Abstract
Riboswitches are RNA regulatory elements that govern gene expression by recognition of small molecule ligands via a high affinity aptamer domain. Molecular recognition can lead to active or attenuated gene expression states by controlling accessibility to mRNA signals necessary for transcription or translation. Key areas of inquiry focus on how an aptamer attains specificity for its effector, the extent to which the aptamer folds prior to encountering its ligand, and how ligand binding alters expression signal accessibility. Here we present crystal structures of the preQ1 riboswitch from Thermoanaerobacter tengcongensis in the preQ1-bound and free states. Although the mode of preQ1 recognition is similar to that observed for preQ0, surface plasmon resonance revealed an apparent KD of 2.1 ± 0.3 nm for preQ1 but a value of 35.1 ± 6.1 nm for preQ0. This difference can be accounted for by interactions between the preQ1 methylamine and base G5 of the aptamer. To explore conformational states in the absence of metabolite, the free-state aptamer structure was determined. A14 from the ceiling of the ligand pocket shifts into the preQ1-binding site, resulting in “closed” access to the metabolite while simultaneously increasing exposure of the ribosome-binding site. Solution scattering data suggest that the free-state aptamer is compact, but the “closed” free-state crystal structure is inadequate to describe the solution scattering data. These observations are distinct from transcriptional preQ1 riboswitches of the same class that exhibit strictly ligand-dependent folding. Implications for gene regulation are discussed.
Keywords: RNA Modification, RNA Structure, Translation Regulation, X-ray Crystallography, X-ray Scattering
Introduction
Riboswitches are small, structured non-coding RNA molecules commonly located in the 5′ leader sequences of mRNA. These motifs are usually organized into a high affinity aptamer domain that binds a ligand and a downstream expression platform that possesses sequences necessary to direct transcription or translation (1). It has been estimated that as many as 4.1% of bacterial genes are controlled by such regulatory RNA elements (2, 3), with emerging evidence suggesting that some are susceptible to antimicrobial agents (4, 5).
At present, structures of nearly a dozen distinct classes of riboswitch aptamers have been solved in complex with their cognate ligands (6–8), but only three have been reported in the ligand-free state (8–11). Despite this disparity, comparisons of bound and free-state aptamers have provided valuable insight into the means by which metabolite binding transmits chemical binding information in the aptamer to distal expression platform signals that activate or attenuate transcription or translation (7, 11–13). Toward understanding this problem, we undertook a structural and functional analysis of a preQ1 riboswitch from Thermoanaerobacter tengcongensis in the metabolite-bound and free states.
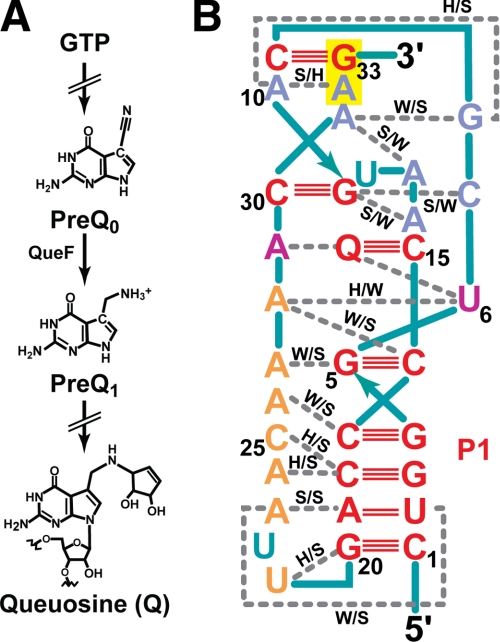
PreQ1 is a metabolic intermediate in the synthetic pathway that produces the hypermodified guanine nucleotide, queuosine (Q)2 (Fig. 1A), which is ubiquitous in the eubacterial and eukaryal domains of life (14). Whereas bacteria synthesize preQ1 de novo in a multistep reaction that starts with GTP (15, 16), eukaryotes are devoid of this pathway and acquire Q from dietary sources or gut flora. A specialized enzyme called tRNA-guanine transglycosylase is then used to insert the Q base at position 34 of select tRNAs (17). The Q modification has been invoked as a source of translational fidelity and has been implicated in bacterial virulence (18) as well as tyrosine bioynthesis in animals (19). The prevalence of Q(minus)-tRNA is correlated with neoplastic transformation and has been proposed as a metric to grade malignancies (20).
FIGURE 1.
Schematic diagrams of queuosine-precursor metabolites and preQ1 riboswitch organization. A, relationship between GTP and the hypermodified base queuosine, whose synthesis proceeds through preQ0 (7-cyano-7-deazaguanine) and preQ1 (7-aminomethyl-7-deazaguanine) intermediates via the nitrile reductase QueF. In eubacteria, TGTase (tRNA transglycosylase) inserts preQ1 into the wobble position of specific tRNAs, where it is modified further to give Q (14). B, secondary structure and nucleotide interaction diagram for the T. tengcongensis aptamer adapted from Ref. 22. The color code is as follows. Red, bases involved in Watson-Crick pairs (triple or double solid lines); orange, A-rich region; purple, strictly conserved, non-canonical base interactions with metabolite; slate, decaloop bases engaged in non-canonical pairing and base triples; teal bases are bulged. Q, the metabolite. Interactions are as follows. W, Watson-Crick face; S, sugar edge; H, Hoogsteen edge. The RBS is highlighted in yellow.
Bacterial genes involved in preQ1 biosynthesis and cellular import were found to be regulated by a small, 34-nucleotide RNA motif known as the class 1 preQ1 riboswitch (21). At present, this is the smallest known riboswitch aptamer, which was predicted to adopt a stem-loop structure followed by an A-rich tail (21). Subclasses of preQ1 aptamers were labeled type I and II based on distinct consensus sequences, although their ligand specificities were reported to be the same (21). Subsequently, three independent laboratories described high resolution preQ1 aptamer structures. The type I aptamer from T. tengcongensis was solved in the presence of preQ0 (22) and is involved in translational regulation. Its organization is notable because a significant portion of the ribosome-binding site (RBS) is located within the aptamer domain. By contrast, the type II aptamer from Bacillus subtilis was solved in complex with preQ1 and exhibits spatially distinct aptamer and expression platform domains responsible for transcriptional regulation (23, 24). Nonetheless, both aptamer types are organized as H-type pseudoknots in which ∼50% of bases engage in triples or quartet interactions (Fig. 1B). Solution analysis of type II aptamers from different species revealed folding that was strictly dependent on the presence of ligand with large structural changes observed upon preQ1 binding (23, 25, 26). Global conformational changes have also been invoked for the thiamine pyrophosphate and glycine riboswitches (27–30).
In this investigation, we conducted crystallographic and solution small angle x-ray scattering (SAXS) analyses of the T. tengcongensis riboswitch in the preQ1-bound and free states. A parallel SAXS analysis was conducted on the type II Fusobacterium nucleatum preQ1 riboswitch, which has demonstrated ligand-dependent folding properties (25, 26). We then used surface plasmon resonance to measure the affinity of ligand interactions with the T. tengcongensis aptamer and to identify key affinity determinants as well as the basis for preQ1 versus preQ0 selectivity. The results suggest a mechanism of translational attenuation in which metabolite binding is coupled structurally to the solvent accessibility of the RBS. The apparent ability of T. tengcongensis aptamer to adopt a compact, folded conformation in the free state stands in sharp contrast to the type II transcriptional aptamers.
EXPERIMENTAL PROCEDURES
Preparation of RNA Aptamers and Crystallization
All RNA strands were synthesized by Dharmacon Inc. (Fayette, CO). The 33-mer used in crystallization (Fig. 1B) was deprotected, purified by HPLC, and desalted as described (31). The 34-mer aptamer from F. nucleatum harbored a double mutation (26) and was prepared similarly. Strands used for surface plasmon resonance (SPR) were prepared with a 5′-biotin modification and comprised the wild type sequence or point variations in Table 3.
TABLE 3.
