accumulating evidence suggesting that when the plasma KKS is activated, the results are a sequential release of proteolytic enzymes and vasoactive peptides, generation of both angiogenic and anti-angiogenic molecules, stabilization of thrombus, and an increase in protease inhibitor activity in blood (4–7). The activation of HK-PK complex on endothelial cells triggers vasodilation through smooth muscle relaxation, inhibits platelet aggregation, and induces proinflammatory responses. Of note, the direct assembly of HK, PK, and FXII on vascular smooth muscle cells (VSMC) also results in the activation of PK to kallikrein (8). The induction of these physiological reactions is caused by the release of the vasoactive peptide bradykinin (BK) from HK by kallikrein. Bradykinin B2 receptor activation by BK mediates the activation of endothelial nitric-oxide synthase (eNOS) and phospholipase A2 (PLA2) leading to production of nitric oxide (NO) and prostacylin (PGI2). Evidence suggests that BK phosphorylates p44/42 mitogen-activated protein kinase in VMSC, which is blocked by BK antagonist HOE-140 (8).
Besides having a direct effect on blood vessels, the HK-PK complex has also been shown to mediate the effects of other pro-inflammatory molecules. Recent study suggests that the inhibitors of both BK and factor XII activity protect from mast cell-induced effects not only in patients but also in genetically engineered mouse models. The authors proposed that this class of inhibitors could be useful to treat allergic diseases (9). Tryptase is a serine protease produced by mast cells during inflammation. Evidence suggests that tryptase-induced increase in vascular permeability is dependent on PK activation to kallikrein and subsequent release of BK from HK (10). Imamura et al. showed that while HK-deficient plasma completely lacks tryptase-induced vascular permeability enhancement activity, tryptase produces a 30% increase in vascular permeability in PK-deficient plasma. Therefore, we asked the question, what is the cause of incomplete abolishment of vascular permeability enhancement from PK-deficient plasma? Possible explanations for this observation could be heterozygosity of the donor cell genome, direct release of BK from HK by tryptase or the presence of a tryptase-independent mechanism. Finally, HK may have the ability to induce vascular permeability. Because the functional role of HK, in the absence of PK, on vascular endothelial cells is not fully defined and because no clinical abnormality is seen in PK-deficient patients, we determined whether HK regulates endothelial cell function (11–13). Thus, we characterized the effect of HK on cultured endothelial cells in the absence of PK.
EXPERIMENTAL PROCEDURES
Materials
HK and BK-free HK (two chain HK, 2-HK) (14) were purchased from Enzyme Research Laboratories (South Bend, IN). HOE140 was purchased from Biomol Research Laboratories Inc. (Plymouth Meeting, PA). Fura-2 AM, Fluo-4 AM, and pluronic F-127 were obtained from Molecular Probes Inc. (Eugene, OR). BK, thapsigargin, gadolinium, verapamil, nifedipine, U73122, 2-APB, quinine, apamin, and ionomycin were purchased from Sigma-Aldrich. Anti-bradykinin B2 receptor (B2) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). NuPAGE 4–12% Bis-Tris gels, MES running buffer, and LDS sample buffer (4×) were purchased from Invitrogen (Carlsbad, CA). Silver Stain Plus and Blotting Grade Blocker Non-Fat Milk were purchased from Bio-Rad. Mouse anti-human HK antibody was purchased from Abcam (Cambridge, MA). HRP-conjugated goat anti-mouse IgG and ECL Western blotting substrate were obtained from Pierce. Autoradiography films were purchased from Midsci (St Louis, MO).
Human Pulmonary Artery Endothelial Cell Culture
Human pulmonary artery endothelial cells (HPAEC), purchased from Clonetics (San Diego, CA), were cultured in Dulbecco's modified Eagle's medium (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) as previously described (15).
Ratiometric Calcium Imaging
Monolayers of HPAEC grown overnight on glass coverslips were loaded with ratiometric fluorescence Ca2+ dye fura-2 AM (10 μm) for 30 min at 37 °C, as described previously (16). Cells were washed three times with HEPES buffer (126 mm NaCl, 5 mm KCl, 0.3 mm NaH2PO4, 10 mm HEPES, 1 mm MgCl2, 2 mm CaCl2, 10 mm glucose, pH 7.4). The slides were fixed on a perfusion chamber (Warner Instruments, Hamden, CT) and continuously perfused with HEPES buffer at a rate of 1 ml/min. The flow rate was controlled by a multichannel ValveBand computerized system connected to pinch valves (Automate Scientific, Berkeley, CA). Changes in [Ca2+]i were monitored as changes in the fluorescence ratio at 340/380 excitation wavelength in a dual excitation digital Ca2+ imaging system (Ionoptix Inc., Milton, MA). A field with no cells was chosen as background and used to generate records of the 340:380 ratio in each cell within the microscope field. At the end of each experimental run, cells were perfused with ionomycin solution (1 μm, (a Ca2+ ionophore)) to obtain the maximal fluorescence intensity.
Confocal Ca2+ Measurements and Image Analysis
To measure intracellular calcium levels, monolayers of HPAEC grown on coverslips were loaded with fluo-4 AM (1 μm). The coverslips were mounted on a perfusion chamber (Warner Instruments, Hamden, CT) and continuously perfused with HEPES-buffer using a perfusion pump driven system at a rate of 1 ml/min. Fluorescence in a field of 10–30 cells was imaged using LSM 510 software. Cells were treated with various concentrations of HK for 3 min with 5-min washes with HEPES buffer in between each stimulation to allow recovery of basal [Ca2+]i levels. Changes in [Ca2+]i levels in cells were measured with a Zeiss LSM 510 META confocal microscope at an excitation wavelength of 488 nm and an emission wavelength of 505 nm.
Biotin-HK Binding Studies
Binding studies were performed on confluent HPAEC (4 × 104 cells/well) cultured in 96-well microtiter plates (Nunclon, Thomas Scientific; Swedesboro, NJ). Briefly, HPAEC were washed three times with HEPES-NaHCO3 buffer (137 mm NaCl, 3 mm KCl, 14.7 mm HEPES, 1 mm MgCl2, 2 mm CaCl2, 5.5 mm glucose, and 0.1% gelatin, pH 7.1) and blocked with 1% gelatin for 1 h at 37 °C. After washing three times, cells were then incubated with biotin-HK (20 nm) in the absence or presence of increasing concentration of HKH20 (0.1–30 μm), HOE140 (0.3–100 μm), or BK (500 μm) for 1 h at 37 °C. Cells were then washed three times to remove the free biotin-HK and incubated with streptavidin horseradish peroxidase (1:500 dilution) in HEPES-NaHCO3 buffer at room temperature for 1 h. At the end of incubation, 100 μl of peroxide specific fast-reacting substrate, turbo-3,3′,5,5′-tetramethylbenzindine dihydrochloride (turbo-TMB) was added and the reaction allowed to proceed for 5 min at room temperature. The reaction was stopped by adding 1 m phosphoric acid (100 μl), and the level of binding was determined by measuring the absorbance of the reaction mixture in each well at OD 450 nm.
NO Assay, HK Dose-Response
Confluent HPAEC cultured in 96-well microtiter plate were washed three times with HEPES-NaHCO3 buffer (137 mm NaCl, 3 mm KCl, 12 mm NaHCO3, 14.7 mm Hepes, 5.5 mm dextrose, 2 mm CaCl2, 1 mm MgCl2, pH 7.1) and blocked with 1% gelatin for 1 h at 37 °C. Cells were then incubated with increasing concentrations (0–300 nm) of HK for 1 h at 37 °C. The samples from each well were collected to measure the amount of nitrate + nitrite (the final products of nitric oxide metabolism) using a fluorometric assay (Cayman Chemicals, MI) according to the manufacturer's protocol.