Affinity constants of the T. tengcongensis preQ1 riboswitch and its variants for metabolites
| kon × 104 | S.E. × 102 | S.D. × 104 | koff × 10−4 | S.E. × 10−6 | S.D. × 10−4 | KDa | S.D. | t½ | Krel (KD(mut)/KD(WT preQ1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| m−1s−1 | s−1 | nm | h | |||||||
| Wild type preQ1 | 7.77 | 3.55 | 3.45 | 1.53 | 1.00 | 0.47 | 2.05 | 0.29 | 1.4 | 1 |
| Wild type preQ0 | 0.65 | 0.27 | 0.13 | 2.22 | 1.04 | 0.14 | 35.10 | 6.07 | 0.9 | 17 |
| G11dG preQ1 | 8.47 | 2.94 | 3.71 | 1.42 | 0.96 | 0.58 | 1.69 | 0.22 | 1.6 | 0.8 |
| G11dG preQ0 | 0.65 | 0.20 | 0.10 | 1.83 | 1.07 | 0.27 | 28.90 | 7.7 | 1.1 | 14 |
| G5DAP/C16U preQ1 | —b | — | — | — | — | — | 2.01 × 103 | 3.32 × 102 | — | 981 |
| G5DAP/C16U preQ0 | — | — | — | — | — | — | 1.63 × 103 | 1.17 × 102 | — | 795 |
| U6C preQ1 | — | — | — | — | — | — | >2.74 × 105 | 3.05 × 104 | — | — |
| U6C preQ0 | — | — | — | — | — | — | >1.99 × 105 | 2.01 × 104 | — | — |
| A29Pur preQ1 | — | — | — | — | — | — | 1.84 × 104 | 3.34 × 103 | — | 8976 |
| A29Pur preQ0 | — | — | — | — | — | — | 1.80 × 104 | 8.42 × 102 | — | 8781 |
a Here we use the nomenclature KD with the clarification that it is an apparent KD.
b —, not determined (apparent KD obtained from equilibrium analysis) or an estimate that precludes Krel calculation.
Enzymatic Synthesis of PreQ0 and PreQ1
Synthetic preQ0 was produced as described (15, 32). PreQ0 was converted to preQ1 by the E. coli enzyme QueF, which was purified as described (33). The reaction for the enzymatic conversion contained 0.050 m potassium phosphate (pH 7.4), 0.10 m KCl, 0.5 mm NADPH, 0.5 mm preQ0, and 10 μm QueF in a final volume of 0.40 liter. The mixture was stirred gently for 8 h at 37 °C and filtered through a YM-10 (Amicon) membrane under N2(g) to remove QueF. The filtrate was lyophilized, resuspended in 0.20 liters of methanol, and stirred for 1 h to dissolve preQ1. Undissolved material was removed by filtration, and methanol was removed by rotary evaporation until only a yellow solid remained. The solid was dissolved in 10 ml of water (pH 3.8) and loaded onto a 60-ml CM Sepharose Fast Flow column (2.6 × 11.3 cm; GE Healthcare) in water (pH 3.8). The column was rinsed with 0.10 liter of water (pH 3.8), and preQ1 was eluted with a linear gradient extending to 0.5 m HCl in a volume of 0.50 liter. Fractions (5 ml) were collected and analyzed for the presence of preQ1 by LC-MS using the following protocol. 25 μl of material was injected onto a 4.6 × 250-mm Eclipse XDB-C18 column (Agilent) that had been pre-equilibrated in water. PreQ1 was eluted by developing the column with a gradient to 40% acetonitrile over 40 min at a flow rate of 0.3 ml/min. The elution was monitored by UV-visible and MS detection. UV-visible spectra were obtained from 220 to 500 nm using a ThermoFinnigan Surveyor photodiode array detector. Mass spectra were obtained in positive mode, scanning the m/z range of 100–300 atomic mass units using an electrospray ionization-equipped LCQ ThermoFinnigan Deca XP mass spectrometer. The instrument was set at a 6 V ionization energy and a 200 °C ion source temperature. Fractions containing preQ1 were pooled, lyophilized, and dissolved in 1 ml of water. The concentration of preQ1 was determined by UV absorbance using extinction coefficients published for 7-deazaguanine at pH 6.8 (34).
Crystallization and X-ray Structure Determination
The free-state riboswitch was crystallized by suspending lyophilized RNA in 0.010 m sodium cacodylate buffer, pH 7.0, to a concentration of 0.5 mm. A total of 20 μl was heated to 65 °C for 2 min before cooling to 30 °C in an aluminum block, followed by the addition of MgCl2 to a final concentration of 0.010 m. The sample was heated again to 65 °C and held for 3 min, followed by slow cooling in a 0.5-liter water bath to 20 °C over 2 h. Crystallization trials were set up using hanging drop vapor diffusion in which 2 μl of RNA was added to 2 μl of mother liquor comprising 2.0 m Li2SO4, 0.050 m MES, pH 6.0, 0.003 m MgSO4 and 0.002 m spermine at 20 °C. Crystals grew as hexagonal rods to 0.4 × 0.15 × 0.15 mm in 6–8 weeks. Metabolite-bound crystals were prepared as described (22) using preQ1 in place of preQ0. Crystals were cryoprotected in equal mixtures of Paratone-N and silicone oils (Hampton Research) and flash frozen (22).
X-ray diffraction data were collected on site at the Cornell High Energy Synchrotron Source (CHESS) (Ithaca, NY), and remotely at the Stanford Synchrotron-Radiation Lightsource (SSRL) (Menlo Park, CA) using the Blu-Ice and Web-Ice interfaces (35). Intensity data were reduced using HKL2000 (36). Structures were solved by difference Fourier methods in PHENIX (37) using the preQ0 structure (Protein Data Bank entry 3gca) as a starting model. Both structures were subjected to manual rebuilding in COOT (38). The free-state structure was subjected to Cartesian simulated annealing with rebuilding into composite omit maps. Intensity and refinement statistics are provided in Table 1. Figure graphics were generated in PyMOL (39). Solvent accessibility was calculated with AREAIMOL (40).
TABLE 1.
X-ray data reduction and refinement statistics
| PreQ1-bound + Mg2 | Free state (Mg2+ only) | |
|---|---|---|
| X-ray data reduction statistics | ||
| Protein Data Bank accession code | 3Q50 | 3Q51 |
| Beamline | CHESS A-1 | SSRL 9–2 |
| Wavelength (Å) | 0.98 | 0.98 |
| Space group | P6322 | P6322 |
| Unit cell (Å) | a = 110.8, c = 59.6 | a = 116.6, c = 57.9 |
| Total observations | 38,982 | 41,267 |
| Unique observations | 6041 | 5771 |
| Resolution (Å) | 22-2.75 (2.80-2.75)a | 27.8-2.85 (2.95-2.85) |
| Completeness (%) | 99 (96) | 99.2 (100) |
| 〈Redundancy〉 | 6.5 (5.1) | 7.2 (8.4) |
| I/σ(I) | 24.7 (2.1) | 20.6 (6.7) |
| Rsym (%)b | 7.1 (43.6) | 7.5 (35.7) |
| Refinement statistics | ||
| Rcryst/Rwork/Rfree (%)c,d,e | 21.4/21.0/25.2 | 23.1/22.6/27.2 |
| Geometry | ||
| Root mean square bonds (Å) | 0.007 | 0.006 |
| Root mean square angles (degrees) | 1.689 | 0.844 |
| Coordinate error (Å) | 0.35 | 0.40 |
| No. of atoms/B-factors | ||
| RNA | 702/56.6 Å2 | 680/56.7 |
| Metabolite | 13/47.7 Å2 | None |
| SO42− | 20/67.5 Å2 | 5/51.8 Å2 |
| Mg2+ | None | 1/60.3 Å2 |
| Anisotropy correction | B11:12.6 B33:−25.2 | B11:10.6 B33:−21.2 |
a Values in parentheses represent high resolution statistics.
b Rsym = [Σ|I(h)j − 〈I(h)〉|/Σ|I(h)j|] × 100, where I(h)j is the intensity of the jth reflection of type h, and 〈I(h)〉 is the average intensity of a reflection of type h.
c Rcryst represents a final cycle of refinement without cross-validation using the residual target and all reflections.
d Rwork = [Σhkl|(Fo) − k(Fc)|]/[Σhkl|(Fo)|] × 100, where k is a scale factor and the test set has been removed.
e Rfree is defined as the Rwork calculated for 9% of the x-ray data selected randomly and excluded from refinement.
SAXS
Experiments were conducted at the G1 station of CHESS. Using a wavelength of 1.29 Å, scattered X-rays were recorded on a CCD detector at a sample-to-detector distance of 94.2 cm. This configuration gave an accessible q range from 0.025 to 0.35 Å−1, where q = 4πsinθ λ−1, in which 2θ is the scattering angle and λ is the radiation wavelength. Samples of the preQ1 aptamer were prepared in the presence or absence of metabolite in 0.010 m sodium cacodylate, pH 6.0, with 0.010 m MgCl2. Three sample concentrations were utilized, including 0.075, 0.20, and 0.55 mm. Microcentrifuged samples were transferred to disposable cells (41) held at 20 °C. Buffer blanks were collected for each cell. Exposures were recorded as 3 × 3 s followed by 3 × 9 s series. Scattering data for the 3 × 9 s exposures were integrated by Data Squeeze, version 2.20 (P. Heiney) to yield the respective one-dimensional intensity profiles. No evidence of radiation damage was observed based on a comparison of initial versus final exposures. Buffer corrections and extrapolation to zero concentration were accomplished using PRIMUS (43); it has been noted that extrapolation to infinite dilution can circumvent problems with interparticle interference that can cause inaccurate measurements (44). Kratky plots were generated for the full range of data, and Guinier plots were calculated with PRIMUS. GNOM was used to produce the paired distance distribution functions, which were optimized to meet visual perception criteria as described (45). Dimensions of crystal structures and fits between experimental and crystallographic scattering profiles were calculated using CRYSOL (46); additional dimensions were obtained by use of MOLEMAN (47). Relevant SAXS parameters are reported in Table 2.
TABLE 2.
Solution dimensions from small angle x-ray scattering
| RgG | RgP | rmax | |
|---|---|---|---|
| Å | Å | Å | |
| Wild type preQ1 + Mg2+ | 16.6 ± 0.02 | 16.5 ± 0.01 | 51 ± 5 |
| Wild type preQ0 + Mg2+ | 16.9 ± 0.02 | 16.9 ± 0.01 | 57 ± 6 |
| Wild type Mg2+ | 17.0 ± 0.02 | 17.0 ± 0.01 | 57 ± 6 |
| F. nucleatum preQ1 + Mg2+ | 19.3 ± 0.03 | 19.3 ± 0.01 | 64 ± 6 |
| F. nucleatum Mg2+ | 31.0 ± 0.07 | 30.9 ± 0.02 | 107 ± 11 |
| Crystal coordinates, preQ0/Q1 | 16.9a | 50b | |
| Crystal coordinates, free state | 17.0a | 52b |
SPR
NeutrAvidin-conjugated CM5 chips were prepared on a Biacore T100 instrument (Biacore Inc., Piscataway, NJ) using reagents from the manufacturer. Biotinylated free-state preQ1 aptamers and variants thereof (Table 3) were suspended in running buffer (0.010 m sodium cacodylate, pH 7.0, and 0.003 m MgCl2) to a concentration of 400 nm. RNA aptamers were injected over NeutrAvidin-conjugated flow cells to give a surface density of 3800–4000 response units, with the reference flow cell having only NeutrAvidin. For kinetic experiments, the association phase consisted of 180-s injections of preQ1 or preQ0 conducted at concentrations ranging from 1 to 250 nm at 75 μl/min; dissociation was monitored for 600 s. Regeneration was conducted using 3 m guanidine HCl for 180 s followed by a 120-s incubation in running buffer flowing at 30 μl/min. Equilibrium binding experiments were carried out as follows. Injections of metabolite lasted for 240 s at 30 μl/min and were conducted with concentrations ranging from 0.470 to 400 μm. A dissociation phase of 400 s ensued in running buffer. Regeneration was accomplished using 3 m guanidine HCl for 90 s, followed by a 160-s stabilization period in running buffer at 30 μl/min. All measurements were made in duplicate at 25 °C with a data collection rate of 10 Hz. Experimental data were processed using Biacore T100 Evaluation software version 2.2 (GE Healthcare) and the double referencing method (48) to remove instrumental and bulk shift effects. The buffer-subtracted sensorgrams for the kinetic binding data were then fit to a 1:1 binding interaction model to determine the rate constants (kon and koff) and the apparent equilibrium binding constant (KD). To evaluate the steady-state equilibrium binding data, the response during the equilibrium binding phase (Req) was calculated by averaging the response over 5 s just before the end of the metabolite injection. Data were fit using non-linear curve fitting using PRISM4 (GraphPad, Inc.) to a one-site binding equation to determine KD.
RESULTS
Quality Indicators and Overall Fold of the PreQ1-bound Riboswitch Aptamer
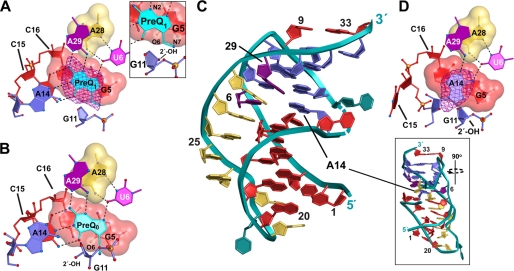
To understand the basis of metabolite specificity and gene regulation by the class 1, type I T. tengcongensis preQ1 translational riboswitch, we undertook a structure determination in the presence of the primary target ligand, preQ1 (Fig. 1A). The entire 33-mer RNA aptamer was observed in electron density maps as well as the preQ1 ligand, whose omit electron density map is representative of the overall quality of the model (Table 1 and Fig. 2A). The hydrogen bonding pattern, global fold, and location of the metabolite closely matched features reported previously for the preQ0-bound state (Figs. 1B and 2B) (22). The quality of the 2.75 Å resolution preQ1-bound structure is indicated by its Rwork and Rfree values of 21.0 and 25.2%, respectively (Table 1), which are substantially lower than the preQ0-aptamer complex, whose Rwork and Rfree values were 24.5 and 27.2% (22). Despite identical diffraction resolutions, B-factors for the preQ1-bound state were 57 Å2 for RNA atoms and 48 Å2 for preQ1 (Table 1) as compared with B-factors of 76 Å2 for RNA atoms and 58 Å2 for preQ0 of the preQ0-bound state.
FIGURE 2.
Schematic representations of the preQ1 riboswitch in the free and preQ1-bound states. A, aptamer interactions with preQ1 looking down the helical axis of the riboswitch. The “floor” of the binding pocket is shown as a space-filling model; putative hydrogen bonds are shown as dashed lines. The location of the ligand is indicated by an averaged “kicked” (66) or AK (mFo − DFc) omit electron density map with σA weighting contoured at 4σ; the ligand (cyan) was removed from the phase calculation. Inset, close-up view of the preQ1 methylamine in relation to the G11 2′-OH group and the G5 O6 keto moiety. Neighboring groups of G5 are highlighted with a semitransparent circle and labeled; the electron density has been removed for clarity. B, aptamer interactions with preQ0 based on Protein Data Bank entry 3cga as described previously (22). C, ribbon diagram of the free-state riboswitch. The phosphate backbone is depicted as a teal ribbon with bases colored as in Fig. 1B; inset, a view rotated 90° to accentuate the location of A14 within the ligand-binding pocket. D, schematic diagram depicting A14 of the free-state structure; the purine base was removed from the phase calculation to generate an AK (mFo − DFc) omit map as in A.
Quality Indicators and Overall Fold for the Free-state Aptamer
To understand the conformational changes involved in RNA-mediated gene regulation by sequestration of the RBS, we determined the ligand-free T. tengcongensis aptamer structure (Fig. 2C). Compared with the preQ1-bound state, free-state crystals showed a 7.6% increase in the unit cell volume (Table 1), suggestive of significant structural differences. Although the overall fold of the free-state structure is comparable with the compact 48 Å × 28 Å × 15 Å preQ1-bound state, changes occurred in the RNA backbone flanking the metabolite-binding pocket. No electron density was observed for A13 of the decaloop, indicated as a dashed line in the backbone of Fig. 2C, and the quality of the electron density in this region was generally poorer than that of either ligand-bound state. The overall RNA B-factors were comparable at 57 Å2, but the Rwork and Rfree values were slightly worse than the preQ1-bound state (Table 1). Significantly, the electron density was of sufficient quality and resolution to observe conformational changes in the decaloop at C15 and A14 that are consistent with changes resulting from ligand-free crystallization as opposed to crystal packing because this area is devoid of lattice contacts. Reduced bias omit-electron density maps, such as that calculated for the A14 base of the free-state structure (Fig. 2D), indicate the quality of the model in this region. The level of change relative to the coordinate error (Table 1) is sufficiently large to permit a meaningful structural comparison on both global and local levels.