Briefly, 10 μl of sample was added to 96-well assay plate and the volume adjusted to 80 μl with the assay buffer. The samples were then incubated at room temperature for 1 h with 10 μl of nitrate reductase enzyme and 10 μl of enzyme cofactors to convert the nitrate to nitrite. 10 μl of DAN (2,3-diaminonaphthalene) reagent was added to each well followed by 10 min of incubation at room temperature. Finally, 20 μl of NaOH was added to enhance the detection of the fluorescent product, 1(H)-naphthotriazole. The fluorescence was read at an excitation wavelength of 360 nm and an emission wavelength of 460 nm using BioTek Synergy HT Multi-Mode Microplate Reader (BioTek, Winooski, VT).
Inhibition of HK-induced NO Production by Anti-B2 Receptor Antibody, HOE-140, U73122, and 2-APB
Confluent HPAEC cultured in 96-well microtiter plate were washed three times with HEPES-NaHCO3 buffer and blocked with 1% gelatin for 1 h at 37 °C. Cells were then treated with 100 nm HK with or without anti-B2 receptor antibody (1:500 dilution), HOE-140 (1 μm), U73122 (100 μm), or 2-APB (100 μm) and incubated for 1 h at 37 °C. The amount of nitrate + nitrite in each sample was measured using the same protocol as described above.
Prostacyclin Assay, HK Dose Response
Confluent HPAEC cultured in 96-well microtiter plate were washed three times with HEPES-NaHCO3 buffer (137 mm NaCl, 3 mm KCl, 12 mm NaHCO3, 14.7 mm Hepes, 5.5 mm dextrose, 2 mm CaCl2, 1 mm MgCl2, and 0.1% gelatin, pH 7.1) and blocked with 1% gelatin for 1 h at 37 °C. Cells were then incubated with increasing concentrations (0–600 nm) of HK for 1 h at 37 °C. The samples from each well were collected to measure the amount of 6-keto prostaglandin F1α (a stable analog of prostacyclin) using a competitive acetylcholinesterase (AChE) enzyme immunoassay (Cayman Chemicals) according to the manufacturer's protocol.
Briefly, 50 μl of sample was added to 96-well microtiter plate pre-coated with mouse monoclonal anti-rabbit IgG, followed by 50 μl of AChE-conjugated 6-keto PGF1α tracer and 50 μl of 6-keto PGF1α-specific rabbit antiserum. After incubating the plate for 18 h at 4 °C, the solution was discarded, and the wells washed five times with a wash buffer containing 0.05% Tween 20. 200 μl of Ellman's Reagent containing the color producing substrate for AChE was then added to each well, and the plate was allowed to develop in the dark for 90–120 min. The absorbance was measured spectrophotometrically at 405 nm using BioTek ELx800 Absorbance Microplate Reader. The data were analyzed using a computer spreadsheet provided on the manufacturer's website.
Inhibition of HK-induced PGI2 Production by HOE 140
Confluent HPAEC cultured in 96-well microtiter plate were washed three times with HEPES-NaHCO3 buffer (137 mm NaCl, 3 mm KCl, 12 mm NaHCO3, 14.7 mm Hepes, 5.5 mm dextrose, 2 mm CaCl2, 1 mm MgCl2, and 0.1% gelatin, pH 7.1) and blocked with 1% gelatin for 1 h at 37 °C. Cells were then treated with HK (300 nm) in the absence or presence of HOE 140 (1 μm); or BK-free HK (300 nm) for 1 h at 37 °C. The amount of 6-keto prostaglandin F1α in each sample was measured using the same protocol as described above.
In Vitro Vascular Permeability Assay
The effect of HK on vascular permeability was assessed using an in vitro vascular permeability assay kit (Chemicon/Millipore, MA) according to the manufacturer's protocol. Briefly, collagen coating solution in 0.2× PBS, pH 7.1 was added to the inserts. After incubating for 1 h at room temperature, the inserts were hydrated with cell growth medium for 15 min and seeded with 200 μl of cell suspension (1.0 × 106 HPAEC/ml). The plate was incubated at 37 °C for 24 h until a cell monolayer was formed. The inserts were then treated with cell basal medium (negative control); 1 μg/ml LPS (positive control); 0.3 μm and 1 μm HK in the absence or presence of HOE-140 (1 μm) and, 0.3 μm and 1 μm BK-free HK and incubated at 37 °C for 18 h. The solution from each insert was removed and the inserts transferred to the permeability detection plate. 500 μl of cell basal medium was added to each plate well and 150 μl 1:20 FITC-Dextran was added to each insert. After 5 min of incubation at room temperature the reaction was stopped by removing the inserts from the wells. 100 μl of the plate well solution was then transferred to a 96-well Greiner Microlon 200 Black fluorescence detection plate (USA Scientific). The fluorescence was read at an excitation wavelength of 485 nm and an emission wavelength of 528 nm using BioTek Synergy 2 Multi-Mode Microplate Reader.
Silver Staining of SDS-PAGE Gel
SDS-PAGE was performed using pure HK (300 nm), PK (300 nm), HK+PK (300 nm each), and 2-HK (two chain BK-free HK, 300 nm) as well as HK (300 nm) and 2-HK (300 nm) incubated with HPAEC. HPAEC cultured overnight in 96-well microtiter plate were washed three times with HEPES-NaHCO3 buffer and blocked with 1% gelatin for 1 h at 37 °C. Cells were then washed and incubated with buffer, lisinopril (1 μm), HK+lisinopril, or 2-HK+lisinopril for 1 h at 37 °C. Lisinopril (ACE inhibitor) was used to inhibit ACE-mediated metabolism of any BK produced. After 1 h, buffer solution (supernatant) from each well was collected for SDS-PAGE. Electrophoresis was carried out using NuPAGE 4–12% Bis-Tris gel and MES running buffer under non-reducing conditions. After electrophoresis, protein bands were detected using Silver Stain Plus, according to the manufacturer's protocol.
Determination of HK Metabolism on HPAEC using Western Blot
Electrophoresis was performed as described above on cell supernatants collected after incubating HPAEC with HEPES, lisinopril (1 μm), HK (300 nm)+lisinopril, or 2-HK (300 nm)+lisinopril for 1 h at 37 °C. After electrophoresis, proteins were transferred onto nitrocellulose membrane at 100 V for 1 h. The membrane was blocked using 3% Blotting Grade Blocker Non-Fat milk overnight and then incubated with mouse anti-human HK antibody (1:500) for 1 h. After washing three times with 1× PBS containing 0.1% Tween, the membrane was then incubated with HRP-conjugated goat anti-mouse IgG (1:1000) for 1 h. The membrane was then washed three times to remove the unbound antibody and incubated with ECL substrate for 5 min. Protein bands were detected using an autoradiography film.
LC/MS Studies
LC/MS experiments for the analysis of bradykinin was conducted based on the method of Chajkowski (17), with minor modifications.
Statistical Methods
The changes in [Ca2+]i were analyzed by paired Student's t test and one-way analysis of variance (ANOVA) followed by the Student Newman-Keuls test for comparison among groups of data. p ≤ 0.05 was considered statistically significant. Data are expressed as the mean ± S.E. of n determinations.