Comparison of the PreQ1-bound and Free-state Crystal Structures Reveals a Network of Localized Conformational Changes
To contrast the preQ1-bound and free state aptamers, an all-atom superposition was conducted (Fig. 3A). Although only a few nucleotides differed significantly in their relative positions, this was sufficient to elevate the root mean square displacement of atoms to 1.7 Å. The maximum displacement was 7.4 Å, which corresponded to the N1 imino of A14. The nucleotide at this position rotates out of a stable G11 to A14 (N3-amino amino-N1) pair flanking the ligand pocket to occupy the preQ1 binding site (Fig. 3B, compare dark blue A14 to light blue A14). Other pronounced changes cluster in the pseudoknotted decaloop surrounding the preQ1 binding site. C15 rotates away from the binding pocket, a 6.6-Å movement of N3, from a location where it base-pairs with preQ1 (Fig. 2, A versus D, and Fig. 3, dark blue C15 versus red). By contrast, U12 shifts by 4.4 Å toward the binding pocket closer to the position vacated by A14. Finally, base A32 moves away from the binding pocket in the free-state structure by 2.3 Å. These changes appear interconnected to the A14 shift, which has the effect of eliminating the continuous base stack that links A32 and G33 of the RBS to preQ1 in the aptamer core in the ligand-bound state.
FIGURE 3.
All-atom superposition of the respective free-state and ligand-bound structures. A, schematic stereo view of the global, free-state structure (colored as in Fig. 2) superimposed upon the preQ1-bound structure (dark blue) using LSQKAB (42). Black arrows indicate the movements of bases in the preQ1-bound structure relative to those of the free state. The start of the ribosome-binding site at A32 is labeled RBS. The RBS portion of the aptamer is highlighted in yellow. The preQ1 metabolite is labeled Q1. A ribbon passing through the phosphate backbone is shown in teal (free state) and blue (preQ1-bound). B, close-up stereo diagram of A (rotated +90° along the x axis) depicting the superposition of select decaloop nucleotides. Putative hydrogen bonds to preQ1 in the ligand-bound structure are drawn as broken lines. Nucleotides that superimpose with a root mean square deviation of >2.0 Å are labeled twice for the respective ligand-bound (blue) or free state (colored as in Fig. 2C) structures. Base G11 was rendered with thin bonds for clarity; here only the preQ1-bound state is shown because of its close similarity to the free-state structure.
Molecular Recognition of PreQ1 and Closure of the Binding Pocket in the Free State
Although the mode of preQ0 binding has been described for the T. tengcongensis riboswitch aptamer (22), the mode of preQ1 binding has remained uncertain until now. The compact architecture of the aptamer buries 330 of 337 Å2 of the preQ1 metabolite fortifying the core structure by generating a network of continuously stacked bases that join the RBS at the “top” of the aptamer to the P1 stem (Figs. 1B and 3A). Like preQ0, the Watson-Crick face of preQ1 is “read out” by C15 (Fig. 2, A and B), and conserved bases U6 and A29 recognize the minor groove edge of both metabolites. The modified guanine base of the metabolite stacks between the G5-C16 base pair of the P1 stem (Fig. 2A) and bases G11 and A14 (Fig. 3). These interactions have been referred to as the respective “floor” and “ceiling” of the binding pocket (22). However, in the absence of metabolite, the pocket adopts a “closed” conformation whereby binding to preQ1 is blocked by A14, which spatially overlaps the ligand-binding site (Fig. 3B) and pairs with key bases involved in ligand recognition, most notably U6 and A29 (Fig. 2D). Because A14 forms part of the binding pocket ceiling, its shift in the free state effectively collapses the ligand recognition site and directly increases the solvent accessibility of the RBS from 468 to 511 Å2, which starts at position A32 of the aptamer (Figs. 1B and 3A and supplemental Fig. S1).
Small Angle X-ray Scattering Analysis of the T. tengcongensis and F. nucleatum PreQ1 Aptamers
An important question regarding the observed preQ1-bound and free state crystal structures is whether they are consistent with (i) the compactness and (ii) the conformations present in the solution state. To address these questions, we subjected the T. tengcongensis riboswitch to SAXS analysis. We also examined the F. nucleatum 34-mer class 1, type II preQ1 aptamer as a control, which was chosen for its pronounced ligand-dependent folding (25, 26).
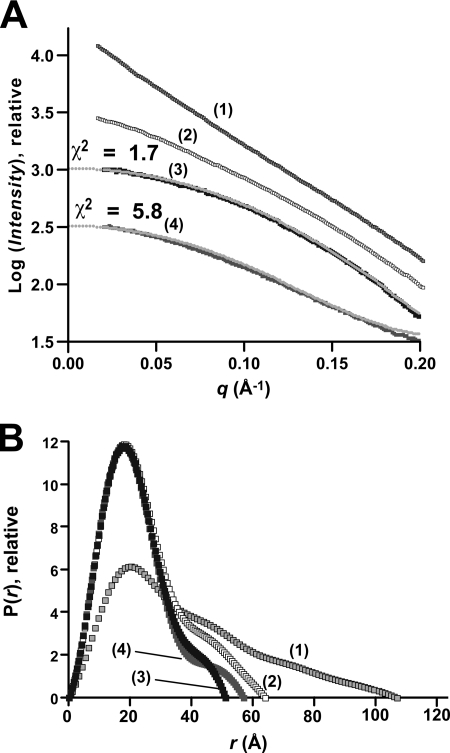
The results revealed smooth intensity distributions with gently changing slopes for both aptamer types (Fig. 4A). Sudden upturns or downturns were not observed, consistent with an absence of aggregation and interparticle repulsion, respectively (49). Visual comparisons indicated that the free-state F. nucleatum aptamer has a more negative slope at low q than the matched sample prepared in the presence of preQ1 (Fig. 4A, curve 1 versus curve 2). In contrast, the F. nucleatum sample in the presence of preQ1 is more similar to the T. tengcongensis samples (Fig. 4A, curves 2–4). Based on visual criteria (49), the quality of the scattering profiles was deemed suitable for dimensional analysis.
FIGURE 4.
Small angle x-ray scattering analysis of preQ1 riboswitches from T. tengcongensis and F. nucleatum in metabolite-bound and free states. A, scattering profiles (thick lines) derived from experimental measurements and those calculated from T. tengcongensis crystal structures (thin gray lines) in Table 1. The samples are free-state F. nucleatum with 10 mm Mg2+ (1), preQ1-bound F. nucleatum with 10 mm Mg2+ (2), preQ1-bound T. tengcongensis with 10 mm Mg2+ (3), and free-state T. tengcongensis with 10 mm Mg2+ (4). The agreement between the scattering profiles from the experimental SAXS data and crystallographic coordinates of the respective T. tengcongensis preQ1-bound and free-state crystal structures is given by χ2 as defined by CRYSOL (46). B, comparison of the paired distance distribution plots, P(r), for the samples in A. The real-space RgP and maximum intraparticle distance, rmax, from these functions are reported in Table 2. The functions were scaled relative to a reference according to the integrated areas of each respective curve.
Comparison of Ligand-induced Compaction of PreQ1 Aptamers
To evaluate the degree to which the T. tengcongensis translational riboswitch undergoes ligand-dependent compaction, we used three criteria: (i) reduction of radius of gyration (Rg) values derived from both Guinier plots (RgG; supplemental Fig. S2B) and paired distance distribution functions (RgP; i.e. P(r) plots in Fig. 4B and supplemental Fig. S2C); (ii) reduction of the longest interatomic distance rmax in a sample from P(r) functions; and (iii) the shapes of Kratky plots, which show distinct dependences of momentum transfer-weighted intensity versus q for folded versus unfolded polymers (50) (supplemental Fig. S2D).
Significant differences in ligand-dependent compaction were observed between the F. nucleatum and T. tengcongensis riboswitch aptamers. The F. nucleatum “control” aptamer in the absence of ligand had a significantly larger Rg of 31.0 Å and longer rmax of 107 ± 11 Å compared with its preQ1-bound state, in which the Rg was 19.3 Å and rmax was 64 ± 6 Å (Fig. 4B and Table 2). The broad, heavy tailed Kratky plots of the free-state F. nucleatum aptamer were consistent with partial folding in the absence of preQ1 (supplemental Fig. S2D), whereas the bell-shaped, light-tailed profile of the preQ1-bound aptamer was characteristic of a well folded polymer (supplemental Fig. S2D) (49, 50). Conversely, the T. tengcongensis aptamer appeared as compact in the free state with RgG = 17.0 Å and rmax = 57 ± 6 Å, as it is in the preQ1- and preQ0-bound states in which the respective RgG and rmax values were 51 ± 5 and 16.6 Å and 57 ± 6 and 16.9 Å (Table 2). Kratky profiles for each of the T. tengcongensis samples were comparable with the preQ1-bound F. nucleatum aptamer, indicative of folded RNA (supplemental Fig. S2D).