RESULTS
High Molecular Weight Kininogen (HK) Induces an Increase in the Endothelial Cell Ion Transport Activity
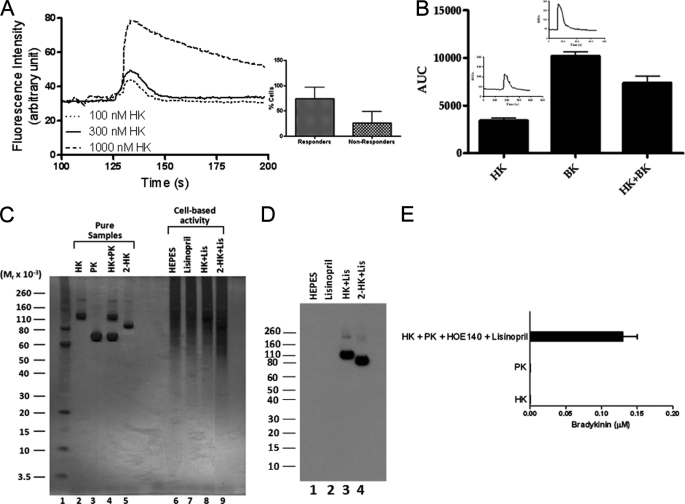
The binding of HK to endothelial cells has been described (18). Because no clinical abnormality is apparent in patients with PK deficiency, investigations were performed to determine the functional role of HK, in the absence of PK, on endothelial cells using a flow-based assay. As shown in Fig. 1A, HK induced a dose-dependent increase in cytosolic Ca2+ in HPAEC. Stimulation of HPAEC with increasing concentrations of HK produced a rapid elevation of cytosolic Ca2+. Endothelial cells exposed to HK concentrations greater than 300 nm showed biphasic calcium kinetics (a rapid transient increase in [Ca2+]i followed by a slow recovery) (Fig. 1A). However, cells exposed to HK concentration lower than 300 nm had a rapid recovery. These differences might be due to an alternative Ca2+-signaling pathway or cell swelling. Changes in fluorescence in HPAEC in response to HK were evaluated by the post-hoc Newman-Keuls's method. The test indicated that HK (300 nm) caused significant fluorescence change in 75% of cells (p < 0.001) (Fig, 1A, inset).
FIGURE 1.
Effect of HK on [Ca2+]i in endothelial cells. Panel A, HK increases [Ca2+]i in HPAEC in a dose-dependent manner. HPAEC grown on coverslips were incubated with 1 μm fluo-4 AM, mounted on a perfusion chamber and continuously perfused with HEPES buffer at the rate of 1 ml/min. Cells were treated with increasing concentrations (100, 300, and 1000 nm) of HK for 3 min each with 5 min wash with HEPES buffer in between each concentration to allow recovery of basal [Ca2+]i. Changes in [Ca2+]i levels in cells were measured with a Zeiss LSM 510 META confocal microscope at an excitation wavelength of 488 nm and an emission wavelength of 505 nm. Change in fluorescence in a field of 10 to 30 endothelial cells was measured. The percentage of endothelial cells which responded to HK stimulation was also determined (inset). Panel B, comparison of the effect of HK, BK, and HK+BK on [Ca2+]i levels in HPAEC. HPAEC were perfused with HK (300 nm), BK (300 nm), or HK+BK (300 nm each). The increase in [Ca2+]i was measured in each cell and expressed as area under curve. Data are presented as mean ± S.E. The changes in [Ca2+]i expressed as change in fluorescence in response to HK or BK in representative endothelial cells are shown (inset). Panel C, characterization of HK by SDS-PAGE. SDS-PAGE was performed with pure HK (300 nm), PK (300 nm), and 2-HK (two chain BK free HK, 300 nm) as well as HK (300 nm), and 2-HK (300 nm) incubated with HPAEC. HPAEC cultured in 96-well microtiter plate were blocked with 1% gelatin for 1 h at 37 °C. Cells were then washed and incubated with HK or 2-HK in the presence of lisinopril (1 μm). After 1 h of incubation at 37 °C, cell supernatant was collected for SDS-PAGE. Electrophoresis was carried out using 4–12% Bis-Tris gel and MES running buffer under non-reducing conditions. After electrophoresis, protein bands were detected using silver staining. Panel D, Western blot analysis of HK metabolism on HPAEC. Electrophoresis was performed as described in panel C on cell supernatants collected after incubating HPAEC with HEPES, lisinopril (1 μm), HK (300 nm)+lisinopril, or 2-HK (300 nm)+lisinopril for 1 h at 37 °C. After electrophoresis, proteins were transferred onto nitrocellulose membrane at 100 V for 1 h. Western blotting was performed using mouse anti-human HK antibody (1:500) and HRP-labeled goat anti-mouse IgG (1:1000) as described under “Experimental Procedures.” Panel E, characterization of HK on HPAEC by LC/MS analysis. HPAEC (104 cells/well) were plated and cultured in 96-well plates. Triplicate samples of cells were incubated with HK (600 nm), PK (600 nm) and HK+PK (600 nm each). The generation of BK in the presence of HOE 140 (1 μm) and lisinopril (1 μm) was determined by LC/MS.
Next, investigations were performed to determine whether HK and BK produce the same effect. The increase in [Ca2+]i was significantly higher in response to BK as compared with HK (Fig. 1B). Further, the combination of HK and BK produced a smaller increase in [Ca2+]i than that produced by BK alone. These data indicated that HK influenced [Ca+2]i levels in HPAEC and that HK and BK could be interacting with a common binding site on endothelial cells.
Although unlikely, the increase in [Ca2+]i in HPAEC treated with HK could be due to BK-like peptide contaminants in the HK preparation used. This possibility was ruled out using 4–12% gradient SDS-PAGE under non-reducing condition and detected by silver staining, Western blot, and a comparative LC/MS study of HK (Fig. 1). On silver stained gels, pure HK (lane 2) appeared as a single prominent 120 kDa band compared with BK-free HK (2-HK, lane 5) (Fig. 1C). No low molecular weight protein bands were observed, suggesting that the HK preparation used in this study was indeed pure and did not contain any BK-like peptide contaminants. Further, PK (lane 3) alone was not activated, suggesting that PK does not undergo autoactivation under our assay condition. Accordingly, PK did not cleave HK as shown in Fig. 1C (lane 4). Next, investigations were performed to determine the metabolites of HK in the supernatant of cells. HK (lane 8) in the presence of lisinopril (to prevent BK metabolism by ACE) incubated with HPAEC for 1 h remained intact, appearing as a 120 kDa band compared with 2-HK (lane 9). The HK-incubating buffer (HEPES-NaHCO3 buffer, lane 6) and lisinopril (lane 7) were pure. While the concentrations of HK and 2-HK for both pure and those added to HPAEC were identical, the intensity of HK band and 2-HK band in HPAEC culture supernatant was fainter than those of pure samples. The underlying reason for having such faint bands for HK (lane 8) and 2-HK (lane 9) is mainly due to their ability to bind to cell surface proteins, as previously reported (19, 20). Further studies were performed to determine the metabolism of HK on cells and then analyzed by Western blot. 2-HK was used as a control. Within the incubation period, HK was not metabolized to 2-HK and BK or smaller peptides on HPAEC as detected by anti-HK antibody (Fig. 1D (lane 3)). This finding is in accord with the silver staining study.
Using a commercially available kit to measure BK level in the samples, we previously showed that no BK was detected in the supernatant when HK was incubated with HPAEC. On the contrary, incubation of HPAEC with HK and PK led to robust BK production (21). In the present study, LC/MS analysis of HK and a mixture of HK and PK were performed to determine proteolytic digests of HK on cultured HPAEC. As shown in Fig. 1E, the assembly and activation of the complex of HK-PK on HPAEC resulted in the generation of BK, which was similar to that seen in previous studies (17). However, no BK was generated when HPAEC were treated with HK (600 nm) or PK (600 nm) alone.
Taken together, these data confirmed that the increase in [Ca2+]i in HPAEC was indeed due to intact HK and not due to the presence of BK or BK-like peptide contaminants in the HK preparation used in this study.
Mechanisms of HK-induced Increase in [Ca2+]i in Endothelial Cells
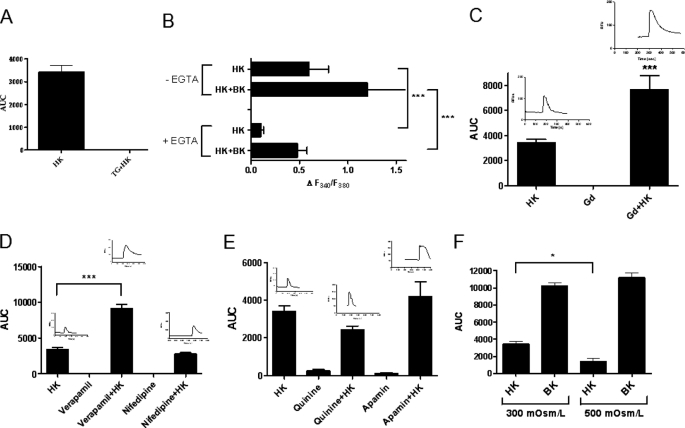
Regulation of endothelial cell processes by Ca2+ is well-established (23). Studies have shown that agonist-induced increase in endothelial cell [Ca2+]i consists of two phases, an initial rapid peak caused by mobilization of Ca2+ from IP3-sensitive intracellular stores (endoplasmic reticulum), and a sustained rise/slow recovery due to influx of extracellular Ca2+ into the cell to refill the intracellular stores (24). Because HK produced a similar biphasic Ca2+ response in endothelial cells, we sought to determine the relative contribution of intracellular and extracellular Ca2+ in HK-induced endothelial cell signaling.
We initially characterized the involvement of intracellular Ca2+ stores in mediating the initial rapid peak of the biphasic Ca2+ response produced by HK in endothelial cells. HPAEC were perfused with thapsigargin (TG, 2 μm) in a Ca2+-free buffer containing EGTA (1 mm) to deplete the intracellular Ca2+ stores (25). The depletion of IP3-sensitive calcium stores by TG completely abolished HK-induced increase in cytosolic Ca2+ in HPAEC (Fig. 2A), suggesting that the release of Ca2+ from intracellular stores is responsible for the rapid endothelial cell stimulation produced by HK.
FIGURE 2.
HK stimulates the mobilization of IP3-sensitive intracellular Ca2+ pool as well as extracellular Ca2+ influx in HPAEC. HPAEC were treated with HK (300 nm) in the presence or absence of inhibitors. Changes in [Ca2+]i levels in cells were measured with a Zeiss LSM 510 META confocal microscope at an excitation wavelength of 488 nm and an emission wavelength of 505 nm, and the data expressed in terms of area under curve. Panel A, effect of thapsigargin (TG, 2 μm) on HK-induced increase in [Ca2+]i. HPAEC were perfused with TG in a Ca2+-free buffer containing EGTA (1 mm) to deplete the intracellular Ca2+ stores. Cells were then perfused with HK (300 nm). Changes in [Ca2+]i were measured using confocal microscopy and expressed as area under curve. Data are presented as mean ± S.E. Panel B, effect of EGTA on HK-induced increase in [Ca2+]i. HPAEC were perfused with either HEPES buffer without EGTA or calcium-free buffer with EGTA (1 mm) throughout the experiment. Cells were then treated with HK (300 nm) or HK+BK (300 nm each, control). Changes in [Ca2+]i were measured in each cell using a dual excitation digital Ca2+ imaging system. Changes in [Ca2+]i are expressed as ΔF340/F380. Data are presented as mean ± S.E. Panels C–E, effect of gadolinum (Gd3+, 10 μm) (C), nifedipine (10 μm) and verapamil (20 μm) (D) and quinine (1 μm) and apamin (1 μm) (E) on HK-induced increase in [Ca2+]i. The increase in [Ca2+]i was measured and expressed as area under curve. Data are presented as mean ± S.E. Panel F, effect of hypertonic stimulation of HPAEC on HK-induced increase in [Ca2+]i. Because Ca2+-activated channels are markedly suppressed by hypertonic stimulation of cells, the osmolarity of cell perfusing medium (300 mOsm/liter) was increased by adding 100 mm NaCl (200 mOsm/liter). Cells were then treated with HK (300 nm) or BK (300 nm) and the changes in [Ca2+]i were measured. ***, p < 0.001. *, p < 0.05.
Next, we determined the significance of extracellular Ca2+ influx in HK-induced endothelial cell signaling. HPAEC were perfused with Ca2+-free buffer containing 1 mm EGTA throughout the experiment. As shown in Fig. 2B, HK-dependent increase in intracellular Ca2+ was significantly reduced in Ca2+-free buffer containing EGTA. These data suggested that extracellular Ca2+ is required for HK-induced increase in cytosolic Ca2+, specifically for the sustained rise/slow recovery phase. The question we asked was which calcium channels might be involved in HK-mediated extracellular Ca2+ influx into endothelial cells?
We initially examined the role of stretch-activated non-selective cation channels in HK-induced increase in [Ca2+]i in HPAEC using gadolinium (Gd3+), an inhibitor of stretch-activated ion channels. Treatment of HPAEC with 10 μm Gd3+ alone resulted in no change in the intracellular Ca2+ levels (Fig. 2C). However, contrary to our assumption, treatment of HPAEC with HK following Gd3+ significantly potentiated the HK-stimulated Ca2+ increase (Fig. 2C). One potential explanation for the robust rise in cytosolic Ca2+ might be due to coupling of stretch-activated ion channels with another channel or inhibition of Ca2+-efflux.
Because experimental evidence suggests that endothelial cells express voltage-dependent L-type Ca2+ channels, we investigated the effects of nifedipine and verapamil (L-type Ca2+ channel blockers) on HK-induced Ca2+ influx in HPAEC (26). It is well-established that nifedipine not only blocks L-type Ca2+ channels in smooth muscle cells but also modulates endothelial functions such as NO production (27, 28). While nifedipine (10 μm) had no significant effect on HK-evoked Ca2+ increase in HPAEC, verapamil (20 μm), like Gd3+, caused a robust potentiation of HK-mediated Ca2+ signaling, which was much greater than that of HK alone (Fig. 2D). Because verapamil reduces external Ca2+ entry, we suggest that the synergistic effect of the combination of HK and verapamil might be due to an increase in the release of Ca2+ from intracellular stores by HK. However, the mechanism underlying HK-evoked Ca2+ influx in the presence of verapamil or Gd3+ needs further elaboration.
Regulation of Calcium-activated Potassium and Chloride Channels by HK in HPAEC
Endothelial cells express intermediate and small conductance calcium-activated potassium channels (IKCa and SKCa, respectively) as well as calcium-activated chloride channels (ClCa) (29). IKCa and SKCa channels are activated in response to BK and contribute to NO production. Calcium-activated potassium and chloride channels play an important role in repolarizing the endothelial cells following agonist-induced Ca2+ influx to maintain an electrochemical gradient for Ca2+ entry to promote the sustained release of NO and PGI2 (24). These channels are also important for cell volume regulation during cell division, wound healing and angiogenesis (31). We therefore determined whether HK modulates IKCa, SKCa, or ClCa channels.
Using confocal microscopy, the influence of the inhibitors of calcium-activated potassium channels on HK-induced increase in [Ca2+]i in HPAEC was investigated. As shown in Fig. 2E, apamin (a SKCa channel blocker) had no significant effect on HK-induced Ca2+ response in HPAEC. However, apamin did prolong the recovery phase (Fig. 2E, inset). To further characterize the activation of calcium-dependent potassium channels by the elevation of cytosolic Ca2+ induced by HK, the effect of quinine (an IKCa channel blocker) on HK-induced Ca2+ influx was studied as well. Contrary to our hypothesis, Ca2+ influx in response to HK was only slightly affected by quinine (Fig. 2E). This finding could potentially be due to non-selectivity of quinine, as previously suggested (32). In sum, no support was found for the involvement of IKCa and SKCa channels in cytosolic Ca2+ changes produced by HK in endothelial cells.