Comparison of Crystal Structures with Solution SAXS Data
We then asked whether our crystal structures were representative of solution dimensions and conformational states. The experimentally measured RgP and rmax values were in reasonable agreement with those calculated from the respective preQ1-bound, preQ0-bound, and free-state crystal structures but with notable differences. The best agreement corresponded to the preQ1-bound state in which the crystal structure RgP and rmax values were 16.9 and 50 Å versus the solution RgP and rmax values of 16.5 and 51.5 Å (Table 2). The solution RgP values for the preQ0-bound and free-state samples agreed exactly with crystal structures at 16.9 and 17.0 Å, respectively (Table 2). However, the corresponding solution rmax values for the preQ0-bound and free-state samples were each 57 Å, which is longer than the corresponding crystal structures, whose values were 50 and 52 Å, respectively. This finding may be the result of greater mobility in solution or conformational restraints imposed on the coordinates by the crystal lattice, which can only accommodate an rmax of ∼52 Å (discussed below).
To assess the agreement between the intensity profiles calculated from crystal structures in Table 1 versus those corresponding to the experimental data, we calculated χ2 values (46). The results revealed that the preQ1-bound crystal structure has a χ2 value of 1.7, indicating reasonable agreement between the atom model and the solution conformation (11). In contrast, the free-state crystal structure yielded a χ2 of 5.8. Deviations in the intensity fit can be seen at several distinct points along the curve, especially a medium and high q (Fig. 4A, curve 4). This level of discrepancy is strong evidence that the crystallographic model is insufficient to describe the solution conformational ensemble and that differences between the bound and free states are more complex than suggested by a comparison of the corresponding crystal structures, despite similarities in compactness demonstrated by RgP and rmax.
Kinetics and Affinity Constants for PreQ1 and PreQ0 Binding to the T. tengcongensis Aptamer
PreQ0 and preQ1 differ by substitution of a cyano group for a methylamine (Fig. 1A). To evaluate the kinetics and thermodynamics of ligand binding, we measured the rates of binding and dissociation to the T. tengcongensis aptamer using surface plasmon resonance (Fig. 5, A and B). The results revealed that preQ1 binds with an on-rate of 7.77 × 104 m−1 s−1, which is 12-fold faster than the on-rate for preQ0 (Table 3). Both metabolites exhibited similar off-rates at 1.53 × 10−4 s−1 and 2.22 × 10−4 s−1, respectively. The kon for the T. tengcongensis aptamer is 8-fold slower than preQ1 binding to the F. nucleatum 34-mer aptamer, but koff for the F. nucleatum aptamer is >103-fold faster (25). The preQ1 on-rate of the T. tengcongensis aptamer falls into a range of values reported for other translational riboswitches, including the Escherichia coli thiamine pyrophosphate aptamer at 8.66 × 104 m−1 s−1 (51) and the Vibrio vulnificus adenine aptamer at 3.75 × 104 m−1 s−1 (52). By contrast, the off-rate of the T. tengcongensis aptamer is 2 orders of magnitude slower than the TTP (0.043 s−1) and adenine (0.017 s−1) aptamers. At present, the slowness of the T. tengcongensis off-rate is surpassed only by the Vibrio cholerae c-di-GMP riboswitch, a transcriptional regulator whose off-rate is 850-fold slower (53).
FIGURE 5.
Kinetic and affinity analysis of the T. tengcongensis riboswitch aptamer for preQ1 and preQ0. A, representative surface plasmon resonance sensorgram for preQ1 interactions with the wild type aptamer. B, representative surface plasmon resonance sensorgram for preQ0 interactions with the wild type aptamer. The thick black lines represent the buffer-subtracted data, and gray lines are the global fit obtained from a 1:1 binding model based on the experimental data. The resulting kon and koff rates and the apparent equilibrium KD values are reported in Table 3. C, A29Pur mutant response at equilibrium (Req) plotted against the concentration of preQ1 or preQ0. D, the U6C mutant as described in C. E, the G5DAP/C16U double mutant as described in C. The plots of Req correspond to preQ1 data points (filled squares) or preQ0 data points (open squares); error bars are shown. The equilibrium binding constant KD was determined for each metabolite by curve fitting (black or gray lines) to the respective metabolite Req data points as described under “Experimental Procedures.” The apparent KD values are reported in Table 3.
The T. tengcongensis aptamer apparent equilibrium dissociation constant (KD) was 2.05 ± 0.29 nm for preQ1 and 35.10 ± 6.07 nm for preQ0. The magnitude and affinity trends mirror values reported previously for these metabolites during an analysis of a longer B. subtilis aptamer (20 and 100 nm, respectively) using equilibrium dialysis (21). By contrast, the F. nucleatum aptamer showed a KD of 283 nm for preQ1, making the T. tengcongensis aptamer a significantly tighter preQ1 binder than either of the transcriptional riboswitches in its class.
Using Mutagenesis and SPR to Probe T. tengcongensis Riboswitch Aptamer Binding to Metabolites
Specific predictions about the molecular interactions between the T. tengcongensis aptamer and preQ1 or preQ0 can be proposed based on new and prior structural observations (Fig. 2, A and B) (22). To test the importance of key aptamer functional groups consistent with hydrogen bonding to preQ1 or preQ0 and to differentiate the modest difference in ligand-binding affinity, we undertook an SPR analysis of specific site-directed mutants that were placed into two categories: (i) those that recognize guanine-like features, and (ii) those that promote preQ1 binding but not necessarily preQ0.
Mutagenesis of T. tengcongensis Aptamer Bases That Recognize Guanine-like Features Shared by PreQ1 and PreQ0
The first class of mutants examined were those predicted to interact with the guanine-like features held in common by preQ1 and preQ0. Prior analysis indicated that position C15 (T. tengcongensis numbering) is a universally conserved base in the class 1 preQ1 aptamer family and that modifications to this base ablated preQ1 binding or could be used to alter ligand specificity in a manner consistent with Watson-Crick pairing (21). This finding was corroborated subsequently by three independent structure determinations (22–24), which also revealed the locations of other key groups involved in metabolite recognition. Of special importance are universally conserved bases U6 and A29 because they each recognize the same minor groove edge features of preQ1 and preQ0 (Fig. 2, A and B) (22).
Our mutagenesis results revealed that the A29Pur and U6C variants each have detrimental effects on preQ1 and preQ0 binding but to varying extents (Fig. 5, C and D). The A29Pur variant is devoid of an exocyclic amine and was predicted to eliminate a single hydrogen bond to the metabolites (Fig. 2, A and B). The resulting KD values were 18.4 μm for preQ1 and 18.0 μm for preQ0, which is nearly 9000-fold worse than wild type binding to preQ1 (Table 3). Loss of the A29 amine also ablates a core folding interaction with the O2 keto of C7 (not shown) that may account for a loss in binding affinity for both ligands that is significantly greater than removal of a single hydrogen bond.
The U6C variant was also predicted to eliminate a single hydrogen bond between the aptamer O4 keto group and the N9 imine of the ligand pyrrolo ring (Fig. 2, A and B). However, the U6C change introduces a steric block from replacement of a keto with an amine. This substitution may account for the greater relative loss in binding affinity of this variant compared with A29Pur in which a functional group was eliminated. As expected, the U6C change proved detrimental for preQ1 and preQ0, whose KD values were estimated to be >274 and >199 μm, respectively (Table 3). Like A29Pur, the U6C mutation also has the potential to eliminate two core hydrogen bonds between the Watson-Crick face of U6 and the Hoogsteen edge of A28 (Fig. 2, A and B).
The 2′-OH group of G11 was observed to be within hydrogen bonding distance of the O6 keto group of preQ0 (Fig. 2B) and was perceived to be a potential contributor to metabolite recognition (22). However, this interaction was not present in the preQ1-bound state due to an apparent change in the ribose pucker (Fig. 2, A (inset) versus B). Instead, the G11 ribose hydroxyl is oriented close to the methylamine of preQ1, although it is not in an optimal hydrogen bonding orientation. Mutation of the 2′-OH group of G11 to 2′-deoxy G11 revealed no significant deviation in either preQ0 or preQ1 binding but showed slightly more favorable binding properties (Table 3). The fact that the 2′-deoxy G11 variant had no substantial effects on the binding affinity or kinetics of either metabolite appears to rule out a role for this group in metabolite selectivity.