Calcium-activated Cl− channels (ClCa) are activated by an increase in intracellular Ca2+ concentration. Studies were performed to characterize whether endothelial cell stimulation by HK involves activation of ClCa channels. ClCa are markedly suppressed by hypertonic stimulation in various cell types (33, 34). Thus, medium osmolarity was increased by adding 100 mm NaCl (200 mOsm/liter) to the bathing medium (300 mOsm/liter) and HK-stimulated increase in intracellular Ca2+ levels in cultured HPAEC was determined. HK-induced increase in intracellular Ca2+ concentration was significantly decreased in the presence of hypertonic medium (p = 0.034) (Fig. 2F), suggesting that ClCa channels contribute to HK-induced Ca2+ response in HPAEC. Thus, HK may play a role in regulating endothelial cell volume via modulation of ClCa channel activity.
HK Is Capable of Activating Bradykinin B2 (B2) Receptors in Endothelial Cells
Because HK is a precursor of BK (domain 4 of HK has the BK sequence), we proposed that HK and BK interact with a common recognition site on endothelial cells to stimulate endothelial cell activation. To test this hypothesis we determined whether HK and BK could act through a common binding site on B2 receptors.
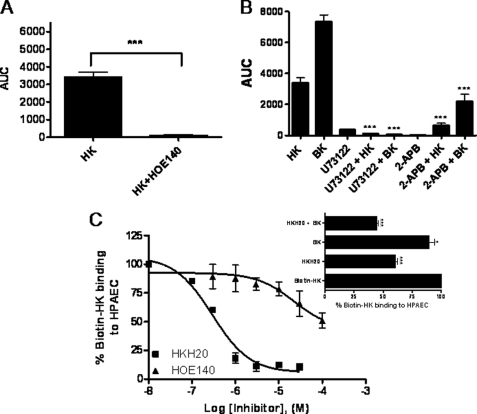
We examined the effect of HOE140 on HK-induced increase in [Ca2+]i in HPAEC. Cytosolic Ca2+ levels were unaffected by HOE140 (1 μm) alone. However, HOE140 (1 μm) significantly inhibited the increase in [Ca2+]i in response to 300 nm HK in endothelial cells (Fig. 3A). These data suggested that HK triggers the activation of B2 receptor signaling pathway in endothelial cells. To test this hypothesis, we determined the effect of HK on endothelial cell signaling pathway downstream of B2 receptors in HPAEC.
FIGURE 3.
HK activates bradykinin B2 receptors on HPAEC. Panel A, effect of HOE140 on HK-induced increase in [Ca2+]i. Panel A, HPAEC were treated with HK (300 nm) in the absence or presence of HOE140 (1 μm). Changes in [Ca2+]i levels in cells were measured using a Zeiss LSM 510 META confocal microscope at an excitation wavelength of 488 nm and an emission wavelength of 505 nm and the expressed as area under curve. Data are presented as mean ± S.E. Panel B, effect of U73122 and 2-APB on HK-induced increase in [Ca2+]i. HPAEC were treated with HK (300 nm) in the absence or presence of U73122 (5 μm) or 2-APB (100 μm). Changes in [Ca2+]i levels in cells were measured as described in panel A. Data are presented as mean ± S.E. Panel C, effect of HKH20, HOE140 and BK on biotin-HK binding to HPAEC. HPAEC (4 × 104 cells/well) were incubated with biotin-HK (20 nm) in the absence or presence of increasing concentration of HKH20 (0.1–30 μm) or HOE140 (0.3–100 μm) for 1 h at 37 °C. The binding of biotin-HK to cells was determined using ImmunoPure streptavidin horseradish peroxidase conjugate and peroxide specific fast-reacting substrate turbo-TMB. The reaction was stopped by adding 1 m phosphoric acid (100 μl) and the level of binding was determined by measuring the absorbance of the reaction mixture in each well at OD 450 nm. Inset, effect of BK and HKH20 + BK on biotin-HK binding to HPAEC. HPAEC (4 × 104 cells/well) were treated with biotin-HK (20 nm) in the absence or presence of HKH20 (0.3 μm), BK (0.5 mm), or HKH20 (0.3 μm) + BK (0.5 mm) and incubated for 1 h at 37 °C. Biotin-HK binding was determined as described in panel C. *, p < 0.05; ***, p < 0.001.
Because the initial step in B2 receptor signaling pathway is the activation of phospholipase C (PLC), experiments were performed to determine whether the inhibitors of PLC would block HK-induced [Ca2+]i increase in HPAEC. U73122 (5 μm, a PLC inhibitor) reduced HK- and BK (control)-mediated [Ca2+]i increase in HPAEC by 90% (Fig. 3B).
Activated PLC cleaves membrane phospholipid PIP2 to produce the two second messengers, IP3 and DAG. IP3 acts on IP3 receptors (IP3-R) in the endoplasmic reticular membrane to stimulate the release of Ca2+ from the intracellular stores. Ca2+ together with DAG leads to protein kinase C activation. We hypothesized that the regulation of PLC by HK would influence endothelial cell function via modulation of IP3-sensitive Ca2+ vesicle dependent signaling cascade downstream of B2 receptors. To test this hypothesis, we determined the effect of 2-aminoethoxydiphenyl borate (2-APB), an IP3-R inhibitor that blocks IP3-induced Ca2+ release from the endoplasmic reticulum, on HK-induced rise in [Ca2+]i in HPAEC. 2-APB (100 μm) significantly inhibited the HK-induced increase in cytosolic Ca2+ (Fig. 3B). These data suggested that HK stimulates B2 receptor-mediated signaling cascade in endothelial cells.
Next, to further characterize the interaction of HK with B2 receptors, the effect of HKH20, HOE140 and BK on biotin-labeled HK (biotin-HK) binding to HPAEC was determined. HKH20 (the endothelial cell binding site in domain 5 of HK) blocked biotin-HK binding to HPAEC by 90% (Fig. 3C). Consistent with previous observations by Hasan et al., HOE140 (100 μm) inhibited biotin-HK binding to HPAEC by 50%. On the other hand, BK (500 μm), in the presence of 1 μm lisinopril, an angiotensin-converting enzyme (ACE) inhibitor, reduced biotin-HK binding to HPAEC by 10% (Fig. 3C, inset). Although HOE140 and BK were much less potent than HKH20 in inhibiting biotin-HK binding to HPAEC, these observations suggest that HK does bind to specific site(s) on the B2 receptor.
HK Stimulates the Production of Nitric Oxide and Prostacyclin
Activation of B2 receptors leads to the production of nitric oxide (NO) and prostacyclin (PGI2) by endothelial cells (35). Because our data showed that HK activates endothelial cells through stimulation of B2 receptors, we determined whether HK can induce the production of NO and PGI2 in HPAEC.
The effect of HK on endothelial NO production was studied by measuring nitrate and nitrite production. When HPAEC were treated with increasing concentrations of HK it was observed that HK acutely enhanced NO production. Non-stimulated endothelial cells produced basal levels of NO which could be a result of physiological role of the constitutively active eNOS or nonspecific stimulation caused by cell growth in the microtiter plate. NO production was linearly elevated in response to increasing concentrations of HK and reached a plateau at 100 nm HK, with a Km of 5.9 nm (data not shown). 100 nm HK caused a 3-fold increase in NO production in HPAEC. Therefore, the HK concentration (100 nm) became the standard in subsequent experiments.
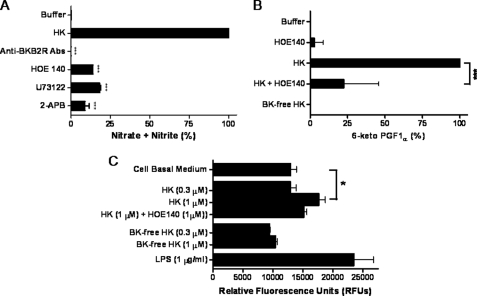
To further characterize the HK-mediated NO generation, the effects of anti-B2 antibody, HOE140 (a B2 receptor antagonist), U73122 (a PLC inhibitor), and 2-APB (an IP3-R inhibitor) were studied. Anti-B2 antibody (1:500 dilution), HOE140 (1 μm), U73122 (100 μm), and 2-APB (100 μm) significantly reduced HK-induced NO production (Fig. 4A).