Mutagenesis of T. tengcongensis Aptamer Base Interactions That Promote PreQ1 Binding but Not PreQ0
A second category of mutants was designed to identify aptamer functional groups that contribute to the higher affinity of preQ1 over preQ0 (2.05 nm versus 35.10 nm, respectively; Table 3). The crystal structure of the T. tengcongensis aptamer bound to preQ1 provides some insight into this question because the methylamine group is within 4.0 Å of the O6 and N7 groups of conserved base G5 (Figs. 2A (inset) and 3B). However, a sulfate ion bound to the preQ1 methylamine precludes a cogent interpretation (Fig. 3B).
Previously, the G5 base was shown to be universally conserved in preQ1 class I aptamers (21). Changes in the J1 stem at position C16 (Fig. 1A) as well as flanking base pairs showed poor responses to ligand binding or failed to elicit metabolite-dependent gene regulation (21). Although no functional analysis was reported for G5, T. tengcongensis crystal structures bound to metabolites suggested that the substitution of 2,6-diaminopurine (DAP) in the context of the double mutation, G5DAP/C16U, would replace the G5 O6 keto group with an N6 amine. A comparable mutational analysis (21) demonstrated that this variant adopts a Watson-Crick-like base pair, which provided confidence that the floor of the binding pocket would remain intact. However, the N6 exocyclic amine from DAP is predicted to disrupt hydrogen bonding to the preQ1 methylamine, whereas this interaction cannot occur with preQ0 due to the presence of its linear cyano moiety (Figs. 1A and 2, A and B).
The results revealed KD values of 2.01 ± 0.33 and 1.63 ± 0.12 μm for preQ1 and preQ0, respectively (Fig. 5E and Table 3). The relative losses in affinity suggest a nearly 103-fold reduction in preQ1 binding but only a 46-fold loss by preQ0, thus providing evidence for involvement of the G5 O6 keto in binding preQ1 but not preQ0.
DISCUSSION
Riboswitches regulate gene expression by altering accessibility to sequences that signal transcription or translation. Prior structural investigations of the preQ1 riboswitch focused on type II aptamers that function transcriptionally by altering the interconversion of terminator and anti-terminator stem-loops (23–25). Here we examined the structural and functional properties of a type I aptamer from T. tengcongensis involved in translational regulation. A32 of the aptamer sequence (Fig. 1B) appears to be the start of the RBS based on the Shine-Dalgarno consensus sequence and base-pairing energy with the 16 S rRNA (supplemental Fig. S1). This assignment places the last nucleotide of our construct, G33, as the second base of the RBS (Fig. 1B) and has implications for how the mode of preQ1 binding by the T. tengcongensis riboswitch can regulate translation.
Crystallographic Conformational Changes Reveal Differences in RBS Accessibility
Comparisons of the preQ1-bound and ligand-free structures of the T. tengcongensis riboswitch provide the basis to identify ligand-dependent conformational changes that may lead to RNA-mediated gene regulation. A superposition of these structures revealed changes in and around the binding pocket that suggested the absence of metabolite causes a shift of base A14 such that it interacts with preQ1 binding determinants U6 and A29 (Figs. 2 (A versus D) and 3). The position of A14 in the free state coincides with unstacking of bases A32 and G33 of the RBS from the core fold, which increases their solvent accessibility relative to the preQ1-bound state. Although this difference is modest in the context of the crystal lattice, the tangible increase in accessibility (Fig. 3A) is illustrative of how the 16 S rRNA of the ribosome could gain greater access to the RBS in the free-state structure, thereby resulting in a “gene-on” state.
Modes of PreQ1 and PreQ0 Binding
The mode of metabolite binding by members of the class I preQ1 riboswitch family appears to be conserved insofar as the B. subtilis (type II) and T. tengcongensis (type I) aptamer structures utilize a common set of core bases for recognition. Our functional analysis supports the importance of U6 and A29 in recognition of both the preQ0 and preQ1 metabolites (Table 3), thus corroborating crystallographic observations. Efforts to understand the molecular basis for the affinity preference of preQ1 over preQ0 led us to examine the G5DAP/C16U double mutant. Our crystal structure shows close proximity between the preQ1 methylamine and the O6 keto of G5 (Fig. 2A, inset). However, a sulfate ion from the mother liquor is bound to the methylamine group of preQ1 (Fig. 3B), thereby obfuscating its potential for recognition by the aptamer. We hypothesized that substitution of the G5 O6 keto with a DAP N6 amine would sterically occlude binding of the preQ1 methylamine and prevent formation of a hydrogen bond. Differences in the KD values between preQ1 and preQ0 correspond to a free energy change of 1.68 kcal/mol. This modest difference suggests no more than 1–2 neutral hydrogen bonds (54). Our results for this mutant suggested a slight favorability in preQ0 binding over preQ1, which seems considerable given the 17-fold greater affinity that wild type exhibits for preQ1 over preQ0 (Table 3). Such a trend supports the expectation that the G5DAP mutation is more detrimental for preQ1 than preQ0 due to the steric block created by the DAP N6 amine. Although the deletion of the G5 keto might appear to be a more conservative probe for hydrogen bonding, we examined a G52AP (2-amino-purine) mutant devoid of an O6 keto moiety and measured the KD for preQ1 at >914 μm (data not shown). Changes in mutant affinity for metabolites relative to wild type suggest that substitutions in the aptamer core have additional long range effects that go beyond energy differences expected for loss of 1–2 core hydrogen bonds.
A comparative structural analysis provided further insight into the basis of preQ1 versus preQ0 specificity. Commonalties are apparent in the mode of preQ1 recognition by conserved bases shared by the T. tengcongensis aptamer and the known B. subtilis aptamer structures. This makes it possible to understand the basis for preQ1 specificity for all class 1 preQ1 riboswitch family members. In each independent B. subtilis structure, the methylamine of preQ1 is sufficiently close to the O6 keto and N7 imino of G5 that the metabolite is within hydrogen bonding distance of these groups (23, 24). These interactions are not present when preQ0 is bound to the T. tengcongensis aptamer (22) and would account for the observed energy difference that favors preQ1 affinity over preQ0. Although structures of the B. subtilis aptamer also show that the preQ1 methylamine is within hydrogen bonding distance of the phosphate backbone, this interaction is not observed in the T. tengcongensis aptamer bound to preQ1 (this work) or preQ0 (22). If this interaction were a means of discriminating the cyano group from the methylamine, the additional energetic difference favoring preQ1 binding would be appreciable, adding another ∼3 kcal/mol (54). Further experiments will be necessary to establish the degree of conservation that this non-base interaction exhibits among family members and its role in preQ1 affinity relative to preQ0.
Despite the lower affinity of the T. tengcongensis aptamer for preQ0 compared with preQ1, the on-rates differ by only 12-fold, whereas the off-rates are comparable with preQ0 dissociating 1.5-fold faster. Kinetic analysis of the QueF nitrile reductase (Fig. 1A) from B. subtilis showed a Km of 237 μm with a kcat of 0.69 min−1, which are values comparable with the kinetic constants of other Q synthesis pathway enzymes (16). Assuming that these enzymes operate at steady-state levels and that Km is a reasonable upper limit for substrate dissociation from the enzyme-substrate complex (55), then it is reasonable that preQ0 concentrations are abundant in the cell at levels far exceeding its measured affinity in the context of the T. tengcongensis aptamer (KD = 35.1 nm). As such, it appears plausible that the T. tengcongensis aptamer could respond to preQ0 in gene regulation. This possibility is in agreement with the prior work of Breaker and colleagues (21), who recognized that the 10-fold affinity difference measured for preQ1 versus preQ0 in the context of the B. subtilis aptamer could not exclude preQ0 as a physiologically relevant target.