FIGURE 4.
Effect of HK on NO and prostacyclin (PGI2) production and endothelial permeability. Panel A, HK induces NO generation in HPAEC. HPAEC were treated with 100 nm HK alone or with anti-B2 receptor antibody (1:500), HOE-140 (1 μm), U73122 (100 μm), or 2-APB (100 μm) and incubated for 1 h at 37 °C. The amount of nitrate + nitrite in each sample was measured using a fluorometric assay. Data are presented as mean ± S.E. Panel B, HK stimulates PGI2 production in HPAEC. HPAEC were incubated with HK (300 nm), bradykinin-free HK (BK-free HK, 300 nm), or HK (300 nm) plus HOE 140 (1 μm) for 1 h at 37 °C. The samples from each well were collected to measure the amount of 6-keto PGF1α using a competitive AChE enzyme immunoassay. The amount of 6-keto PGF1α was determined my measuring the change in absorbance at 405 nm using BioTek ELx800 Microplate Reader. Data are presented as mean ± S.E. Panel C, HK influences endothelial permeability. HPAEC monolayer grown on collagen-coated inserts was treated with cell basal medium (negative control); 1 μg/ml LPS (positive control); 0.3 μm and 1 μm HK in the presence or absence of HOE-140 (1 μm); 0.3 μm and 1 μm BK-free HK and incubated at 37 °C for 18 h. The effect on endothelial permeability was determined by adding 150 μl of 1:20 FITC-Dextran to each insert and incubating for 5 min at room temperature.100 μl of solution from each well was then transferred to a 96-well Greiner Microlon 200 Black fluorescence detection plate and the fluorescence read at an excitation wavelength of 485 nm and an emission wavelength of 528 nm. Data are presented as mean ± S.E.
6-Keto PGF1α is a stable metabolite of PGI2, which provides an accurate estimate of both resting and activated endothelial cells. We therefore examined the effect of HK on PGI2 production through the activation of B2 in HPAEC using a 6-keto PGF1α colorimetric assay. HK produced a dose-dependent increase in PGI2 production in HPAEC with a Km of 5.2 nm. As shown in Fig. 4B, treatment of HPAEC with 300 nm HK caused increased production of 6-keto PGF1α, whereas BK-free HK had no effect. HOE 140 inhibited the production of 6-keto PGF1α by HK. Further, BK-free HK was ineffective in promoting PGI2 production in HPAEC. This study demonstrated that the exposure of HPAEC to HK results in cyclooxygenase 1 (COX-1)-dependent PGI2 production.
Effect of HK on HPAEC Monolayer Permeability in Culture
Investigations were performed to determine whether HK-induced production of NO and PGI2 might be an additional mechanism for modulating endothelial permeability. While treatment of HPAEC monolayer with 0.3 μm of HK did not alter the endothelial permeability, 1 μm of HK (a concentration above the physiological range) produced a significant increase in endothelial permeability, which was blocked with the addition of HOE140 (1 μm) (Fig. 4C). These data suggested that at physiological concentration, HK increased [Ca2+]i level and NO as well as PGI2 production without influencing endothelial monolayer permeability.
DISCUSSION
We report that HK regulates endothelial cell function in the absence of PK. The major findings of this study are: 1) HK induced a concentration- and time-dependent increase in [Ca2+]i in endothelial cells; 2) endothelial cell activation by HK was mediated via its binding to B2 receptors and promoted NO and PGI2 production without influencing endothelial monolayer permeability at physiological concentration; 3) BK-free HK was ineffective in stimulating the production of PGI2 suggesting that the BK sequence within intact HK is important for the interaction of HK with B2 receptor and is responsible for HK-induced endothelial cell activation; 4) HK-induced increase in [Ca2+]i likely mediates responses such as endothelial cell volume regulation; and 5) Verapamil and Gd3+ enhanced HK-induced increase in [Ca2+]i in HPAEC.
The present study aimed at determining the effect of HK on endothelial cell function in the absence of PK. Upon the discovery that HK influences the kinetics of intracellular Ca2+ in HPAEC, we studied the mechanisms involved in HK-induced increase in [Ca2+]i. Using the inhibitors of both intracellular Ca2+ mobilization and extracellular Ca2+ influx, we identified that HK modulates endothelial cell [Ca2+]i levels through the release of intracellular Ca2+ from IP3-sensitive vesicles as well as the influx of extracellular Ca2+.
Studies have shown that extracellular Ca2+ can enter endothelial cells via five distinct pathways: (i) receptor operated Ca2+ channels (ii) store operated Ca2+ channels (iii) stretch-activated non-selective cation channels (iv) voltage-gated L-type Ca2+ channels and (v) Ca2+ leak channels (31). Based on previous studies and our current findings it could be suggested that the interaction of HK with endothelial cell membrane is very selective. The underlying mechanisms of HK interaction with cell membrane proteins have not been completely unraveled. However, this current study suggests that while other mechanisms could be involved, HK-induced influx of extracellular Ca2+ is primarily mediated through receptor (B2) and store operated Ca2+ channels. The proposed mechanism of modulation of endothelial cell function by HK and the identified signaling pathway intermediates are summarized in Fig. 5.
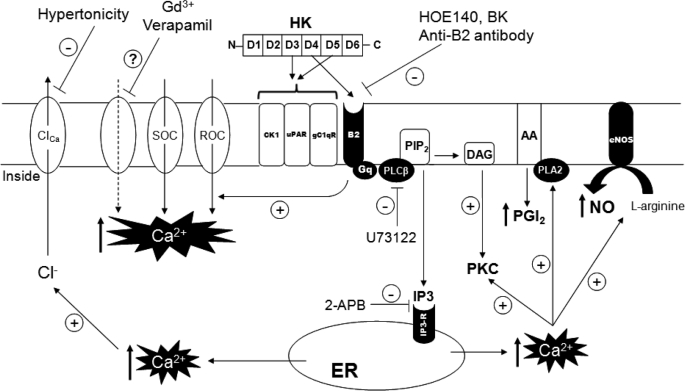
FIGURE 5.
Proposed mechanisms of HK-mediated Ca2+ signaling in endothelial cells. The binding of HK (domains 3 and 5) to cytokeratin 1 (CK1), urokinase plasminogen activator receptor (uPAR) and complement C1q receptor (gC1qR) on endothelial cells is well established. Our novel findings suggest that in addition to these proteins, HK (through its BK sequence in domain 4) directly interacts with BK B2 (B2) receptors to regulate endothelial cell functions. HK-mediated stimulation of Gq-coupled B2 receptors on endothelial cells triggers the activation of PLC-IP3-DAG second messenger signaling pathway. IP3 acts on IP3 receptors (IP3-R) on the endoplasmic reticular (ER) membrane leading to mobilization of intracellular Ca2+ from the IP3-sensitive Ca2+ pool. An increase in [Ca2+]i activates eNOS and phospholipase A2 (PLA2) to NO and PGI2 production, respectively. The known inhibitors of B2 receptor signaling pathway block NO and PGI2 formation by uncoupling the upstream signaling steps involved in the synthesis of these mediators. Endothelial cell activation in response to HK also involves extracellular Ca2+ influx into cells, mainly through B2 receptor-operated (ROC) and/or store-operated (SOC) Ca2+ channels. Gd3+, a stretch-activated Ca2+ channel inhibitor and verapamil, a L-type voltage gated Ca2+ channel inhibitor, potentiate the HK-mediated increase in [Ca2+]i through a yet unidentified mechanism (?). The increase in [Ca2+]i in response to HK activates Ca2+-activated Cl− channels (ClCa), and the inhibition of these channels is associated with a decrease in HK-induced rise in [Ca2+]i.