Proposed Model of Translational Attenuation
The T. tengcongensis preQ1 riboswitch represents an example of direct translational attenuation by the criteria that the aptamer is located fewer than 15 nucleotides upstream of the start codon (supplemental Fig. S1) and the sequestration of RBS sequences occurs within the aptamer domain (1). The preponderance of investigations on genetic regulation by riboswitches has focused on transcriptional control, thus making the T. tengcongensis aptamer a notable exception. Although the cellular concentrations of preQ1 are not available in the literature, we can invoke the known Km of 390 nm for the E. coli tRNA transglycosylase (56), which incorporates preQ1 into tRNA and provides a reasonable upper limit for the metabolite's cellular concentration. Because the T. tengcongensis aptamer exhibits a KD for preQ1 of 2.05 nm (Table 3), it would appear that the aptamer senses preQ1 at concentrations ∼200-fold lower than its steady-state consumption level. This seems plausible because the regulated gene product, COG1564, is a putative membrane protein invoked in preQ1 transport (21, 57). Because GTP levels in E. coli are as high as 1.3–4.9 mm (58), the cell may need to be significantly depleted of GTP before it abandons de novo Q synthesis to undertake preQ1 scavenging. With an off-rate of 1.53 × 10−4 s−1, the half-life for dissociation of preQ1 from the T. tengcongensis aptamer is 1.4 h. By contrast, the half-life for decay of the average E. coli message is 5 min (59), with individual half-lives ranging from seconds to as long as 1 h (59). This time frame suggests that once preQ1 binds to the T. tengcongensis aptamer, the associated mRNA will undergo significant translational attenuation. However, translational attenuation of riboswitches such as the T. tengcongensis aptamer must also consider kinetic control. In E. coli, transcription and translation are closely coupled via the NusE-NusG protein interaction (32), which would require regulation by the T. tengcongensis aptamer to occur at preQ1 levels far above its KD. Such models require additional testing and validation but are worth considering in light of the different KD values associated with members of the preQ1 riboswitch family.
Differences in Ligand-induced Conformational Changes among PreQ1 Aptamers from Different Genera
The preQ1 riboswitch represents the first opportunity to compare metabolite-induced conformational changes by aptamers from three different organisms. Prior analysis of the B. subtilis and F. nucleatum preQ1 riboswitches suggested that aptamer folding was dependent strictly on the presence of ligand (23, 25, 26). Our SAXS analysis of the F. nucleatum aptamer supports such a ligand-induced conformational change (Fig. 4), and the associated Kratky analysis suggests only partial folding in the absence of preQ1 (supplemental Fig. S2D). This finding is consistent with other free-state analyses of these aptamers in solution that showed formation of a hairpin structure attached to a disordered 3′-tail (23, 26). By contrast, the T. tengcongensis aptamer is highly compact in the free state (RgP = 17.0 Å, rmax = 57 Å) and indicates little change in compactness in the presence of preQ1 (RgP = 16.5, rmax = 51 Å). These results demonstrate that small riboswitch aptamers can exhibit highly compact free states in contrast to prior assumptions (60) and that variable degrees of ligand-dependent folding among riboswitches of the same class may be commonplace, suggesting that the mechanism of action for a given aptamer must be evaluated on a case-by-case basis (61).
Expectations for Molecular Level Conformational Features in the Free State
Crystal structures of the T. tengcongensis aptamer exhibited compact conformations in the bound and free states with dimensions that concur with those measured in solution (Table 2). However, efforts to fit observed and calculated scattering profiles (Fig. 4A) revealed that the preQ1-bound state is a significantly better descriptor of the solution conformation (with a χ2 of 1.7, indicating a reasonable fit (11)) as compared with the free-state structure with a χ2 of 5.8, which is considered poor (Fig. 4A). The discrepancy between the preQ1-free-state crystal structure and the solution data most likely originates from the constraints of the crystal lattice rather than incompleteness of the free-state model, which is missing nucleotide A13 (Fig. 2C). Our test calculations indicated that such a deletion has only a nominal effect on χ2. In contrast, the free-state crystal structure fits better to the preQ1-bound SAXS data than the free-state scattering data. This result is best explained by the similar crystal-packing environments of both crystal forms, which produced rmax values of ∼51 Å (Table 2). In solution, only the preQ1-bound sample has an rmax of 51 ± 5 Å, whereas the free state showed an rmax of 57 ± 6 A. This suggests that crystal packing favors a more bound-state-like rmax, which may be the main source of the discrepancy in the free-state scattering profiles. An analogous result was observed previously for the SAM I riboswitch in which the SAM-bound state showed a χ2 value of 1.6 between the crystal structure and solution data, whereas the free-state aptamer had a χ2 value of 5.1. Like position A14 of the T. tengcongensis aptamer, position A46 of the SAM I aptamer moves 7.5 Å in the free state to occlude SAM access (supplemental Fig. S3, A versus B) (11).
Although the T. tengcongensis and SAM I free-state crystal structures are not adequate descriptors of solution scattering data, it is worth noting some general structural expectations for their free-state conformations. The observation that their ligand-binding pockets are blocked (Fig. 2D versus supplemental Fig. S3B) also has been observed for free-state crystal structures of the lysine riboswitch (9, 10) and the U65C mutant of the pbuE adenine riboswitch (62). Because such conformations are ill suited to ligand binding, it is most plausible that they are in equilibrium with open states in solution (11). The plausibility of this model came from the conformational ensemble analysis of the SAM-I riboswitch in which both open and closed conformations were required to deconvolute the free-state solution scattering profile, producing a χ2 value of 0.83 (11). Members of the ensemble exhibited substantial changes in the orientation of helical segments, demonstrating folding differences beyond localized open and closed states. We envision a similar ensemble for the preQ1 riboswitch.
Implications for Long Range Signaling between the Ligand Pocket and the RBS
Our mutagenesis results on various bases in and around the metabolite-binding pocket suggested that single functional group changes often exhibit deleterious binding effects incommensurate with those expected from the loss of 1–2 hydrogen bonds. Investigations of protein and RNA enzymes have shown that changes distant from the active site can influence catalytic function because they are part of an interaction network that limits dynamics and provide proper orientation of catalytic functional groups (63, 64).
It is conceivable that riboswitch aptamers are subject to control by similar interaction networks and that these help sample conformations conducive to ligand binding while maintaining a fold that couples ligand binding to distant regions of the riboswitch. This organization could serve as a basis to control accessibility to gene regulatory sequences, such as the RBS. We favor this interpretation for the T. tengcongensis preQ1 riboswitch because a series of interconnected movements were implied by the free-state structure as a result of inserting base A14 into the ligand-binding pocket (Fig. 3A, arrows). Of special note is the greater accessibility to the RBS in the free-state structure and its apparent uncoupling from the aptamer core through the loss of base stacking. It remains to be seen whether this mode of interaction will be a general phenomenon for riboswitches that utilize direct translation attenuation to regulate gene expression.
Supplementary Material
Acknowledgments
We thank Nils Walter and Robert Batey for critical remarks on the manuscript. We thank Jason Salter and other members of the Wedekind laboratory for helpful discussions. We thank Clara L. Kielkopf for assistance with SPR, which was obtained through National Institutes of Health Grant RR027241 (to Clara L. Kielkopf). We thank Richard Gillilan and the staff of MacCHESS for assistance with SAXS and crystallography. We thank the staff of SSRL for help with remote data collection. MacCHESS is supported by National Science Foundation Award DMR-0225180 and NCRR, National Institutes of Health, Award RR-01646SSRL. SSRL is operated by Stanford on behalf of the United States Department of Energy. The SSRL Structural Molecular Biology Program is supported by the Department of Energy and by NCRR and NIGMS, National Institutes of Health.
This work was supported, in whole or in part, by National Institutes of Health Grants GM072623 (to V. B.) and GM063162 and RR026501 (to J. E. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
The atomic coordinates and structure factors (codes 3Q50 and 3Q51) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- Q
- queuosine
- RBS
- ribosome-binding site
- SAXS
- small angle x-ray scattering
- SPR
- surface plasmon resonance
- CHESS
- Cornell High Energy Synchrotron Source
- SSRL
- Stanford Synchrotron-Radiation Lightsource
- DAP
- 2,6-diaminopurine.