Ca2+ regulates cell volume by modulating the release of ions such as potassium and chloride (Fig. 5). Intermediate calcium-activated potassium channels (IKCa) and small calcium-activated potassium channels (SKCa) are expressed in endothelial cells and contribute to NO generation (36). These channels are also important for cell volume regulation during cell division, wound healing and angiogenesis (31). The activation of IKCa and SKCa currents by BK has been reported in endothelium (37). Understanding the effect of HK on the intracellular signals that control cell volume was important because both extracellular and intracellular Ca2+ have been shown to appreciably affect the cell volume. Calcium-activated potassium channels are activated by an elevation of [Ca2+]i and membrane depolarization. HK-induced increase in [Ca2+]i was not affected by quinine (IKCa blocker) or apamin (SKCa blocker), suggesting that endothelial cell activation by HK does not involve the activation of calcium-activated potassium channels. However, hypertonic stimulation of HPAEC significantly reduced the HK-mediated Ca2+ response. These findings led us to suggest that HK may play a role in regulating endothelial cell volume via modulation of calcium-activated chloride channel activity.
The binding of HK to endothelial cells via cytokeratin 1 (CK1) (38) and complement C1q receptor (gC1qR) (39, 40) is well established and plays an important role in activation of the plasma KKS on cell surface (41–43). Although previous studies indicated that HK did not bind to urokinase plasminogen activator receptor (uPAR) (41), emerging evidence suggests that the unique regions of HK might serve as urokinase-binding sites (45). Recently, it has been reported that both HK and cleaved HK bound to gC1qR, cytokeratin 1 and the soluble extracellular form of urokinase plasminogen activator receptor (uPAR) (46). These studies indicated that HK binds to the soluble extracellular form of uPAR with a much lower affinity than does HKa. The direct binding of HK to these cell membrane proteins indicated that gC1qR is dominant for binding using surface plasmon resonance (46). Furthermore, these cell membrane proteins are not directly coupled with G proteins and there is no evidence suggesting that the interaction of HK with uPAR, CK1 or gC1qR leads to changes in [Ca2+]i in endothelial cells. Further, it has been shown that optimum binding of HK to endothelial cells under physiological condition requires three regions on HK domain 3, domain 5 and BK (domain 4) (47). The existence of a novel endothelial cell binding site that recognizes a part of BK in the context of its parent molecule HK and the close apposition of HK and BK receptors to regulate the bioavailability of BK at endothelial cell surface has been previously suggested by Hasan (47). Because BK, acting via Gq coupled B2 receptors, is known to induce a robust increase in [Ca2+]i and since HK is the precursor of BK, we investigated whether the effects of HK on [Ca2+]i are mediated through its interaction with B2 receptors. We demonstrated that HK mediated its effects through the activation of B2 receptors and that B2 receptor antagonist (HOE140), PLC inhibitor (U73122) and IP3-R inhibitor (2-APB) inhibited HK-induced endothelial cell activation (Fig. 3, A and B). Competition studies using biotin-HK, HKH20 (as a control), HOE140 and BK confirmed that HK binds to B2 receptors, although with a much lesser affinity than to uPAR, CK1, and gC1qR. Although HKH20 inhibited biotin-HK binding to cell surface in a dose-dependent manner, HKH20 appears to act as an atypical synthetic peptide that does not just interact with cytokeratin 1 (38), uPAR (45) and gC1qR (48) but tends to influence the binding of HK to B2 receptors at high concentration (Fig. 3C). Of note, HKH20 did not completely abolish HK binding to cells, confirming previous finding that HK binds to cells also via domain 3 (40). Alternatively, HKH20 may directly interact with B2. Further investigations are needed to address this potential interaction. However, this property of HKH20 could be useful in treating BK-induced inflammatory (48) response and treating bacterial infections (49, 50). Nonetheless, HK interaction with B2 is strong enough to trigger the B2 receptor signaling cascade. This is a novel finding with an important physiological implication. HK, although to a lesser extent than BK, might have a direct cardioprotective effect. It has been reported that deficiency of plasma HK in the genetically susceptible Lewis rat results in decreased chronic enterocolitis and systemic inflammation (22, 51). These studies support our hypothesis and points to the importance of HK-induced transient signals in endothelial cells, although the in vivo significance of this finding is difficult to determine at this point.
HK-deficient rats are protected from peptidoglycan-polysaccharide (PG-PS)-induced inflammatory arthritis as well as endototxin-induced hypotension (30, 44). We, therefore, hypothesized that HK promotes inflammation. To test this hypothesis, we determined the effect of HK on NO and PGI2 generation as well as induction of endothelial permeability. We showed that HK stimulated B2 receptor-mediated increase in the production of NO and PGI2, the two mediators involved in inflammation. However, contrary to our hypothesis, HK at physiological concentration had no effect on endothelial monolayer permeability as determined using an in vitro assay.
In conclusion, the present study provides evidence that HK has the capability to stimulate production of endogenous vasodilators NO and PGI2 by endothelial cells. The significance of this finding might be at least 3-fold: 1) HK regulates endothelial function, 2) HK-induced increase in the production of NO, PGI2 might have a cardioprotective role in patients with PK deficiency, and 3) inhibition of both kallikrein and HK should produce more complete blockade of this pathway in patients with hereditary angioedema (HAE).
Acknowledgments
We thank Dr. Sean Wilson and Scotty Taylor for technical assistance.
This work was supported, in whole or in part, by NCRR/National Institutes of Health Grant P20RR021929 (to Z. S.-M.), National Science Foundation Grant MRI 0619774, and American Heart Association Grant 0330193N.
- KKS
- plasma kallikrein-kinin system
- HK
- high molecular weight kininogen
- BK
- bradykinin
- B2
- bradykinin B2 receptors
- AChE
- acetylcholinesterase
- 2-APB
- 2-aminoethoxydiphenyl borate
- ACE
- angiotensin-converting enzyme
- turbo-TMB
- turbo-3,3′,5,5′-tetramethylbenzindine dihydrochloride
- DAN
- 2,3-diaminonaphthalene
- PGI2
- prostacyclin
- eNOS
- endothelial nitric-oxide synthase.
REFERENCES
- 1. Smith P. L., Kagey-Sobotka A., Bleecker E. R., Traystman R., Kaplan A. P., Gralnick H., Valentine M. D., Permutt S., Lichtenstein L. M. (1980) J. Clin. Invest. 66, 1072–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma J. X., Song Q., Hatcher H. C., Crouch R. K., Chao L., Chao J. (1996) Exp. Eye Res. 63, 19–26 [DOI] [PubMed] [Google Scholar]
- 3. Takano M., Kondo J., Yayama K., Otani M., Sano K., Okamoto H. (1997) Biochim. Biophys. Acta. 1352, 222–230 [DOI] [PubMed] [Google Scholar]
- 4. Bryant J. W., Shariat-Madar Z. (2009) Cardiovasc. Hematol. Agents Med. Chem. 7, 234–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bior A. D., Pixley R. A., Colman R. W. (2007) J. Thromb. Haemost. 5, 403–411 [DOI] [PubMed] [Google Scholar]
- 6. Sainz I. M., Pixley R. A., Colman R. W. (2007) Thromb. Haemost. 98, 77–83 [PubMed] [Google Scholar]
- 7. Schmaier A. H. (2008) Int. Immunopharmacol. 8, 161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernando A. N., Fernando L. P., Fukuda Y., Kaplan A. P. (2005) Am. J. Physiol. Heart Circ. Physiol 289, H251–H257 [DOI] [PubMed] [Google Scholar]
- 9. Oschatz C., Maas C., Lecher B., Jansen T., Björkqvist J., Tradler T., Sedlmeier R., Burfeind P., Cichon S., Hammerschmidt S., Müller-Esterl W., Wuillemin W. A., Nilsson G., Renné T. (2011) Immunity 34, 258–268 [DOI] [PubMed] [Google Scholar]
- 10. Imamura T., Dubin A., Moore W., Tanaka R., Travis J. (1996) Lab. Invest. 74, 861–870 [PubMed] [Google Scholar]
- 11. Lombardi A. M., Sartori M. T., Cabrio L., Fadin M., Zanon E., Girolami A. (2003) Thromb. Haemost. 90, 1040–1045 [DOI] [PubMed] [Google Scholar]
- 12. Patrassi G. M., Martinelli S., Vianello C., Girolami A. (1982) Folia. Haematol. Int. Mag. Klin. Morphol. Blutforsch. 109, 644–654 [PubMed] [Google Scholar]
- 13. Poon M. C., Moore M. R., Castleberry R. P., Lurie A., Huang S. T., Lehmeyer J. (1982) Am. J. Hematol. 12, 261–270 [DOI] [PubMed] [Google Scholar]
- 14. Mashiko H., Miyamoto K., Takahashi H. (1999) Immunopharmacology 45, 103–105 [DOI] [PubMed] [Google Scholar]
- 15. Zhao Y., Qiu Q., Mahdi F., Shariat-Madar Z., Røojkjaer R., Schmaier A. H. (2001) Am. J. Physiol. Heart Circ. Physiol. 280, H1821–H1829 [DOI] [PubMed] [Google Scholar]
- 16. McGinnis K. M., Shariat-Madar Z., Gnegy M. E. (1998) J. Neurochem. 70, 139–146 [DOI] [PubMed] [Google Scholar]
- 17. Chajkowski S. M., Mallela J., Watson D. E., Wang J., McCurdy C. R., Rimoldi J. M., Shariat-Madar Z. (2011) Biochem. Biophys. Res. Commun. 405, 338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shariat-Madar Z., Schmaier A. H. (1999) Trends Cardiovasc. Med. 9, 238–244 [DOI] [PubMed] [Google Scholar]
- 19. Zhang J. C., Claffey K., Sakthivel R., Darzynkiewicz Z., Shaw D. E., Leal J., Wang Y. C., Lu F. M., McCrae K. R. (2000) FASEB J. 14, 2589–2600 [DOI] [PubMed] [Google Scholar]
- 20. Shariat-Madar Z., Mahdi F., Schmaier A. H. (1999) J. Biol. Chem. 274, 7137–7145 [DOI] [PubMed] [Google Scholar]
- 21. Ngo M. L., Mahdi F., Kolte D., Shariat-Madar Z. (2009) J. Inflamm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isordia-Salas I., Pixley R. A., Sáinz I. M., Martínez-Murillo C., Colman R. W. (2005) Arch. Med. Res. 36, 87–95 [DOI] [PubMed] [Google Scholar]
- 23. Dolor R. J., Hurwitz L. M., Mirza Z., Strauss H. C., Whorton A. R. (1992) Am. J. Physiol. 262, C171–C181 [DOI] [PubMed] [Google Scholar]
- 24. Adams D. J., Barakeh J., Laskey R., Van B. C. (1989) FASEB J. 3, 2389–2400 [DOI] [PubMed] [Google Scholar]
- 25. Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. (1988) Biochem. J. 253, 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy H. S., Maroughi M., Till G. O., Ward P. A. (1994) Am. J. Physiol. 267, L145–L151 [DOI] [PubMed] [Google Scholar]
- 27. Berkels R., Mueller A., Roesen R., Klaus W. (1999) J. Cardiovasc. Pharmacol. Ther. 4, 175–181 [DOI] [PubMed] [Google Scholar]
- 28. Berkels R., Taubert D., Rosenkranz A., Rösen R. (2003) Pharmacology 69, 171–176 [DOI] [PubMed] [Google Scholar]
- 29. Sauvé R., Chahine M., Tremblay J., Hamet P. (1990) J. Hypertens. Suppl. 8, S193–S201 [PubMed] [Google Scholar]
- 30. Sainz I. M., Isordia-Salas I., Castaneda J. L., Agelan A., Liu B., De La Cadena R. A., Pixley R. A., Adam A., Sartor R. B., Colman R. W. (2005) Arthritis. Rheum. 52, 2549–2552 [DOI] [PubMed] [Google Scholar]
- 31. Nilius B., Viana F., Droogmans G. (1997) Annu. Rev. Physiol. 59, 145–170 [DOI] [PubMed] [Google Scholar]
- 32. White P. J. (1996) J. Exp. Botany 47, 713–716 [Google Scholar]
- 33. O'Connor E. R., Kimelberg H. K. (1993) J. Neurosci. 13, 2638–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nilius B., Prenen J., Szücs G., Wei L., Tanzi F., Voets T., Droogmans G. (1997) J. Physiol. 498, 381–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gryglewski R. J., Uracz W., Chlopicki S., Marcinkiewicz E. (2002) Pediatr. Pathol. Mol. Med. 21, 279–290 [DOI] [PubMed] [Google Scholar]
- 36. Félétou M. (2009) Br. J. Pharmacol. 156, 545–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. (1987) Circ. Res. 61, 632–640 [DOI] [PubMed] [Google Scholar]
- 38. Hasan A. A., Zisman T., Schmaier A. H. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dedio J., Müller-Esterl W. (1996) FEBS Lett. 399, 255–258 [DOI] [PubMed] [Google Scholar]
- 40. Joseph K., Ghebrehiwet B., Peerschke E. I., Reid K. B., Kaplan A. P. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 8552–8557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colman R. W., Pixley R. A., Najamunnisa S., Yan W., Wang J., Mazar A., McCrae K. R. (1997) J. Clin. Invest. 100, 1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Joseph K., Ghebrehiwet B., Kaplan A. P. (1999) Clin. Immunol. 92, 246–255 [DOI] [PubMed] [Google Scholar]
- 43. Shariat-Madar Z., Mahdi F., Schmaier A. H. (2002) J. Biol. Chem. 277, 17962–17969 [DOI] [PubMed] [Google Scholar]
- 44. Ueno A., Ishida H., Oh-ishi S. (1995) Br. J. Pharmacol. 114, 1250–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mahdi F., Shariat-Madar Z., Kuo A., Carinato M., Cines D. B., Schmaier A. H. (2004) J. Biol. Chem. 279, 16621–16628 [DOI] [PubMed] [Google Scholar]
- 46. Pixley R. A., Espinola R. G., Ghebrehiwet B., Joseph K., Kao A., Bdeir K., Cines D. B., Colman R. W. (2011) Thromb. Haemost. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hasan A. A., Cines D. B., Zhang J., Schmaier A. H. (1994) J. Biol. Chem. 269, 31822–31830 [PubMed] [Google Scholar]
- 48. Nakazawa Y., Joseph K., Kaplan A. P. (2002) Int. Immunopharmacol. 2, 1875–1885 [DOI] [PubMed] [Google Scholar]
- 49. Oehmcke S., Shannon O., von Köckritz-Blickwede M., Mörgelin M., Linder A., Olin A. I., Björck L., Herwald H. (2009) Blood. 114, 444–451 [DOI] [PubMed] [Google Scholar]
- 50. Perkins R., Ngo M. D., Mahdi F., Shariat-Madar Z. (2008) Biochem. Biophys. Res. Commun. 366, 938–943 [DOI] [PubMed] [Google Scholar]
- 51. Isordia-Salas I., Pixley R. A., Li F., Sainz I., Balfour S. R., Adam A., Colman R. W. (2002) Int. Immunopharmacol. 2, 1895–1905 [DOI] [PubMed] [Google Scholar]