REFERENCES
- 1. Barrick J. E., Breaker R. R. (2007) Genome Biol. 8, R239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Winkler W. C., Breaker R. R. (2005) Annu. Rev. Microbiol. 59, 487–517 [DOI] [PubMed] [Google Scholar]
- 3. Irnov Kertsburg A., Winkler W. C. (2006) Cold Spring Harb. Symp. Quant. Biol. 71, 239–249 [DOI] [PubMed] [Google Scholar]
- 4. Blount K. F., Breaker R. R. (2006) Nat. Biotechnol. 24, 1558–1564 [DOI] [PubMed] [Google Scholar]
- 5. Lee E. R., Blount K. F., Breaker R. R. (2009) RNA Biol. 6, 187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serganov A. (2010) RNA Biol. 7, 98–103 [DOI] [PubMed] [Google Scholar]
- 7. Edwards A. L., Reyes F. E., Héroux A., Batey R. T. (2010) RNA 16, 2144–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang L., Serganov A., Patel D. J. (2010) Mol. Cell 40, 774–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garst A. D., Héroux A., Rambo R. P., Batey R. T. (2008) J. Biol. Chem. 283, 22347–22351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serganov A., Huang L., Patel D. J. (2008) Nature 455, 1263–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stoddard C. D., Montange R. K., Hennelly S. P., Rambo R. P., Sanbonmatsu K. Y., Batey R. T. (2010) Structure 18, 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ottink O. M., Rampersad S. M., Tessari M., Zaman G. J., Heus H. A., Wijmenga S. S. (2007) RNA 13, 2202–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stoddard C. D., Gilbert S. D., Batey R. T. (2008) RNA 14, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grosjean H., de Crécy-Lagard V., Björk G. R. (2004) Trends Biochem. Sci. 29, 519–522 [DOI] [PubMed] [Google Scholar]
- 15. McCarty R. M., Somogyi A., Lin G., Jacobsen N. E., Bandarian V. (2009) Biochemistry 48, 3847–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee B. W., Van Lanen S. G., Iwata-Reuyl D. (2007) Biochemistry 46, 12844–12854 [DOI] [PubMed] [Google Scholar]
- 17. Iwata-Reuyl D. (2003) Bioorg. Chem. 31, 24–43 [DOI] [PubMed] [Google Scholar]
- 18. Hurt J. K., Olgen S., Garcia G. A. (2007) Nucleic Acids Res. 35, 4905–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marks T., Farkas W. R. (1997) Biochem. Biophys. Res. Commun. 230, 233–237 [DOI] [PubMed] [Google Scholar]
- 20. Huang B. S., Wu R. T., Chien K. Y. (1992) Cancer Res. 52, 4696–4700 [PubMed] [Google Scholar]
- 21. Roth A., Winkler W. C., Regulski E. E., Lee B. W., Lim J., Jona I., Barrick J. E., Ritwik A., Kim J. N., Welz R., Iwata-Reuyl D., Breaker R. R. (2007) Nat. Struct. Mol. Biol. 14, 308–317 [DOI] [PubMed] [Google Scholar]
- 22. Spitale R. C., Torelli A. T., Krucinska J., Bandarian V., Wedekind J. E. (2009) J. Biol. Chem. 284, 11012–11016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kang M., Peterson R., Feigon J. (2009) Mol. Cell 33, 784–790 [DOI] [PubMed] [Google Scholar]
- 24. Klein D. J., Edwards T. E., Ferré-D'Amaré A. R. (2009) Nat. Struct. Mol. Biol. 16, 343–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rieder U., Kreutz C., Micura R. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 10804–10809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rieder U., Lang K., Kreutz C., Polacek N., Micura R. (2009) Chembiochem 10, 1141–1144 [DOI] [PubMed] [Google Scholar]
- 27. Ali M., Lipfert J., Seifert S., Herschlag D., Doniach S. (2010) J. Mol. Biol. 396, 153–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Serganov A., Polonskaia A., Phan A. T., Breaker R. R., Patel D. J. (2006) Nature 441, 1167–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thore S., Leibundgut M., Ban N. (2006) Science 312, 1208–1211 [DOI] [PubMed] [Google Scholar]
- 30. Lipfert J., Das R., Chu V. B., Kudaravalli M., Boyd N., Herschlag D., Doniach S. (2007) J. Mol. Biol. 365, 1393–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wedekind J. E., McKay D. B. (2000) Methods Enzymol. 317, 149–168 [DOI] [PubMed] [Google Scholar]
- 32. McCarty R. M., Bandarian V. (2008) Chem. Biol. 15, 790–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quaranta D., McCarty R., Bandarian V., Rensing C. (2007) J. Bacteriol. 189, 5361–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davoll J. (1960) J. Chem. Soc., 131–138 [Google Scholar]
- 35. Soltis S. M., Cohen A. E., Deacon A., Eriksson T., González A., McPhillips S., Chui H., Dunten P., Hollenbeck M., Mathews I., Miller M., Moorhead P., Phizackerley R. P., Smith C., Song J., van dem Bedem H., Ellis P., Kuhn P., McPhillips T., Sauter N., Sharp K., Tsyba I., Wolf G. (2008) Acta Crystallogr. D Biol. Crystallogr. 64, 1210–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 37. Adams P. D., Gopal K., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Pai R. K., Read R. J., Romo T. D., Sacchettini J. C., Sauter N. K., Storoni L. C., Terwilliger T. C. (2004) J. Synchrotron Radiat. 11, 53–55 [DOI] [PubMed] [Google Scholar]
- 38. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific, San Carlos, CA [Google Scholar]
- 40. CCP4 (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 41. Ando N., Chenevier P., Novak M., Tate M. W., Gruner S. M. (2008) J. Appl. Crystallogr. 41, 167–175 [Google Scholar]
- 42. Kabsch W. (1976) Acta Crystallogr. Sect. A 32, 922–923 [Google Scholar]
- 43. Konarev P. V., Volkov V. V., Sokolova A. V., Koch M. H., Svergun D. I. (2003) J. Appl. Crystallogr. 36, 1277–1282 [Google Scholar]
- 44. Feigin L., Svergun D. (1987) Structure Analysis by Small-Angle X-Ray and Neutron Scattering, Plenum Press, New York [Google Scholar]
- 45. Svergun D. I. (1992) J. Appl. Crystallogr. 25, 495–503 [Google Scholar]
- 46. Svergun D., Barberato C., Koch M. H. (1995) J. Appl. Crystallogr. 28, 768–773 [Google Scholar]
- 47. Kleywegt G., Zou J., Kjeldgaard M., Jones T. (2001) in International Tables for Crystallography (Rossmann M., Arnold E. eds) pp. 353–356 and 366–367, Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 48. Myszka D. G. (1999) J. Mol. Recognit. 12, 279–284 [DOI] [PubMed] [Google Scholar]
- 49. Mertens H. D., Svergun D. I. (2010) J. Struct. Biol. 172, 128–141 [DOI] [PubMed] [Google Scholar]
- 50. Doniach S. (2001) Chem. Rev. 101, 1763–1778 [DOI] [PubMed] [Google Scholar]
- 51. Lang K., Rieder R., Micura R. (2007) Nucleic Acids Res. 35, 5370–5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rieder R., Lang K., Graber D., Micura R. (2007) Chembiochem 8, 896–902 [DOI] [PubMed] [Google Scholar]
- 53. Smith K. D., Lipchock S. V., Ames T. D., Wang J., Breaker R. R., Strobel S. A. (2009) Nat. Struct. Mol. Biol. 16, 1218–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fersht A. R., Shi J. P., Knill-Jones J., Lowe D. M., Wilkinson A. J., Blow D. M., Brick P., Carter P., Waye M. M., Winter G. (1985) Nature 314, 235–238 [DOI] [PubMed] [Google Scholar]
- 55. Northrop D. B. (1998) J. Chem. Educ. 75, 1153–1157 [Google Scholar]
- 56. Hoops G. C., Townsend L. B., Garcia G. A. (1995) Biochemistry 34, 15381–15387 [DOI] [PubMed] [Google Scholar]
- 57. Meyer M. M., Roth A., Chervin S. M., Garcia G. A., Breaker R. R. (2008) RNA 14, 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bennett B. D., Kimball E. H., Gao M., Osterhout R., Van Dien S. J., Rabinowitz J. D. (2009) Nat. Chem. Biol. 5, 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Celesnik H., Deana A., Belasco J. G. (2007) Mol. Cell 27, 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang J., Lau M. W., Ferré-D'Amaré A. R. (2010) Biochemistry 49, 9123–9131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baird N. J., Kulshina N., Ferré-D'Amaré A. R. (2010) RNA Biol. 7, 328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Delfosse V., Bouchard P., Bonneau E., Dagenais P., Lemay J. F., Lafontaine D. A., Legault P. (2010) Nucleic Acids Res. 38, 2057–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rueda D., Bokinsky G., Rhodes M. M., Rust M. J., Zhuang X., Walter N. G. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10066–10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Forconi M., Sengupta R. N., Piccirilli J. A., Herschlag D. (2010) Biochemistry 49, 2753–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. García De La Torre J., Huertas M. L., Carrasco B. (2000) Biophys. J. 78, 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Praaenikar J., Afonine P. V., Guncar G., Adams P. D., Turk D. (2009) Acta Crystallogr. D Biol. Crystallogr. 65, 921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.